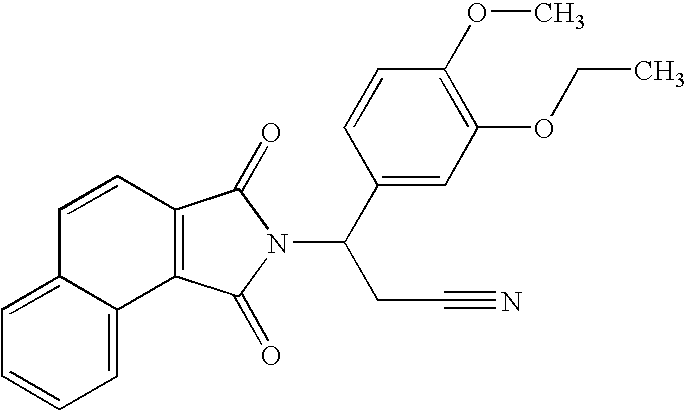
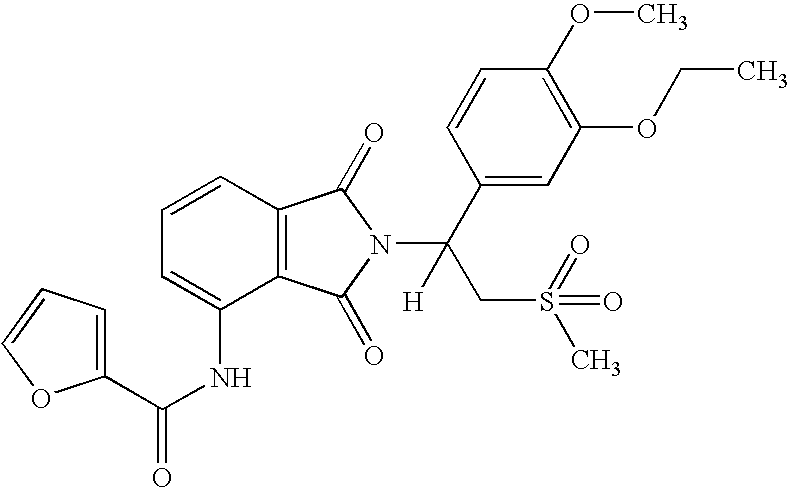
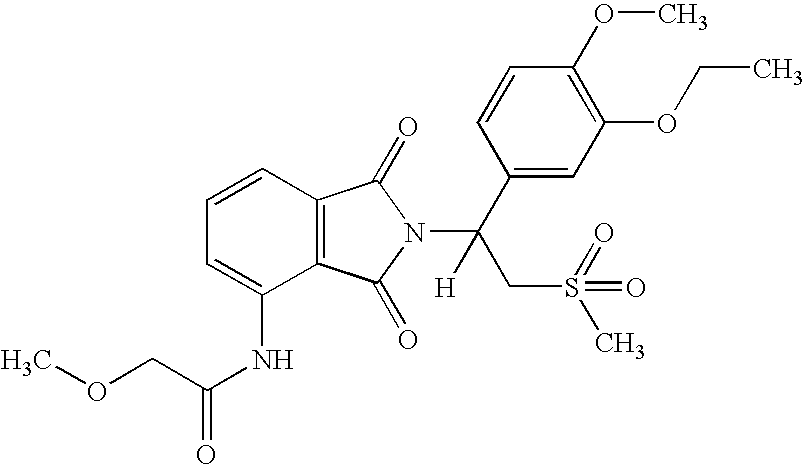
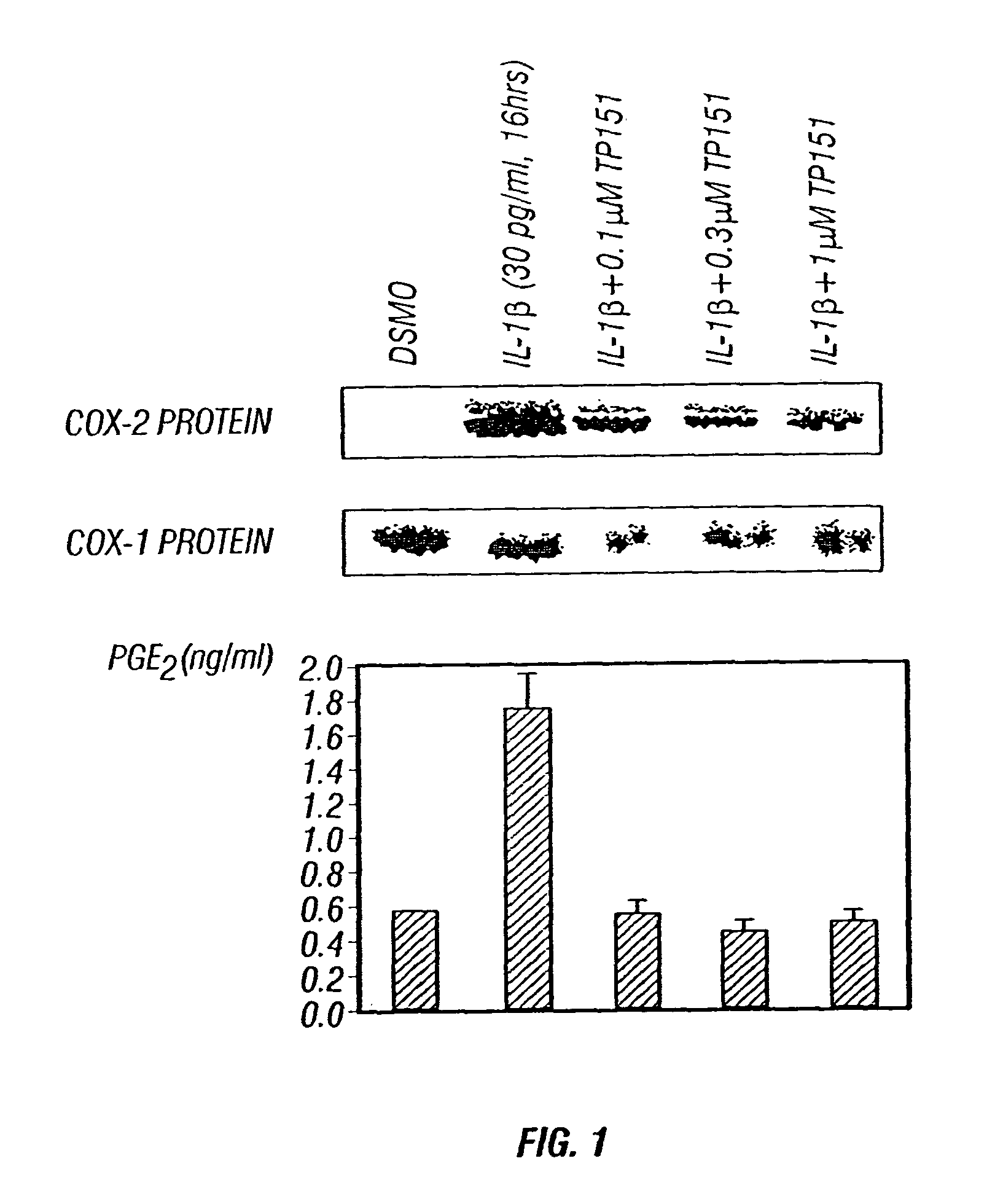
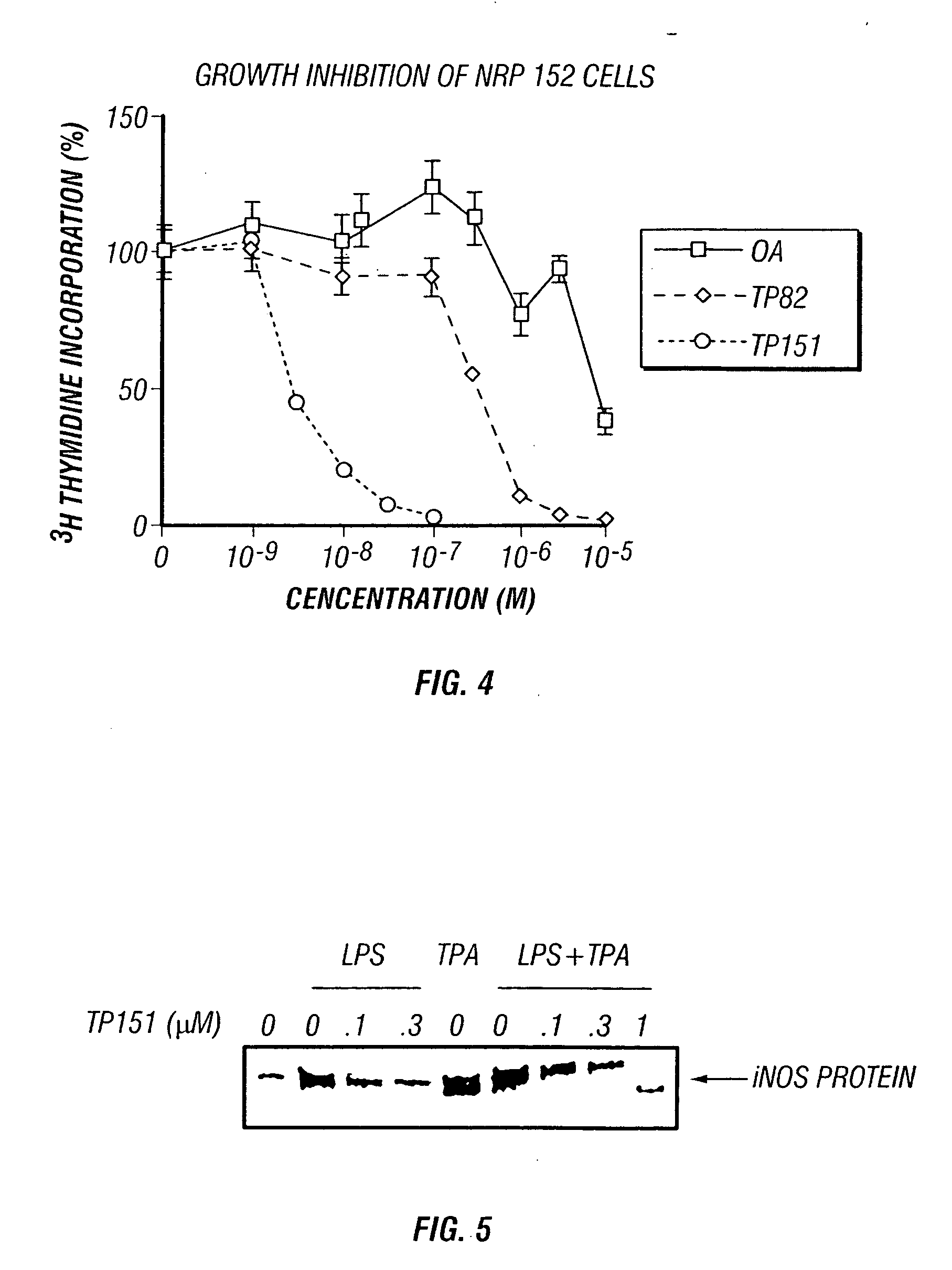
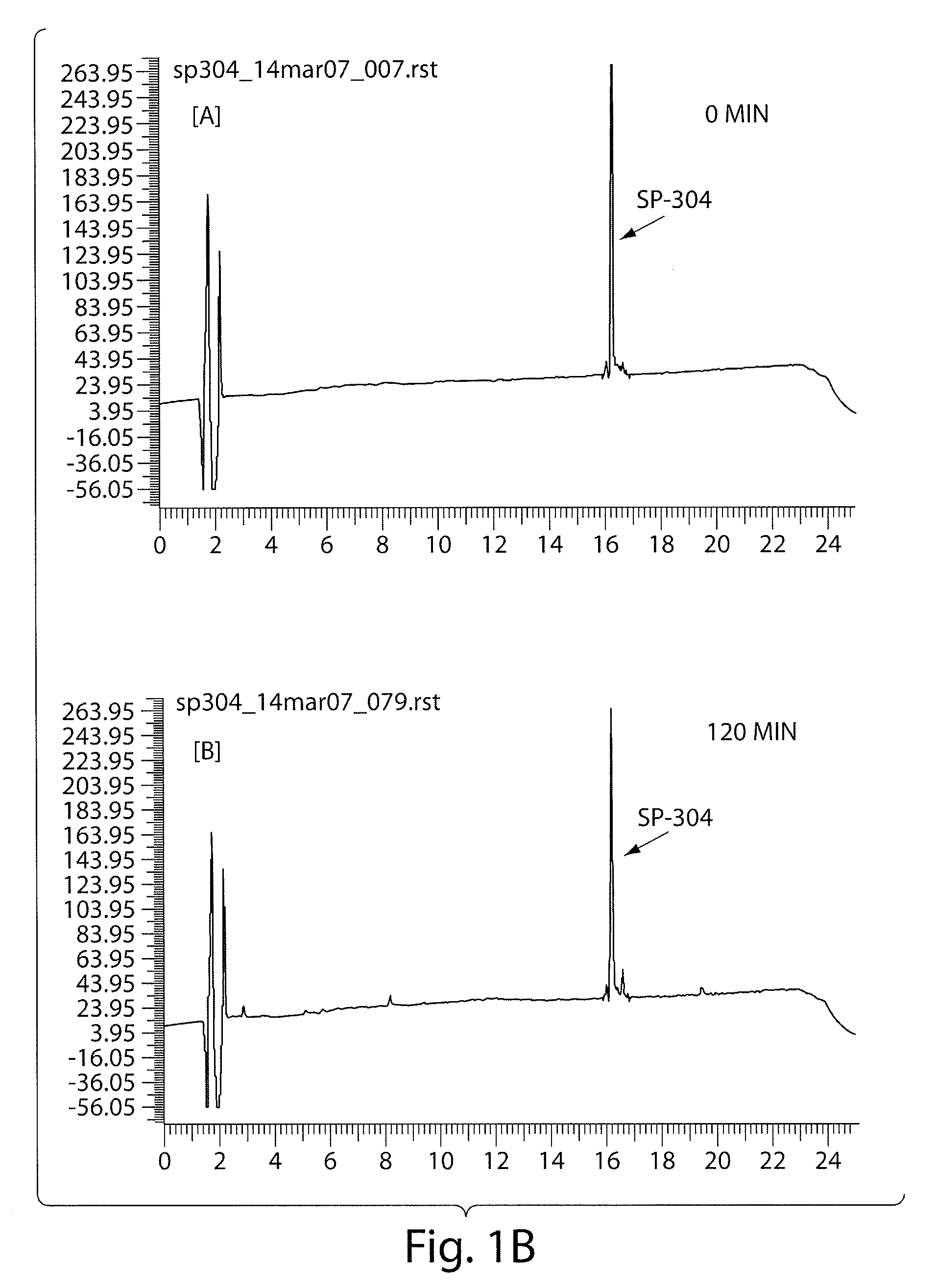
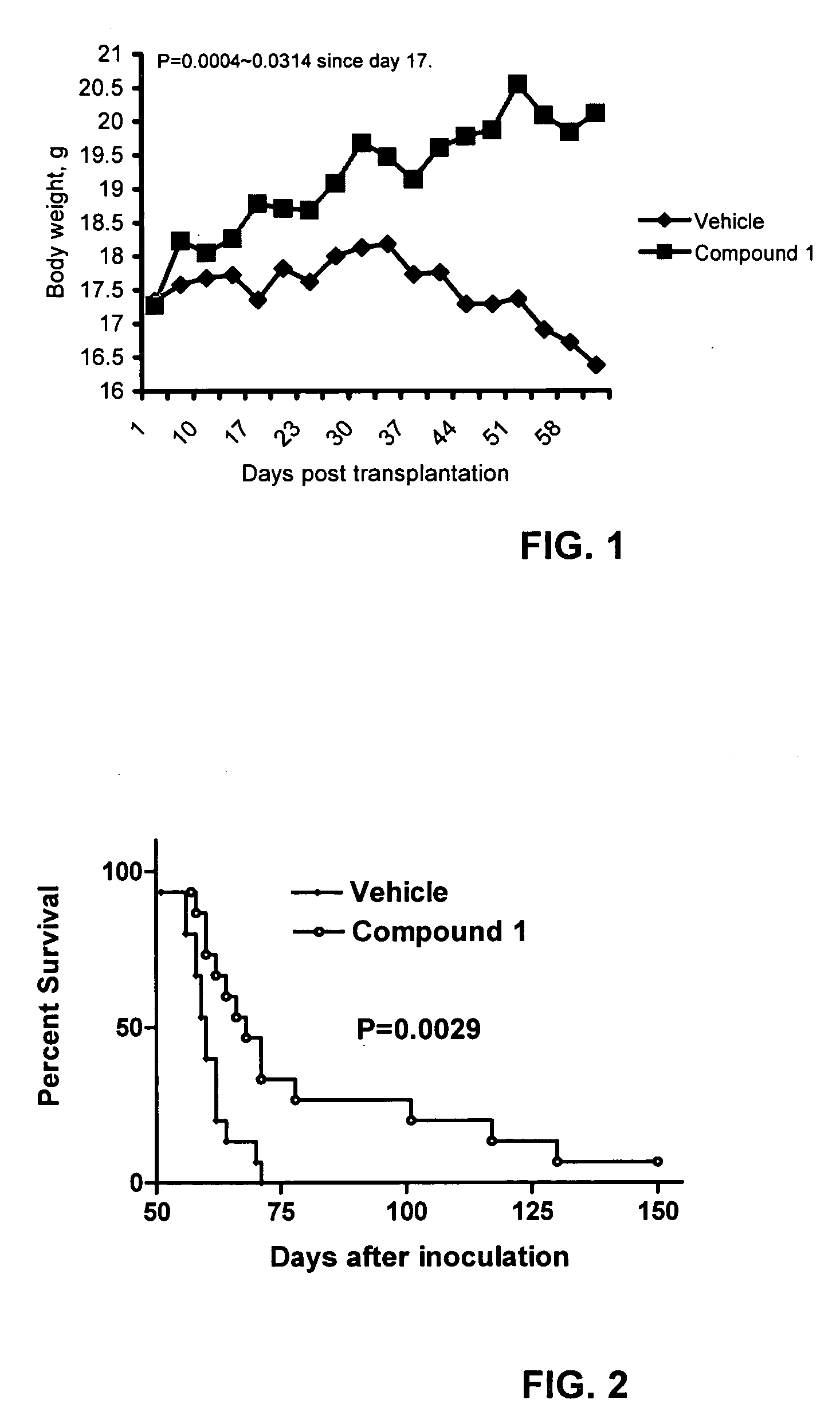
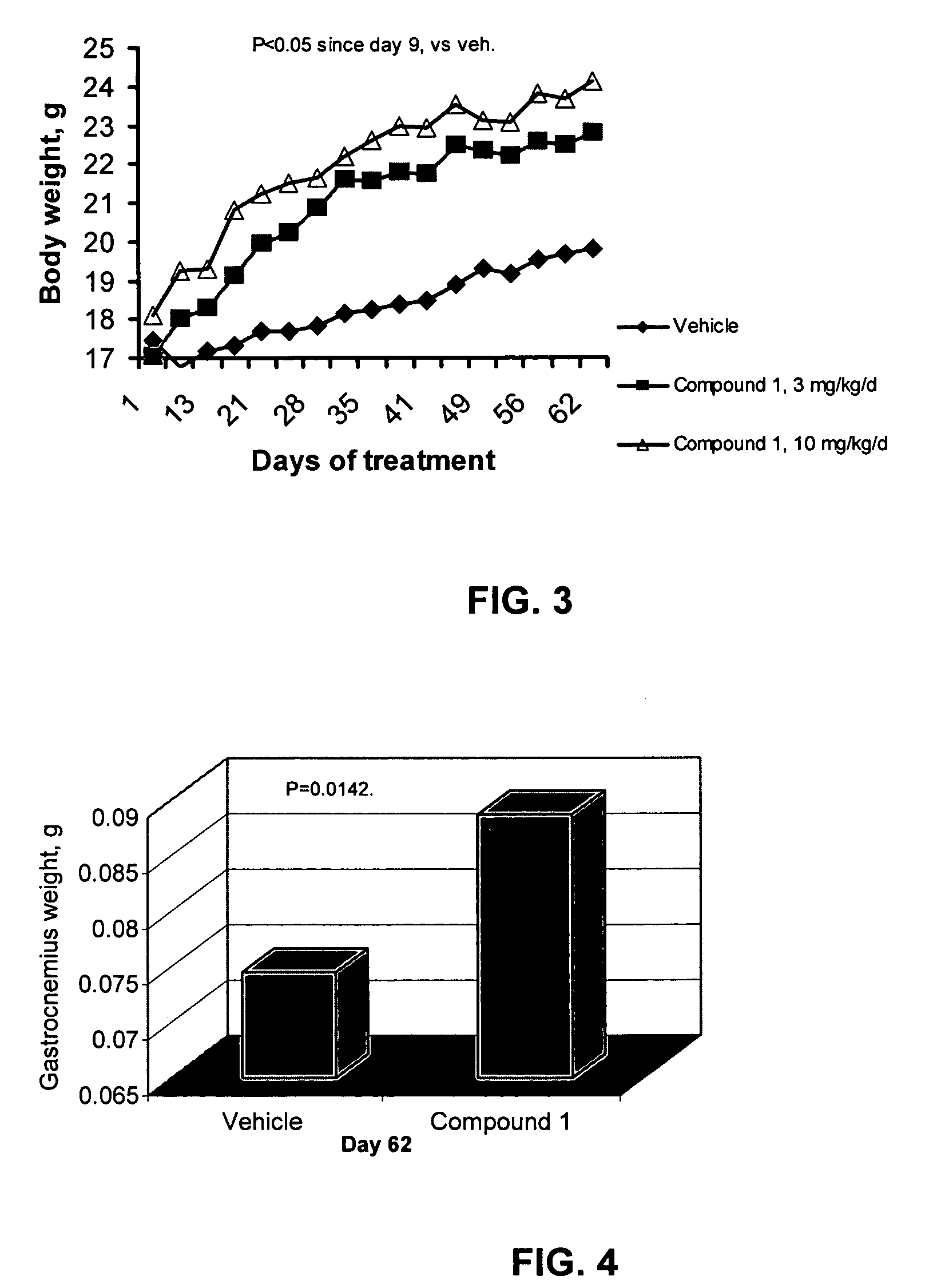
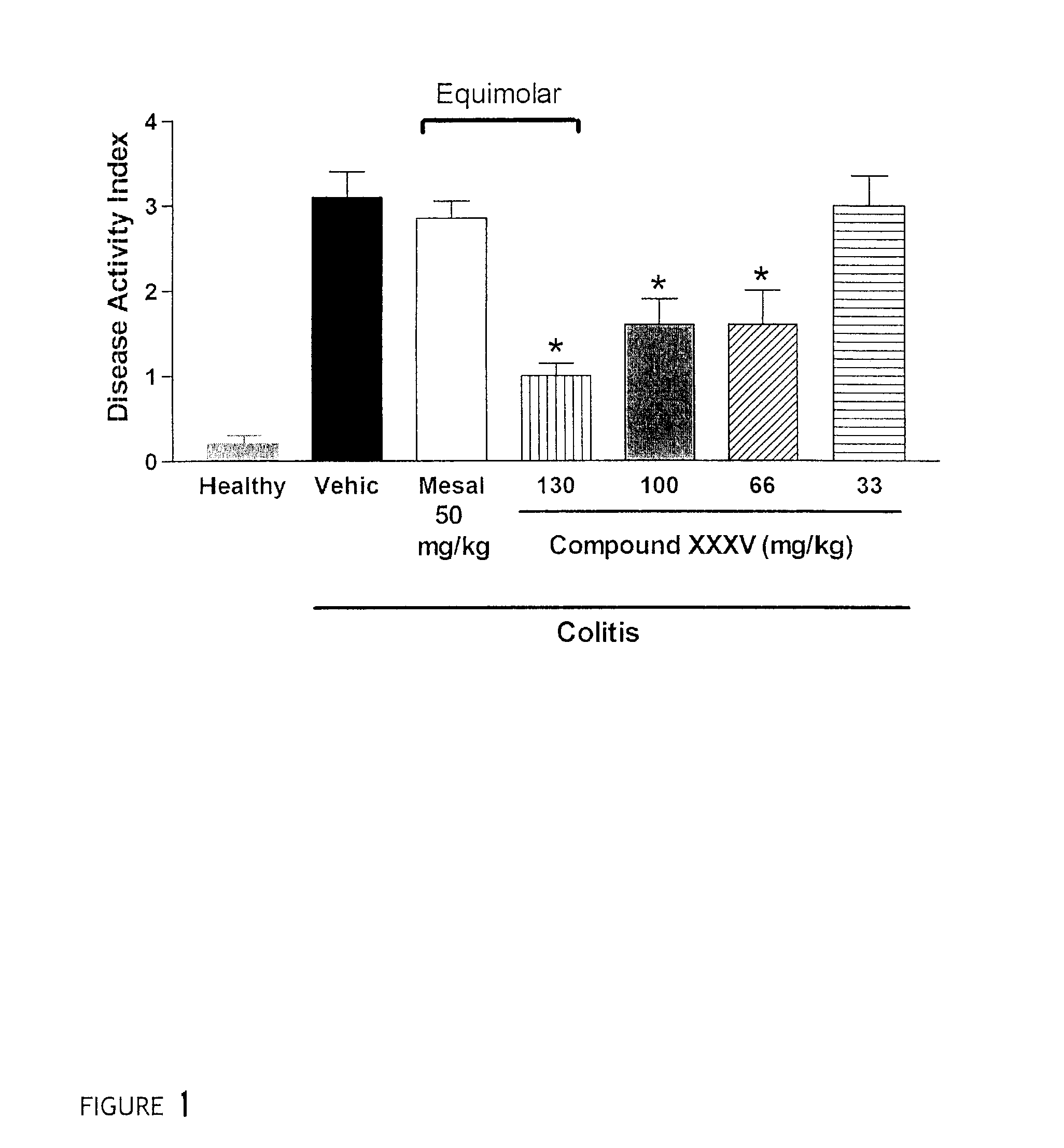
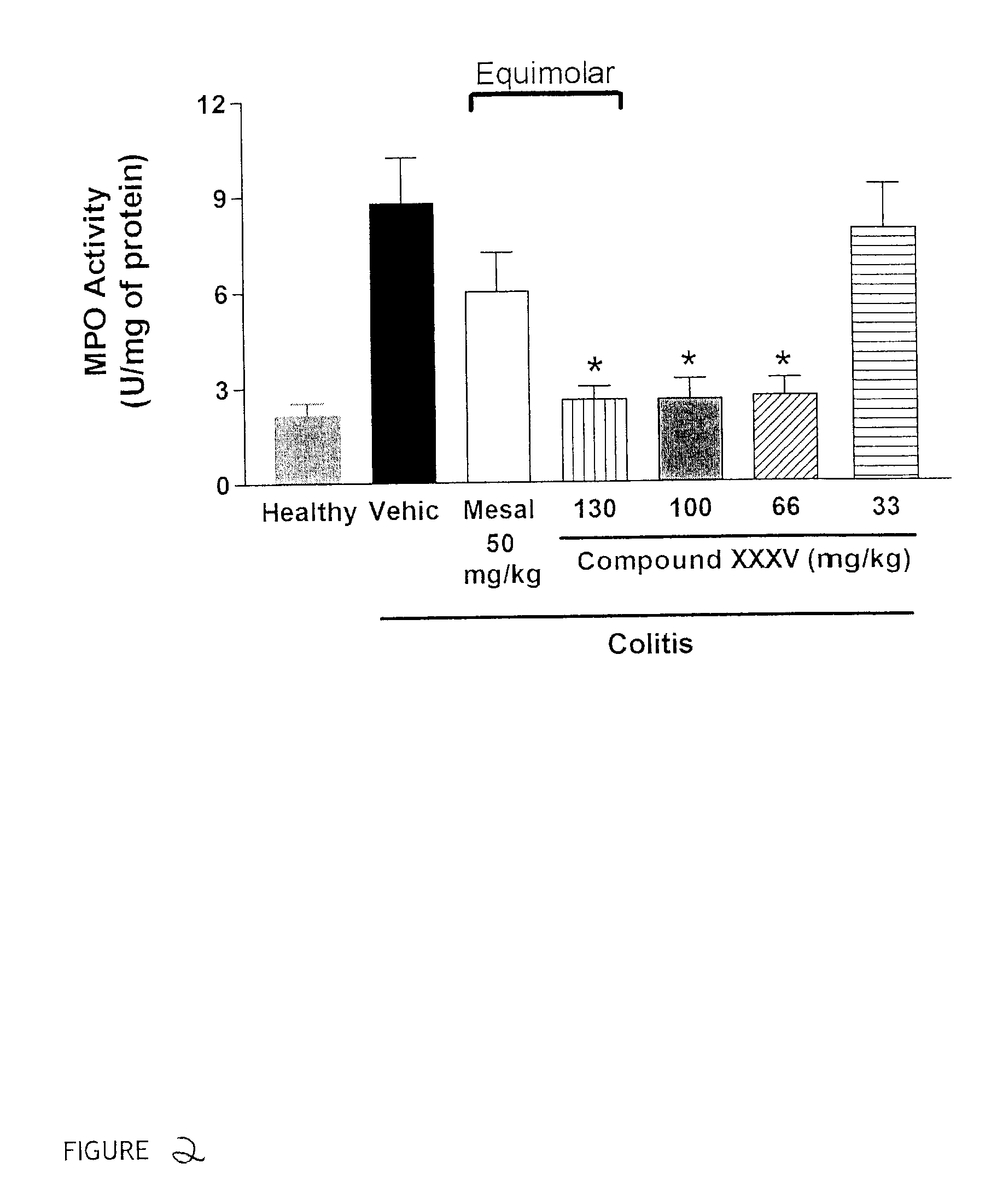
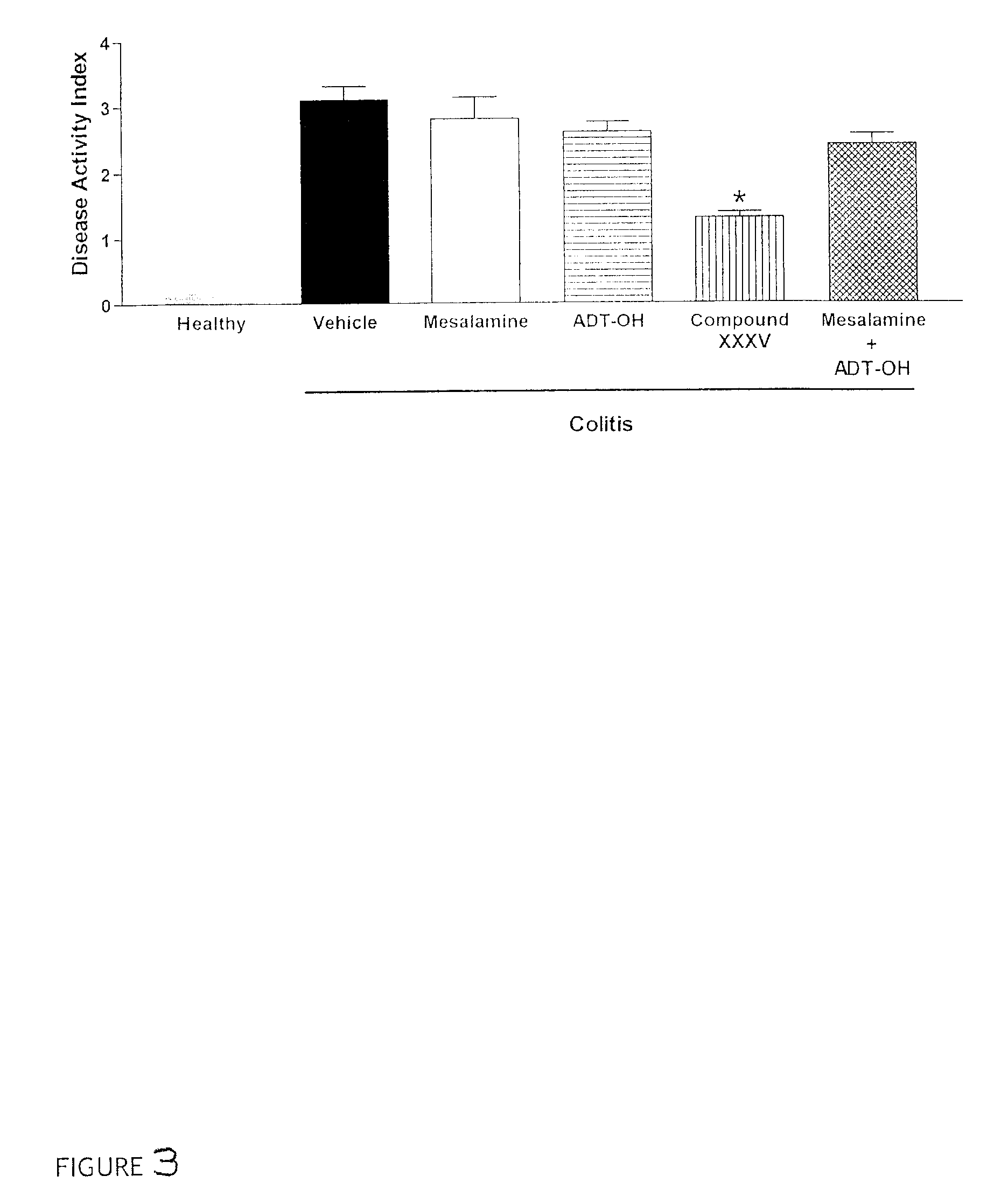
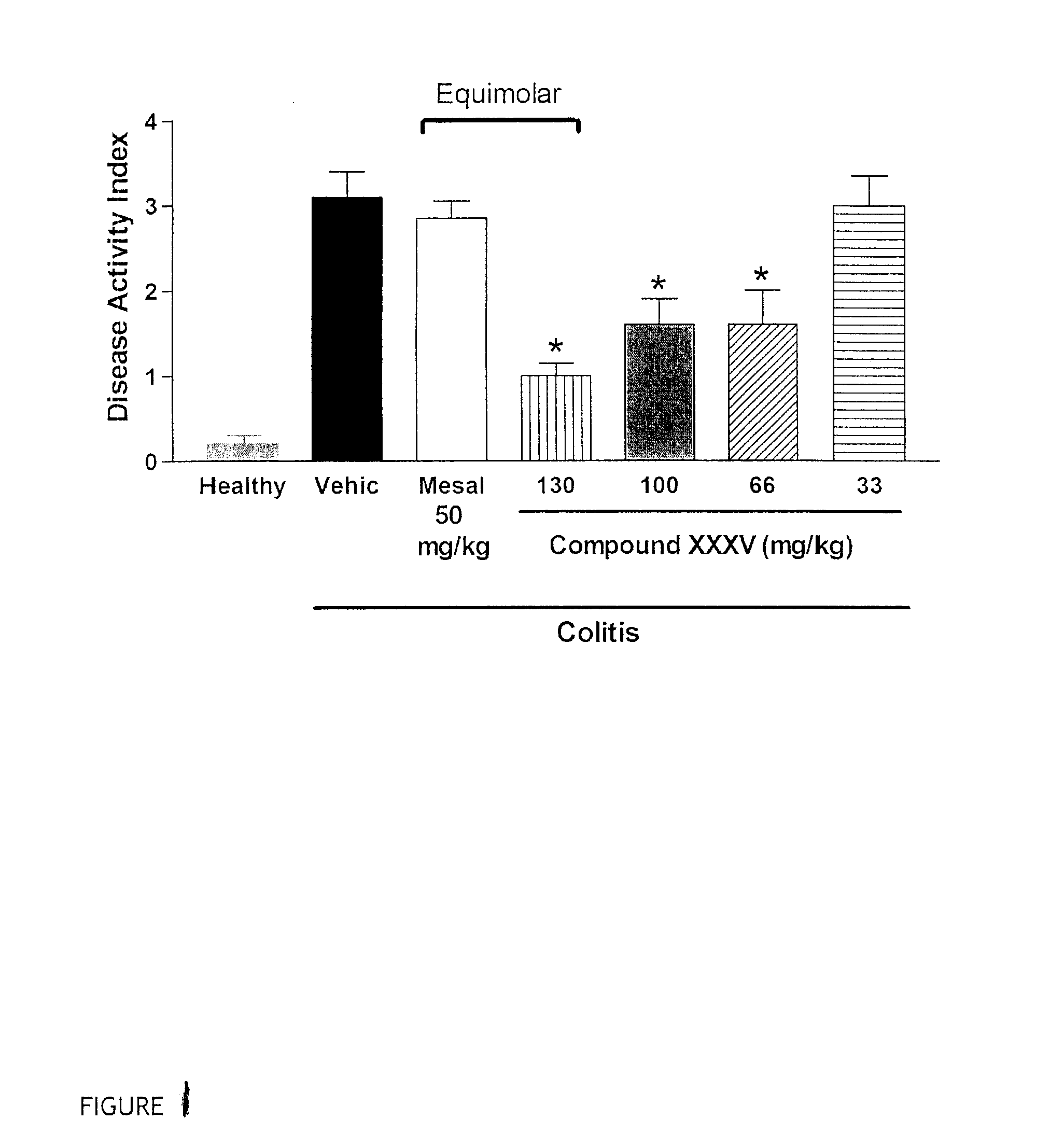
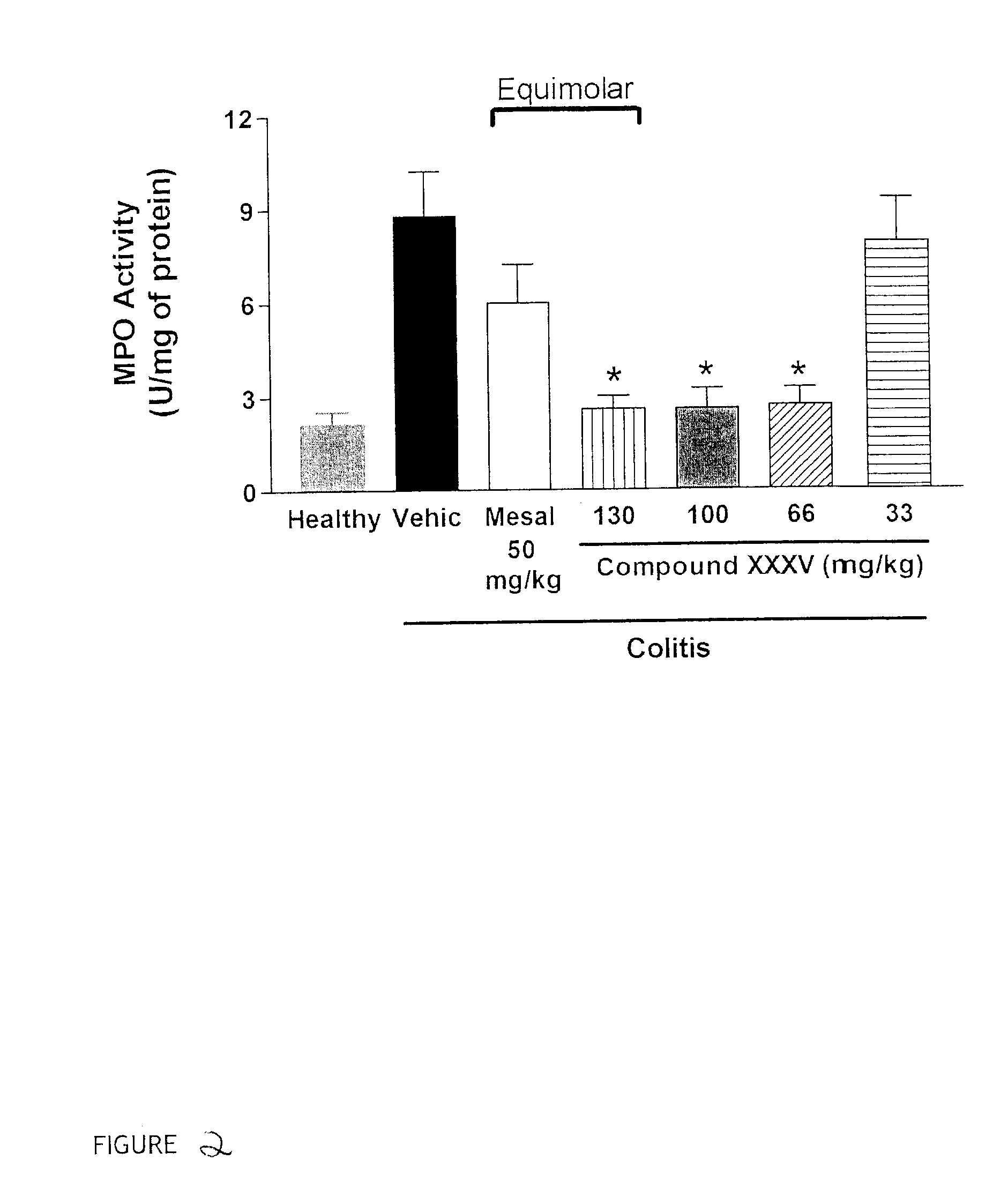
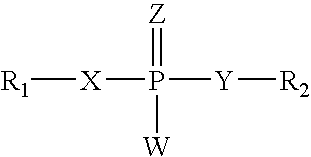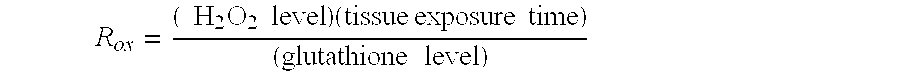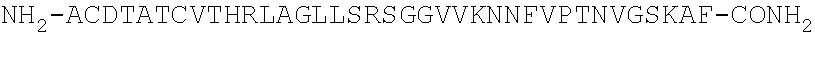Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1065 results about "Autoimmune inflammatory bowel disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The chief types of inflammatory bowel disease are Crohn's disease and ulcerative colitis (UC). Inflammatory bowel diseases fall into the class of autoimmune diseases, in which the body's own immune system attacks elements of the digestive system.
Method for treatment of disorders of the gastrointestinal system
There are provided novel synthetic stool preparations comprising bacteria isolated from a fecal sample from a healthy donor. The synthetic stool preparations are used for treating disorders of the gastrointestinal tract, including dysbiosis, Clostridium difficile infection and recurrent Clostridium difficile infection, prevention of recurrence of Clostridium difficile infection, treatment of Crohn's disease, ulcerative colitis, irritable bowel syndrome, inflammatory bowel disease, and diverticular disease, and treatment of food poisoning such as salmonella. Methods of preparation and methods of use of the synthetic stool preparations are also provided.
Owner:UNIVERSITY OF GUELPH +2
Isoindoline compounds and methods of making and using the same
The invention encompasses isoindoline compounds, pharmaceutical compositions comprising them, and methods of their use for the treatment, prevention or management of various diseases and disorders. Examples include, but are not limited to, cancer, inflammatory bowel disease and myelodysplastic syndrome.
Owner:AMGEN INC
IBD-associated microbial antigens and methods of using same
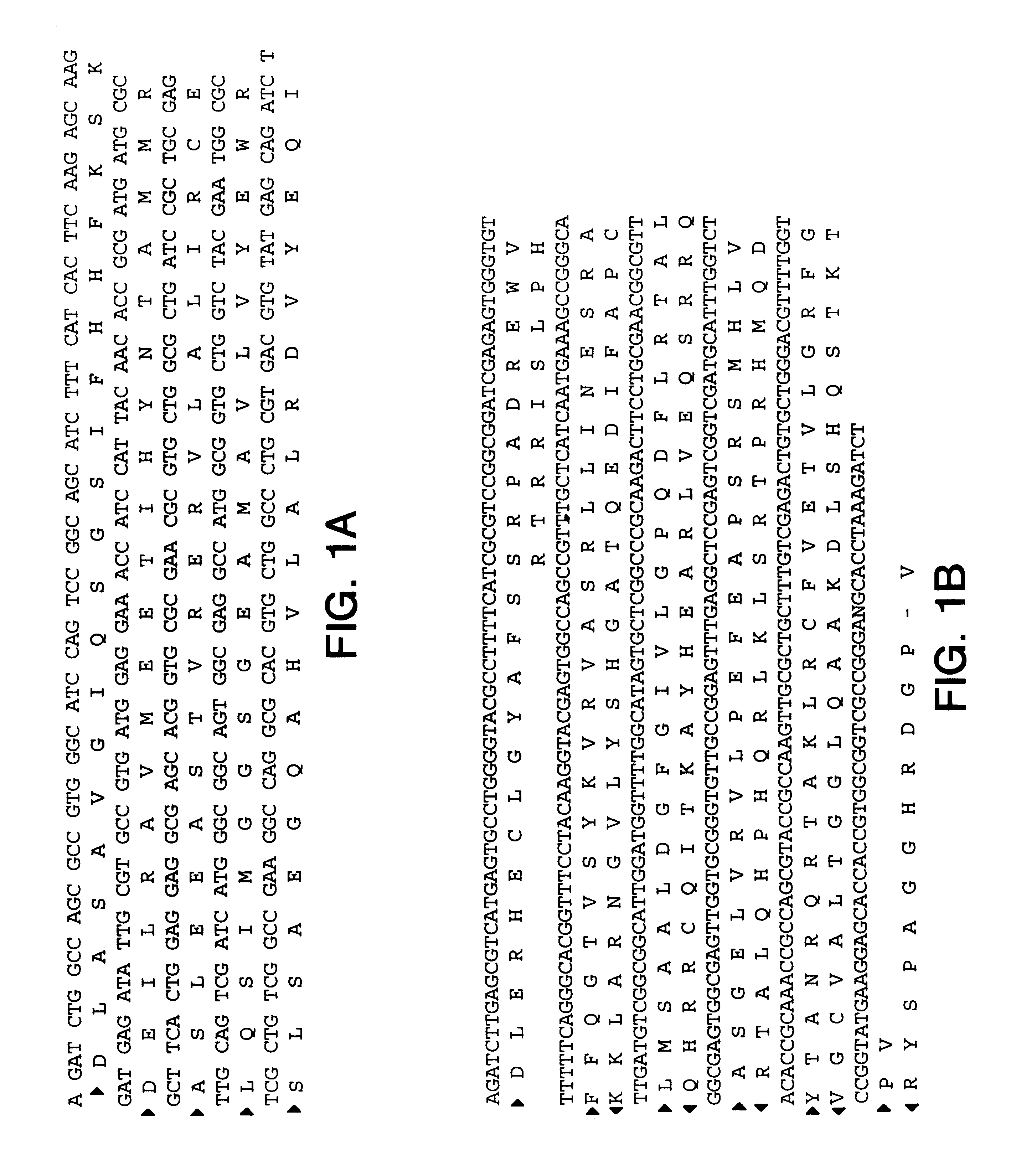
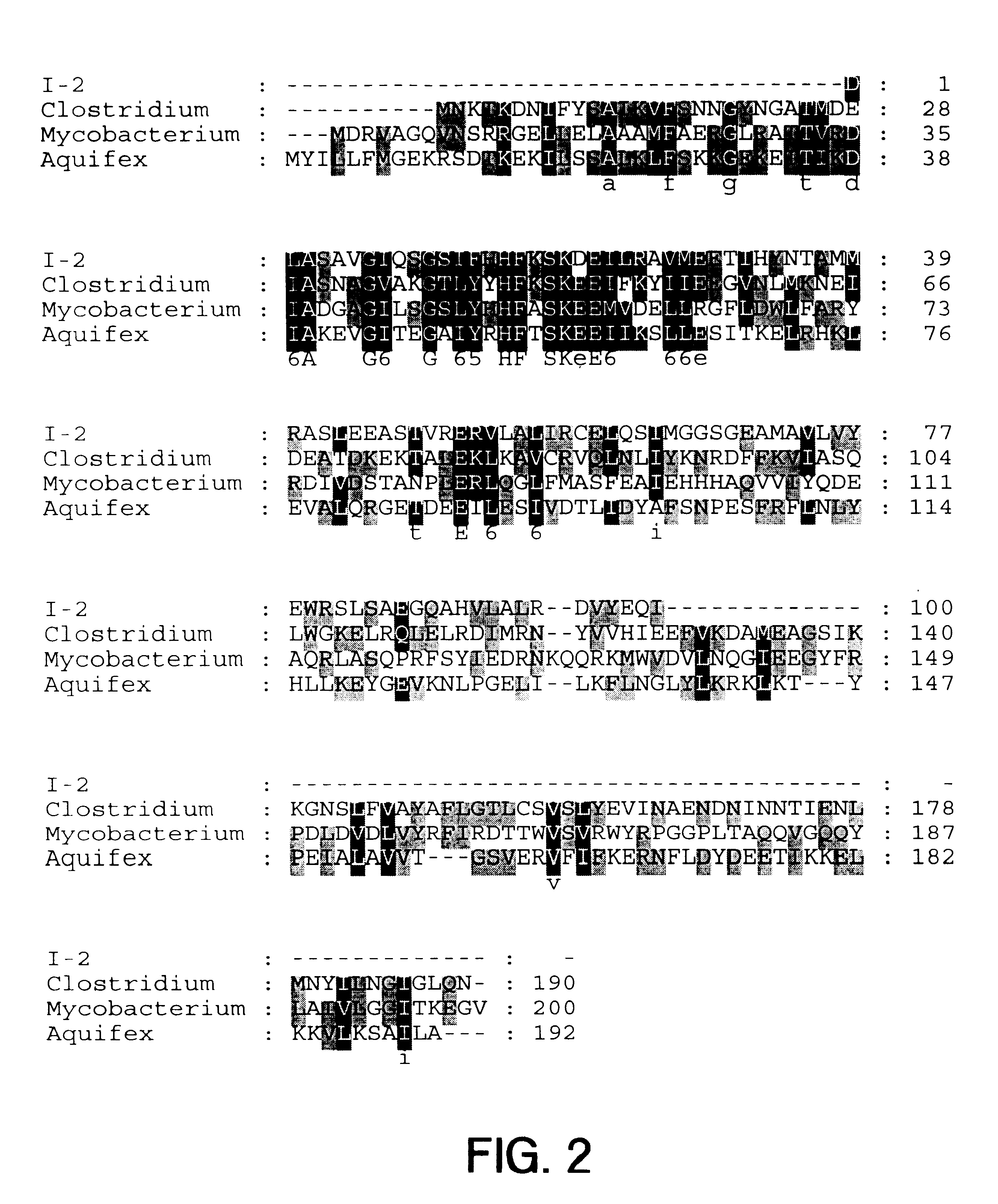
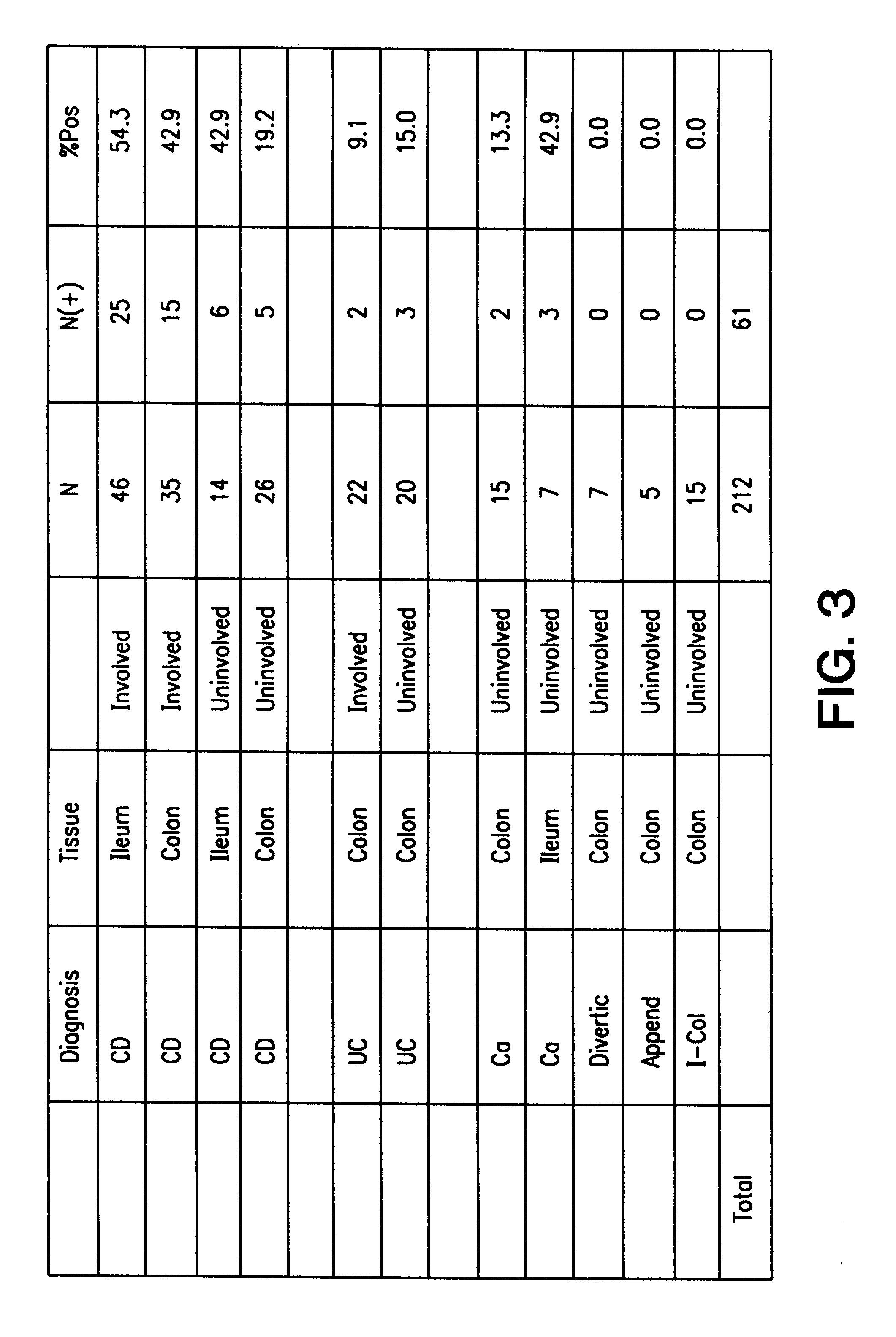
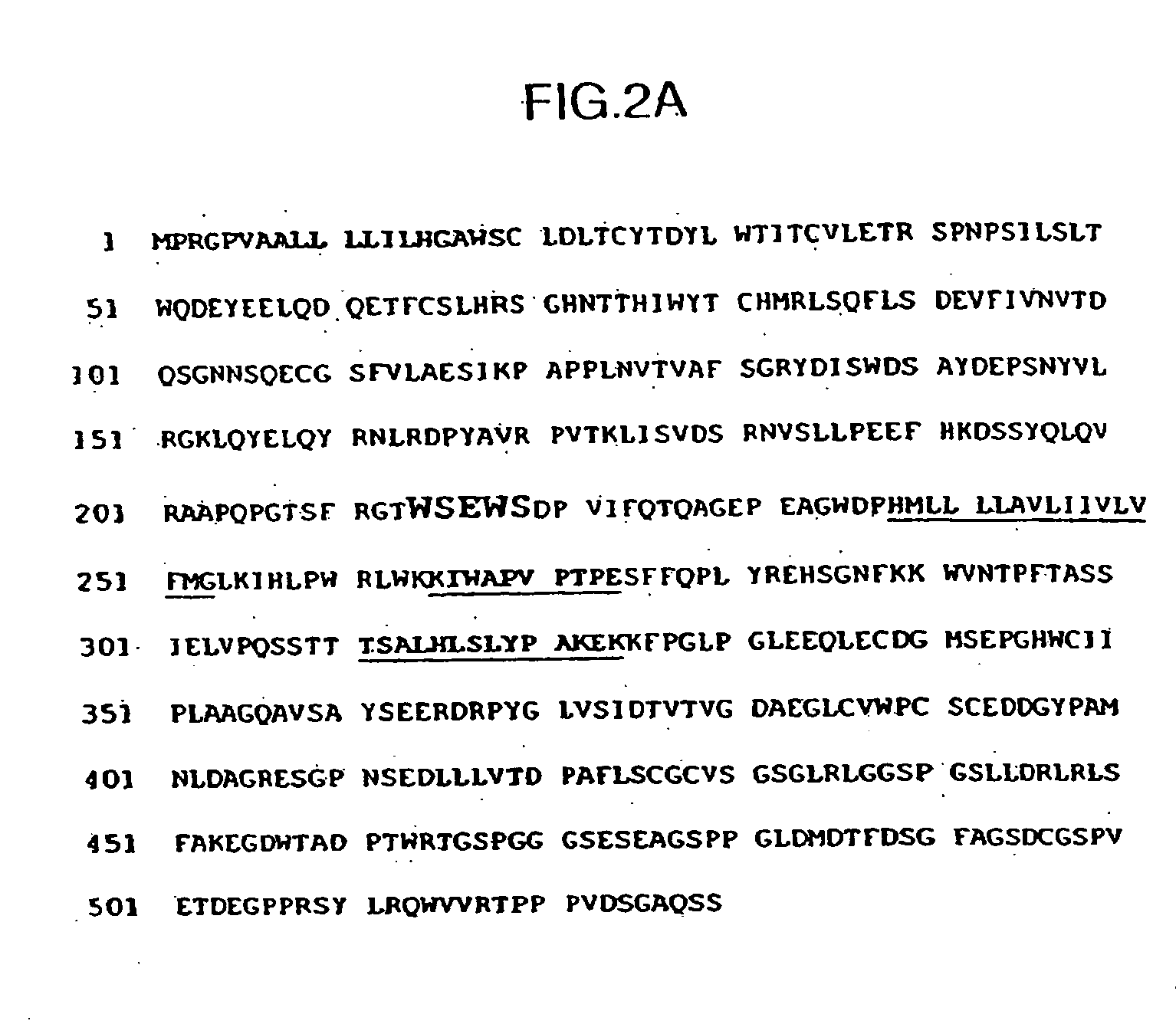
The present invention provides nucleic acid and amino acid sequence of the novel I-1 and I-2 polypeptides, which are associated with human inflammatory bowel disease (IBD). Methods of diagnosing and treating inflammatory bowel disease using the IBD-associated I-1 and I-2 antigens also are provided.
Owner:RGT UNIV OF CALIFORNIA
Antagonizing interleukin-21 receptor activity
InactiveUS20060039902A1Reduce riskSufficient amountCompounds screening/testingCompound screeningWhite blood cellFibrosis
Methods and compositions for inhibiting interleukin-21 (IL-21) / IL-21 receptor (MU-1) activity using antagonists of IL-21 or IL-21 receptor (“IL-21R” or “MU-1”), are disclosed. IL-21 / IL-21R antagonists can be used to induce immune suppression in vivo, e.g., for treating, ameliorating or preventing autoimmune or inflammatory disorders, including, e.g., inflammatory bowel disease (IBD), rheumatoid arthritis (RA), transplant / graft rejection, psoriasis, asthma, fibrosis, and systemic lupus erythematosus (SLE).
Owner:WYETH LLC
Derivatives of 4- or 5-aminosalicylic acid
InactiveUS20060270635A1Less readily absorbedEasily reach colonBiocidePhosphorous compound active ingredientsThioester synthesisSalicylic acid
The present invention provides new derivatives of 4- or 5-aminosalicylic acid, and a pharmaceutical composition containing these derivatives of 4- or 5-aminosalicylic acid as active ingredients, useful for the treatment of intestinal diseases such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) and for the prevention / treatment of colon cancer. More particularly, these derivatives comprise a hydrogen sulfide releasing moiety linked via an azo, an ester, an anhydride, a thioester or an amide linkage to a molecule of 4- or 5-aminosalicylic acid. Furthermore, the present invention provides a process for preparing these compounds and their use for treating IBD and IBS and the prevention / treatment of colon cancer.
Owner:ANTIBE THERAPEUTICS INC
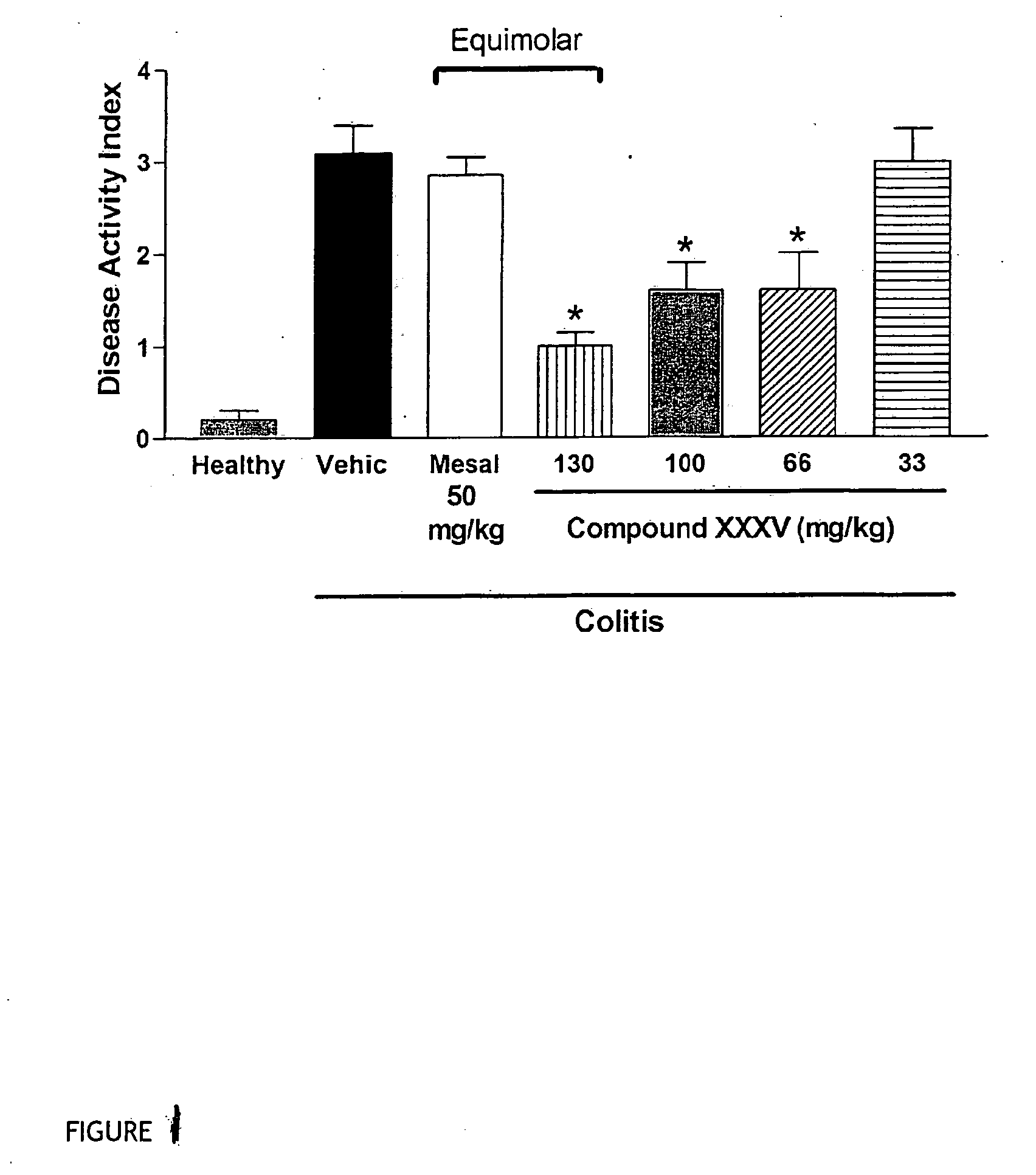
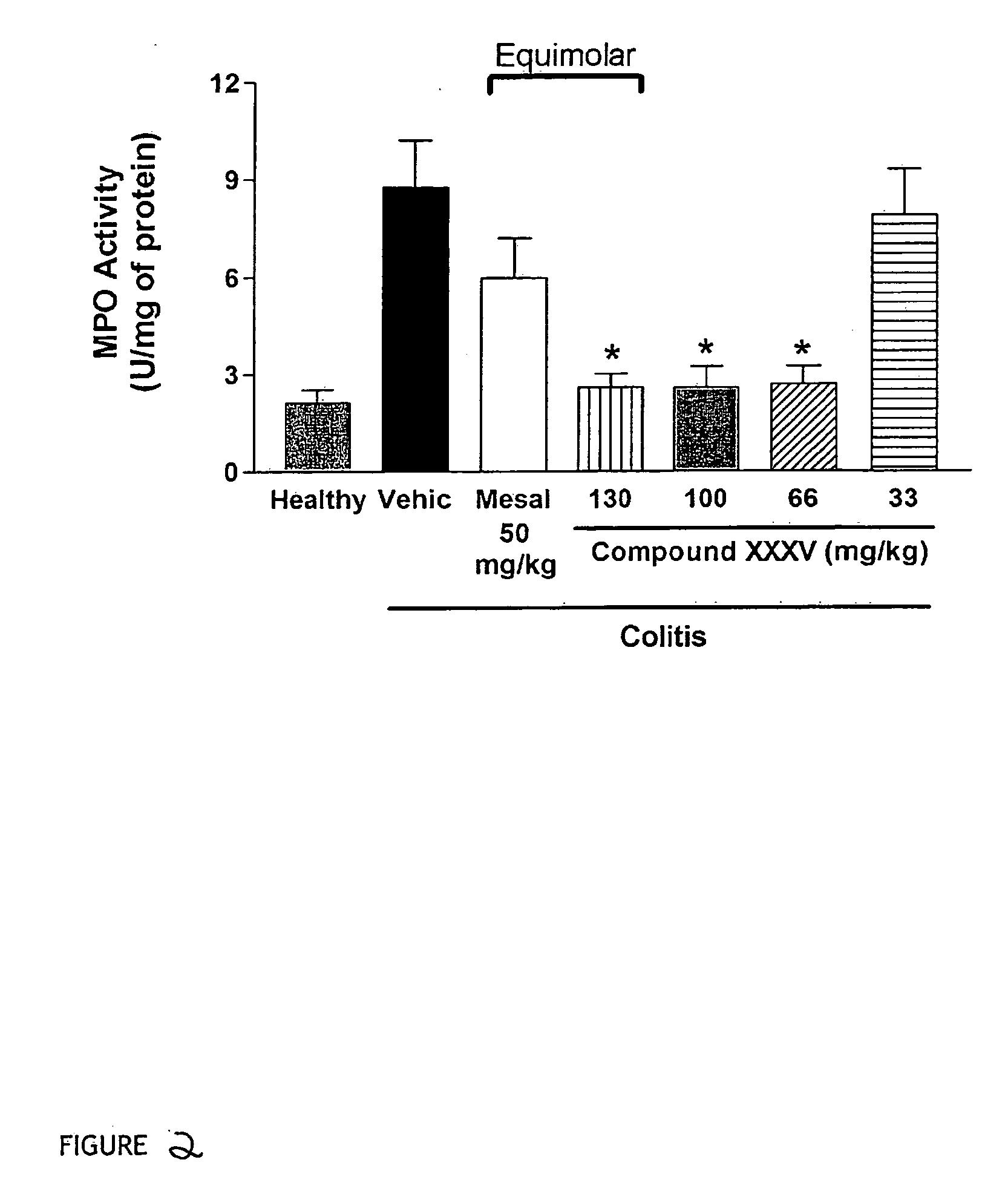
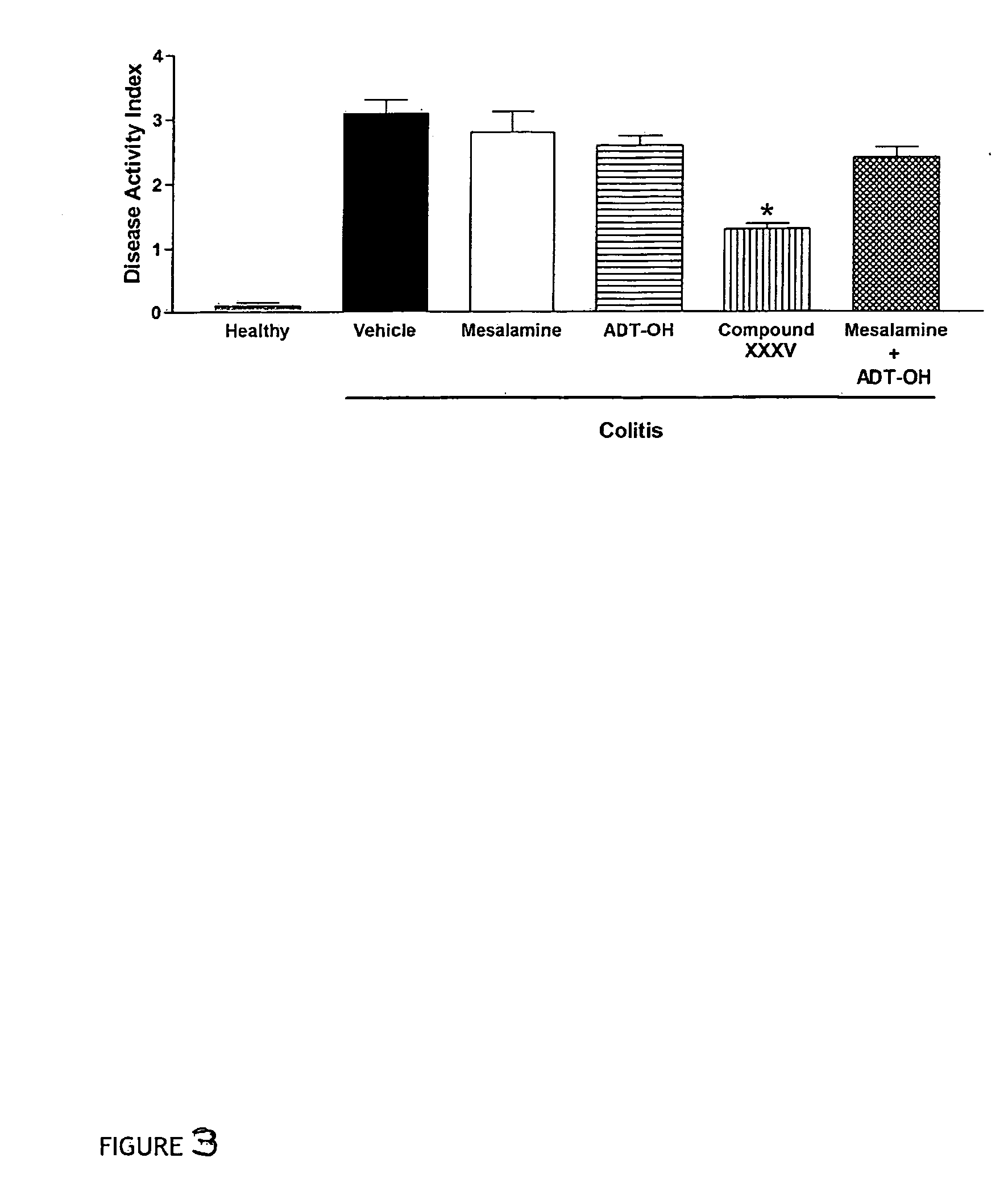
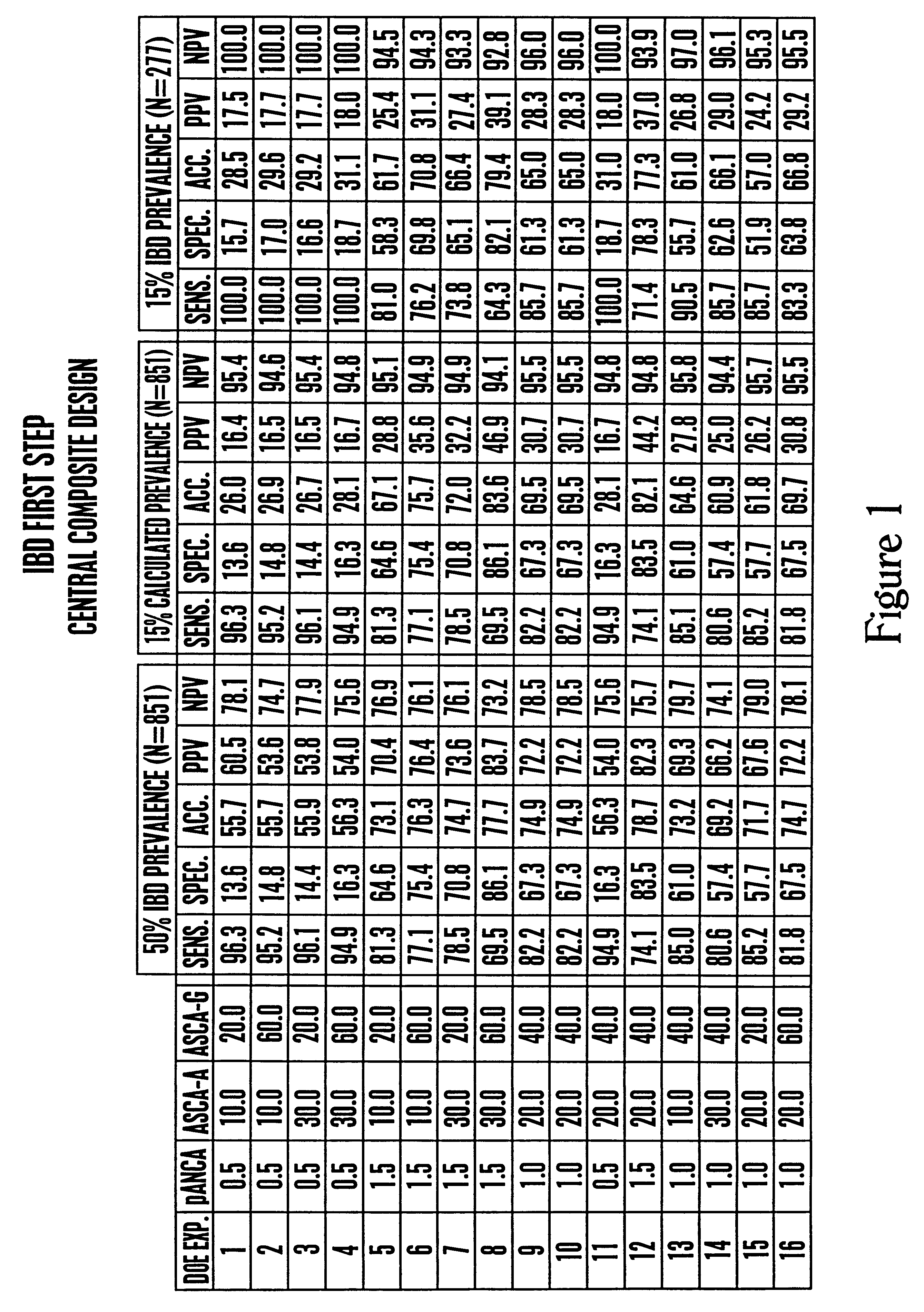
Inflammatory bowel disease first step assay system
The present invention provides a highly sensitive method of diagnosing inflammatory bowel disease (IBD) in an individual. The method includes the steps of isolating a sample from the individual; determining by non-histological means whether the sample is positive for anti-neutrophil cytoplasmic antibodies (ANCA); determining whether the sample is positive for anti-Saccharomyces cerevisiae immunoglobulin A (ASCA-IgA); determining whether the sample is positive for anti-Saccharomyces cerevisiae immunoglobulin G (ASCA-IgG); and diagnosing the individual as having IBD when the sample is positive for ANCA, ASCA-IgA or ASCA-IgG, and diagnosing the individual as not having IBD when the sample is negative for ANCA, ASCA-IgA and ASCA-IgG, provided that the method does not include histological analysis of neutrophils.
Owner:PROMETHEUS LAB +1
Application of bacteroides fragilis in preparation of composition for treating inflammatory bowel diseases
InactiveCN103156888AEnhance pharmacological effectsGood treatment effectMilk preparationBacteria material medical ingredientsPharmacologic actionBowels diseases
The invention relates to the technical field of application of bacteroides fragilis, and in particular relates to application of bacteroides fragilis in preparation of a composition for treating inflammatory bowel diseases. The experiments show that bacteroides fragilis is safe and nontoxic and strong in pharmacologic action, and has good treating effect of treating inflammatory bowel diseases, thereby indicating that the bacteroides fragilis has good edible and medicinal prospects. According to the invention, a novel use of bacteroides fragilis is explored and a novel application field is developed. Bacteroides fragilis as a probiotic can be used for preparing foods or medical compositions for treating inflammatory bowel diseases so as to provide health-cared foods or treating medicines suitable for human body to take.
Owner:广东知光生物科技有限公司
Isoindoline compounds and methods of making and using the same
The invention encompasses isoindoline compounds, pharmaceutical compositions comprising them, and methods of their use for the treatment, prevention or management of various diseases and disorders. Examples include, but are not limited to, cancer, inflammatory bowel disease and myelodysplastic syndrome.
Owner:AMGEN INC
Therapeutic compositions and methods of use
Compounds and methods useful for chemopreventative treatment of diseases such as cancer, Alzheimer's disease, Parkinson's disease, inflammatory bowel diseases, and multiple sclerosis.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Therapeutic compositions and methods of use
Compounds and methods useful for chemopreventative treatment of diseases such as cancer, Alzheimer's disease, Parkinson's disease, inflammatory bowel diseases, and multiple sclerosis.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Method and compositions for the treatment of gastrointestinal disorders
ActiveUS20060094658A1Reduce accumulationIncrease gastrointestinal motilityPeptide/protein ingredientsMetabolism disorderGastroparesisInflammatory Bowel Diseases
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
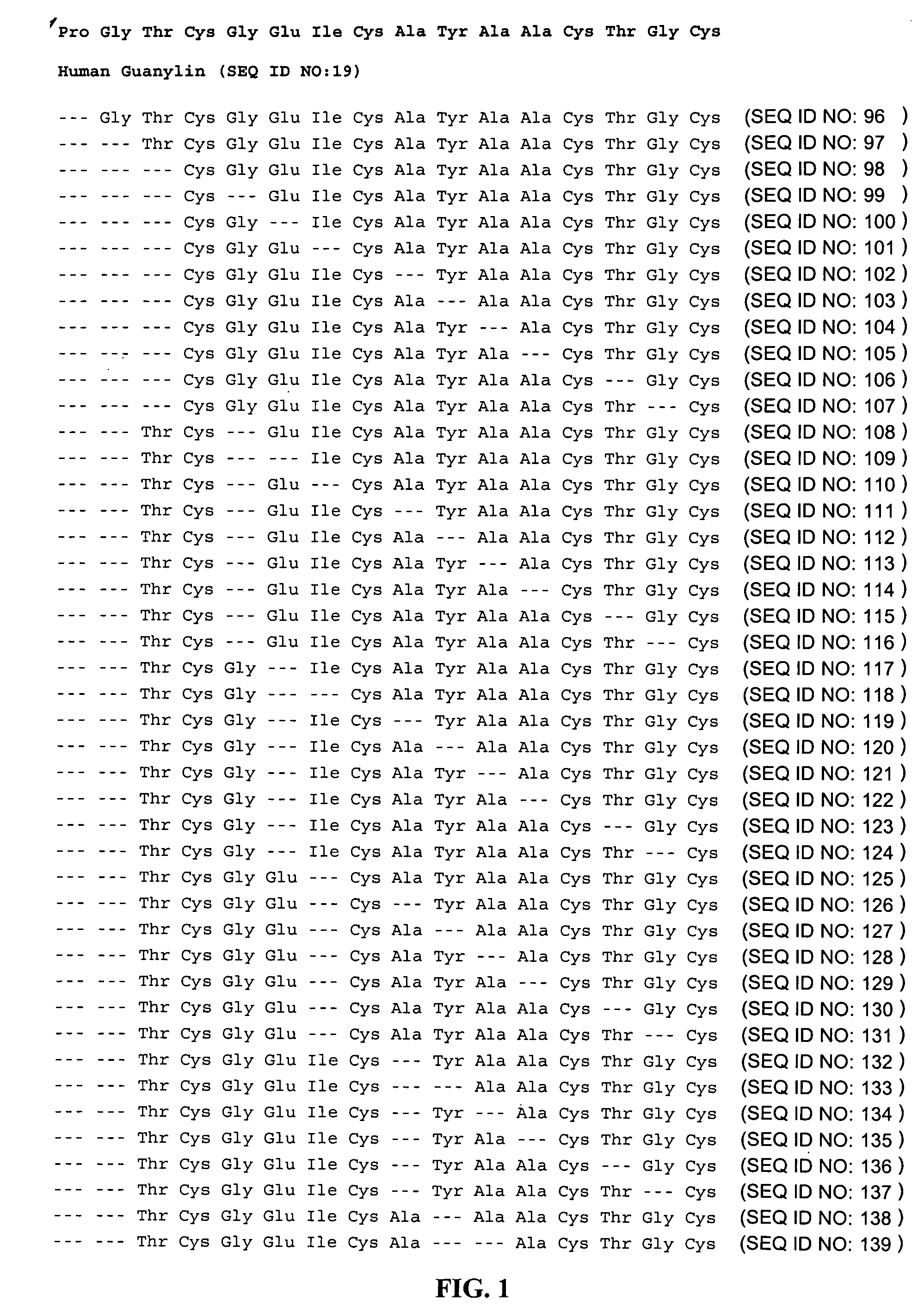
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS20090048175A1Maintain good propertiesIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition, including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
RNA interference mediated inhibition of STAT3 gene expression using short interfering nucleic acid (siNA)
InactiveUS20050196781A1Improve bioavailabilityMinimize the possibilitySugar derivativesMicrobiological testing/measurementDouble strandOrganism
This invention relates to compounds, compositions, and methods useful for modulating STAT3 gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of STAT3 gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (mRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of STAT3 genes. Such small nucleic acid molecules are useful, for example, for treating, preventing, inhibiting, or reducing cancer, proliferative, and / or inflammatory diseases, disorders, or conditions in a subject or organism, such as psoriasis, eczema, dermatitis, Crohn's disease, and inflammatory bowel disease, and for any other disease, trait, or condition that is related to or will respond to the levels of STAT3 in a cell or tissue, alone or in combination with other treatments or therapies.
Owner:SIRNA THERAPEUTICS INC
Multimeric VLA-4 antagonists comprising polymer moieties
InactiveUS20060013799A1Minimize degradationSenses disorderNervous disorderLymphatic SpreadWhite blood cell
Disclosed are conjugates which bind VLA-4. Certain of these conjugates also inhibit leukocyte adhesion and, in particular, leukocyte adhesion mediated by VLA-4. Such conjugates are useful in the treatment of inflammatory diseases in a mammalian patient, e.g., human, such as asthma, Alzheimer's disease, atherosclerosis, AIDS dementia, diabetes, inflammatory bowel disease, rheumatoid arthritis, tissue transplantation, tumor metastasis and myocardial ischemia. The conjugates can also be administered for the treatment of inflammatory brain diseases such as multiple sclerosis.
Owner:ELAN PHARM INC
Humanized immunoglobulin reactive with alpha4beta7 integrin
The present invention relates to a humanized immunoglobulin that has binding specificity for α4β7 integrin and comprises the complementarity determining regions (CDRs) of mouse Act-1 antibody, and to the humanized light chain of the humanized immunoglobulin. The present invention further relates to a humanized immunoglobulin light chain. The invention also relates to isolated nucleic acids, recombinant vectors and host cells that comprise a sequence which encodes a humanized immunoglobulin or immunoglobulin light chain, and to a method of preparing a humanized immunoglobulin. The humanized immunoglobulins can be used in therapeutic applications, for example to control lymphocyte infiltration to mucosal tissue or to treat inflammatory bowel disease.
Owner:MILLENNIUM PHARMA INC
Methods and compositions for the treatment of gastrointestinal disorders
InactiveUS20070010450A1Increase gastrointestinal motilityReduce inflammationPeptide/protein ingredientsMetabolism disorderIntestinal tract diseasesGastroparesis
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
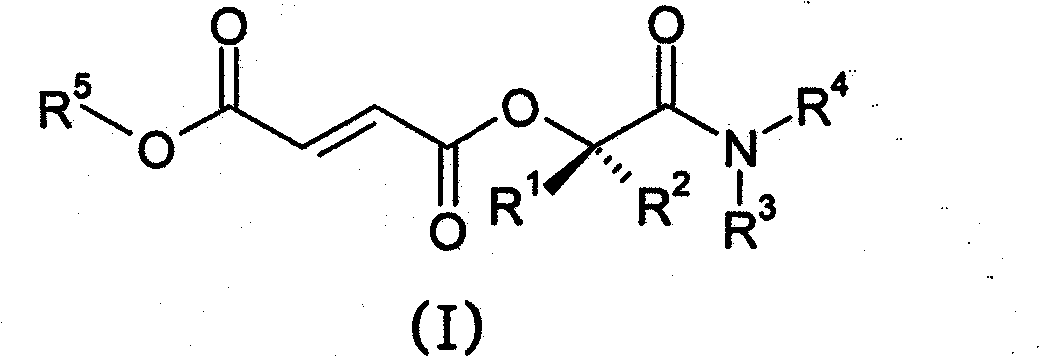
Prodrugs of methyl hydrogen fumarate, pharmaceutical compositions thereof, and methods of use
InactiveCN102123763AInhibition of TNF functionNervous disorderOrganic chemistryDiseaseMS multiple sclerosis
Prodrugs of methyl hydrogen fumarate, pharmaceutical compositions comprising the prodrugs of methyl hydrogen fumarate, and methods of using the prodrugs of methyl hydrogen fumarate and the pharmaceutical compositions thereof for treating diseases such as psoriasis, asthma, multiple sclerosis, inflammatory bowel disease, and arthritis are disclosed.
Owner:XENOPORT
Method for treating cachexia with retinoid ligands
InactiveUS20070185055A1BiocideSilicon compound active ingredientsRetinoidObstructive Pulmonary Diseases
The present invention relates to a method of treatment of cachexia in a subject in need of treatment. More specifically, the present invention relates to the use of retinoid compounds that act on retinoid X receptors (RXRs) for the treatment of cachexia in a subject in need of treatment. The cachexia is associated with, in other words a complication of, a primary disease, condition or disorder. Primary diseases, conditions and disorders include, but are not limited to, cancer, AIDS, liver cirrhosis, diabetes mellitus, chronic renal failure, chronic obstructive pulmonary disease, chronic cardiac failure, immune system diseases (e.g., rheumatoid arthritis and systemic lupus erythematosus), tuberculosis, cystic fibrosis, gastrointestinal disorders (e.g., irritable bowel syndrome and inflammatory bowel disease), Parkinson's disease, anorexia nervosa, dementia, major depression, an aged condition and sarcopenia.
Owner:JIANG GUANG LIANG +2
Il-21 antagonists
InactiveUS20070122413A1Increasing in vivo serum half-lifeModulate antibody responseNervous disorderAntibody mimetics/scaffoldsAutoimmune conditionAutoimmune disease
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
Materials and methods for treatment of gastrointestinal disorders
InactiveUS7312243B1Reduced activityIncrease incidenceBiocideHeavy metal active ingredientsColonic epitheliumUlcerative colitis
The subject invention pertains to materials and methods for the prevention and treatment of gastrointestinal diseases, including inflammatory bowel diseases such as Crohn's disease and ulcerative colitis. Therapeutic compositions of the invention include compositions that can neutralize hydrogen peroxide, such as reducing agents and oxidizing agents. In one embodiment, a therapeutic composition of the invention comprises a reducing agent such as sodium thiosulfate. Therapeutic compositions of the invention can optionally include compounds with antibacterial activity, compositions that inhibit bacterial adherence to cells and tissue, compositions that inhibits epithelial lipid peroxidation, compositions that add viscosity to a solution, compositions that inhibit most cells, and / or compositions that help to seal or repair tight junctions between cells of the colonic epithelium of the gastrointestinal tract. Methods of the invention include administration of compounds or compositions of the invention. In one embodiment, compounds or compositions of the invention are rectally instilled in a patient.
Owner:THERAPEUTIC RES
Derivatives of 4- or 5-aminosalicylic acid
InactiveUS7910568B2Easily reach colonPromote absorptionBiocideAntipyreticSalicylic acidBULK ACTIVE INGREDIENT
Owner:ANTIBE THERAPEUTICS INC
Derivaitves of 4-Or 5-Aminosalicylic Acid
InactiveUS20080207564A1Less readily absorbedEasily reach colonBiocideAntipyreticThioester synthesisSalicylic acid
The present invention provides new derivatives of 4- or 5-aminosalicylic acid, and a pharmaceutical composition containing these derivatives of 4- or 5-aminosalicylic acid as active ingredients, useful for the treatment of intestinal diseases such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) and for the prevention / treatment of colon cancer. More particularly, these derivatives comprise a hydrogen sulfide releasing moiety linked via an azo, an ester, an anhydride, a thioester or an amide linkage to a molecule of 4- or 5-aminosalicylic acid. Furthermore, the present invention provides a process for preparing these compounds and their use for treating IBD and IBS and the prevention / treatment of colon cancer.
Owner:ANTIBE THERAPEUTICS INC
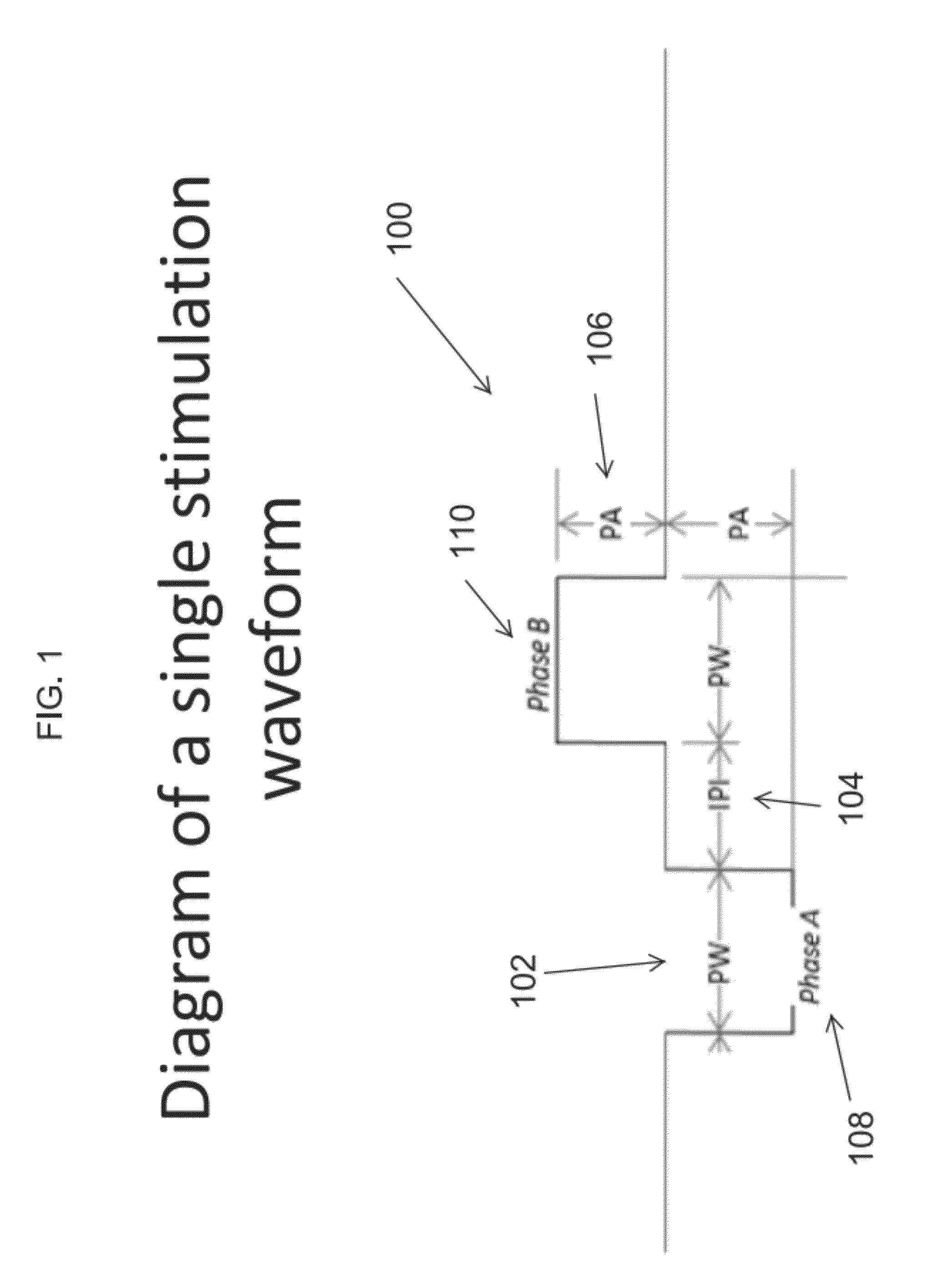
Single-pulse activation of the cholinergic anti-inflammatory pathway to treat chronic inflammation
ActiveUS8788034B2Implantable neurostimulatorsMachines/enginesCholinergic anti-inflammatory pathwayAnatomy
Described herein are methods and systems for applying extremely low duty-cycle stimulation sufficient to treat chronic inflammation. In particular, described herein are single supra-threshold pulses of electrical stimulation sufficient to result in a long-lasting (e.g., >4 hours, greater than 12 hours, greater than 24 hours, greater than 48 hours) inhibition of pro-inflammatory cytokines and / or effects of chronic inflammation. These methods and devices are particularly of interest in treatment of inflammatory bowel disease (IBD).
Owner:SETPOINT MEDICAL CORP
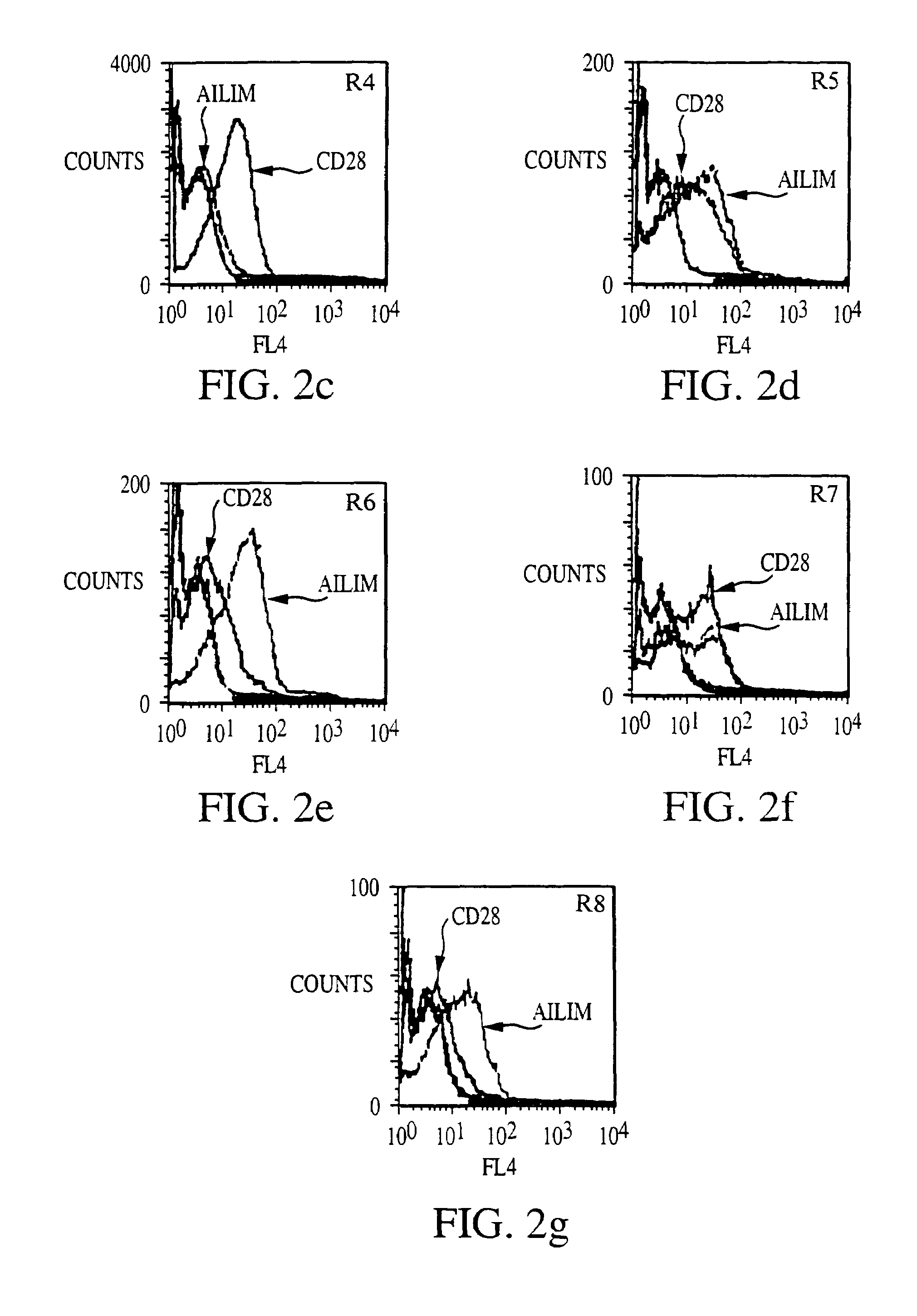
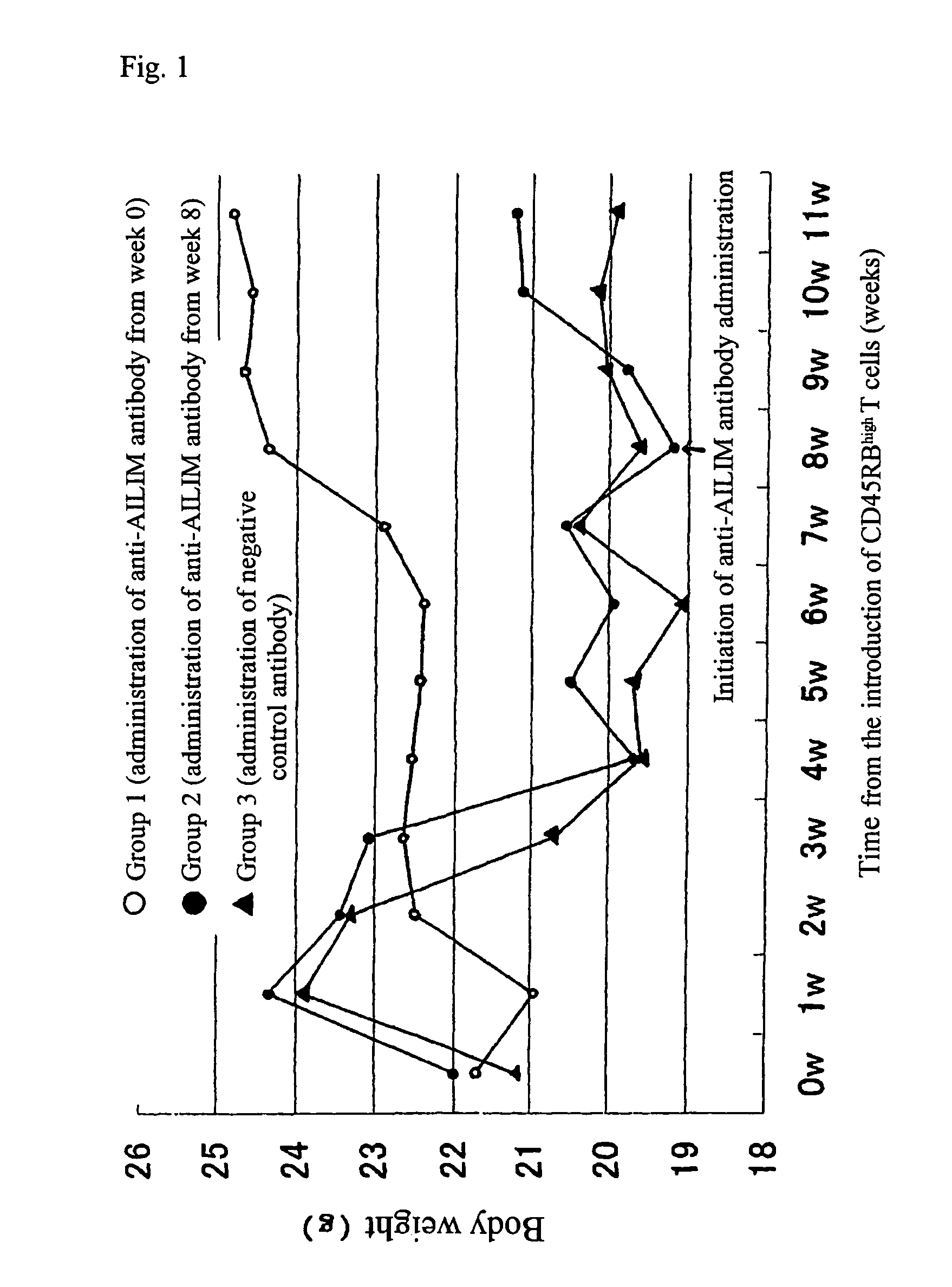
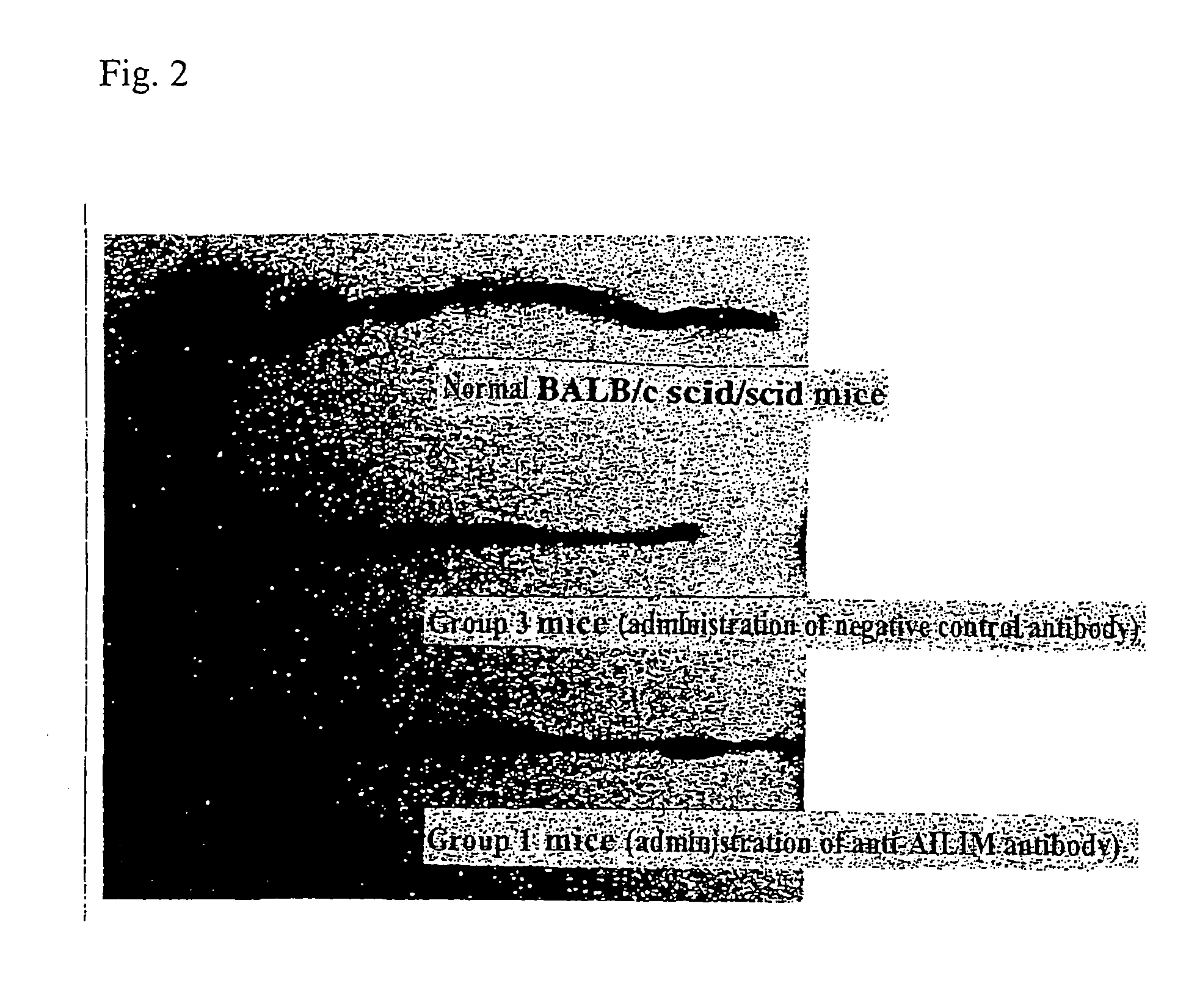
Methods of preventing or treating graft versus host reaction by administering an antibody or portion thereof that binds to AILIM
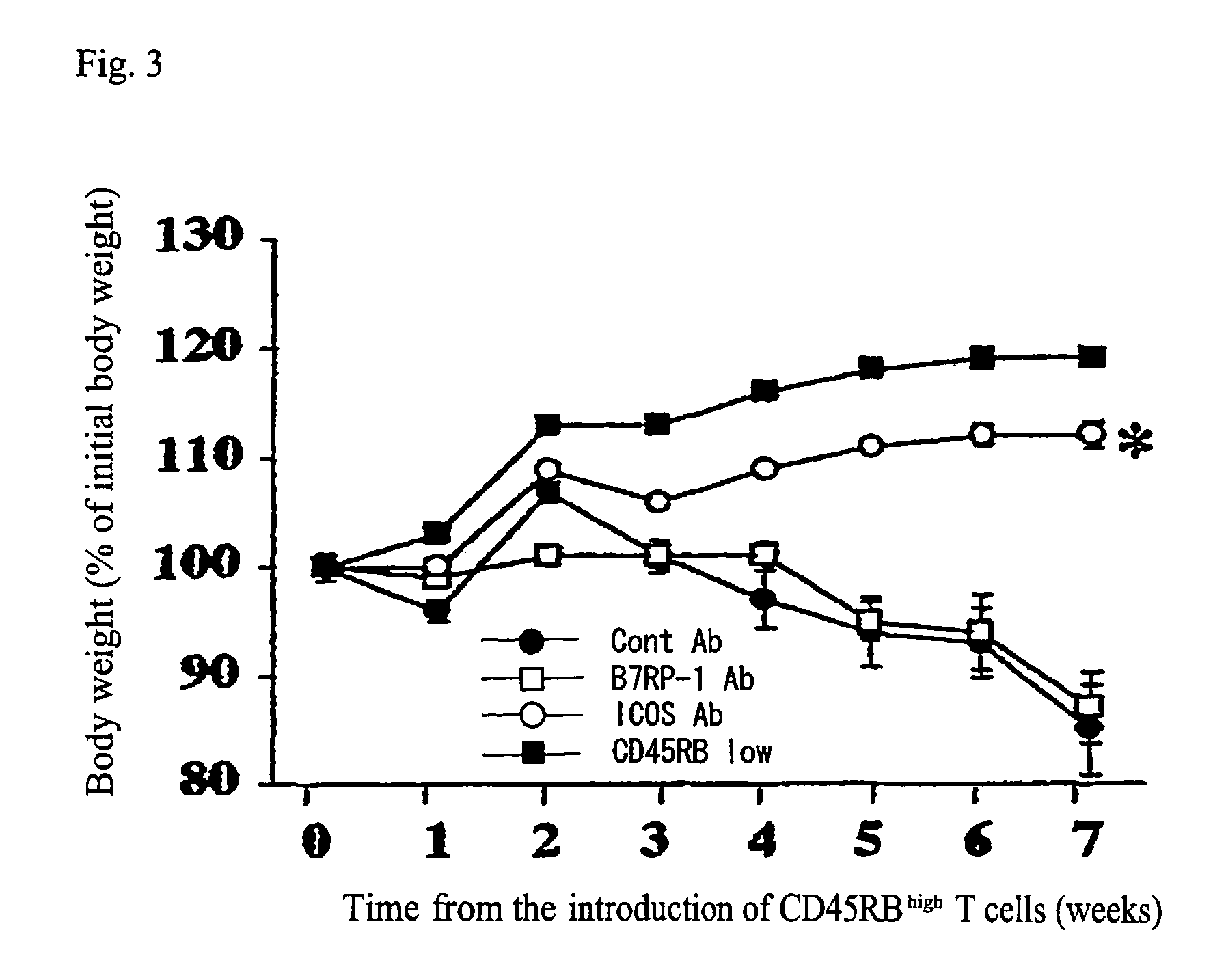
InactiveUS7465445B2Modulate productionPrevent diseaseOrganic active ingredientsBiocideAntigenTherapeutic effect
An antibody against AILIM (alternatively called JTT-1 antigen, JTT-2 antigen, ICOS and 8F4) was found to have a significant therapeutic effect on arthrosis, for example, rheumatoid arthritis and osteoarthritis, graft versus host disease, graft immune rejection, inflammation (hepatitis and inflammatory bowel diseases), diseased condition accompanied by the excessive production of an antibody against a foreign antigen triggered by immunological sensitization by the antigen.
Owner:JAPAN TOBACCO INC
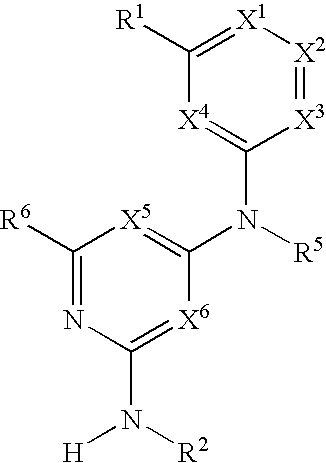
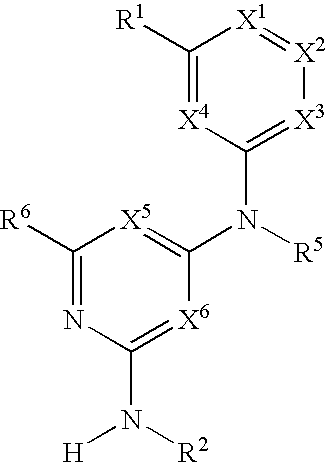
Substituted heterocyclic compounds and methods of use
The present invention relates to pyridines, pyrimidines and derivatives thereof, and pharmaceutically acceptable salts thereof. Also included is a method of treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
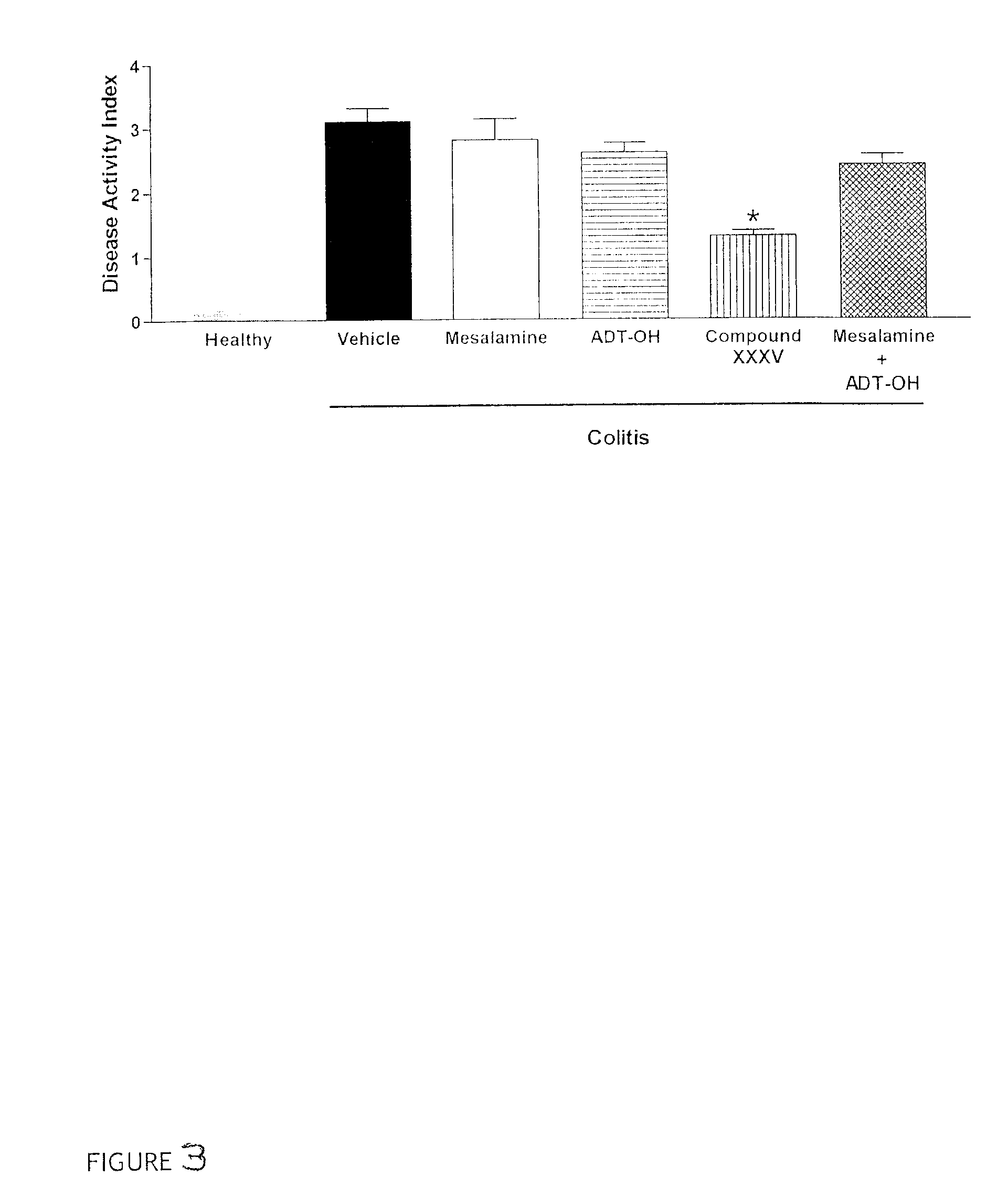
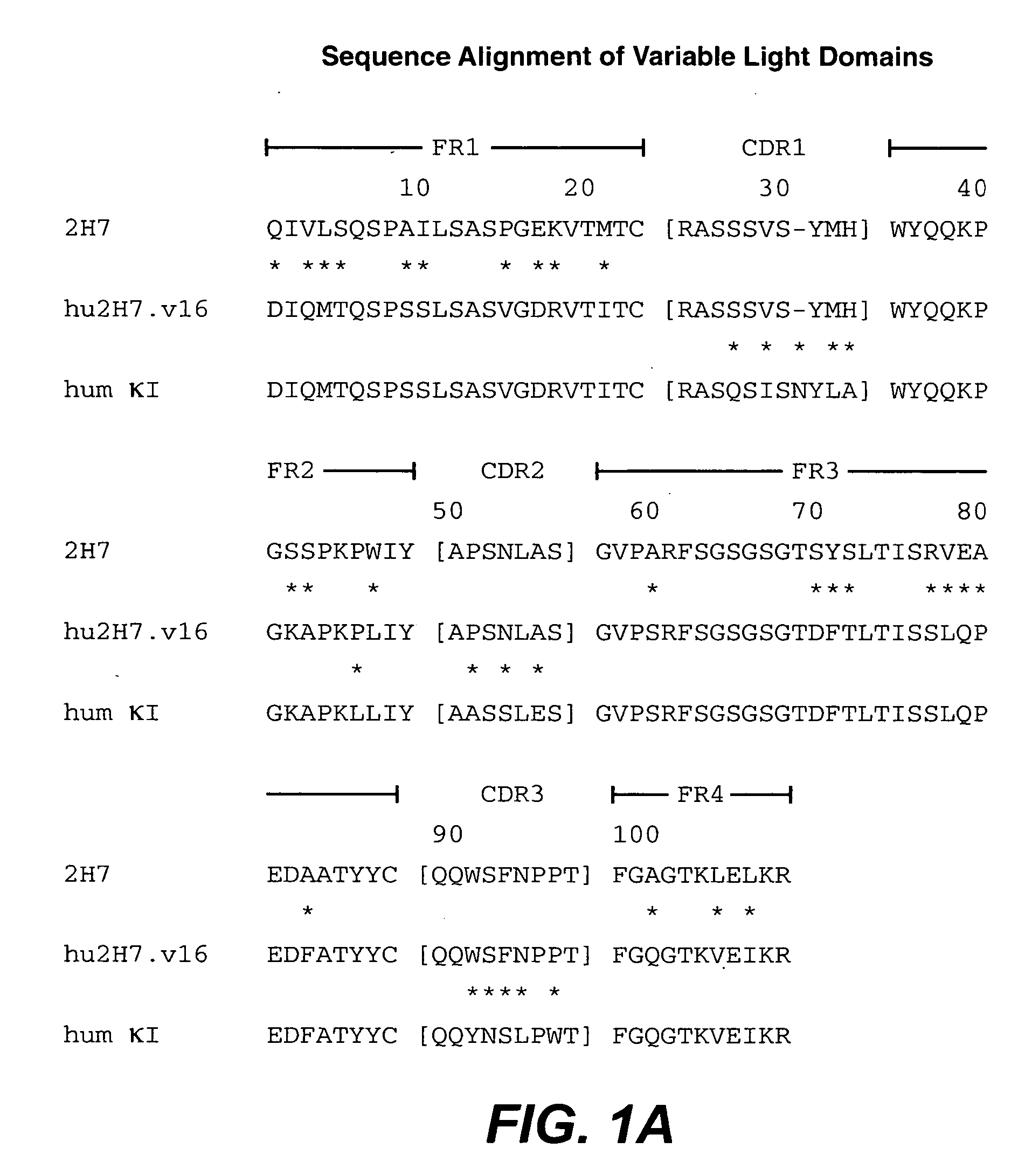
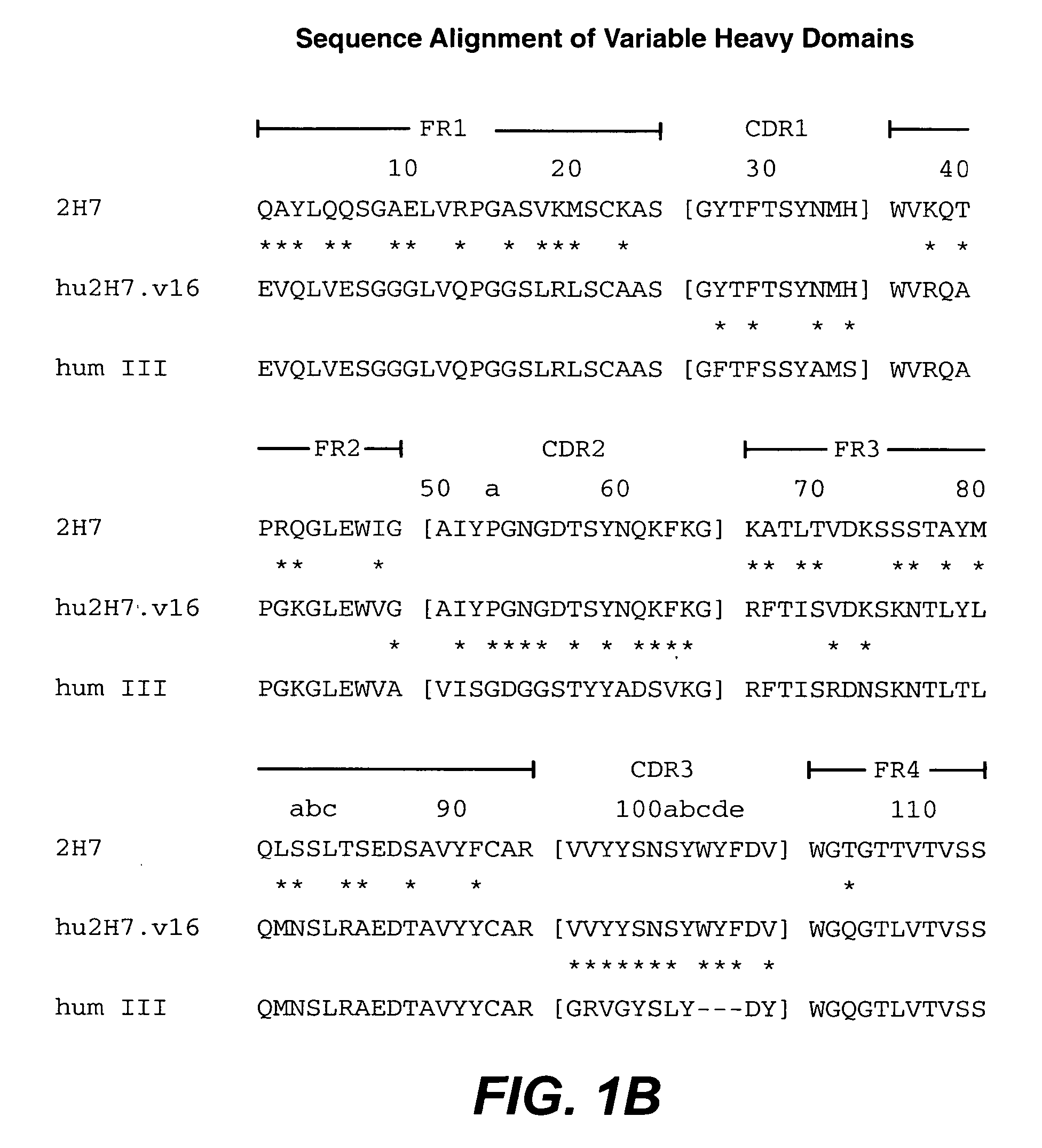
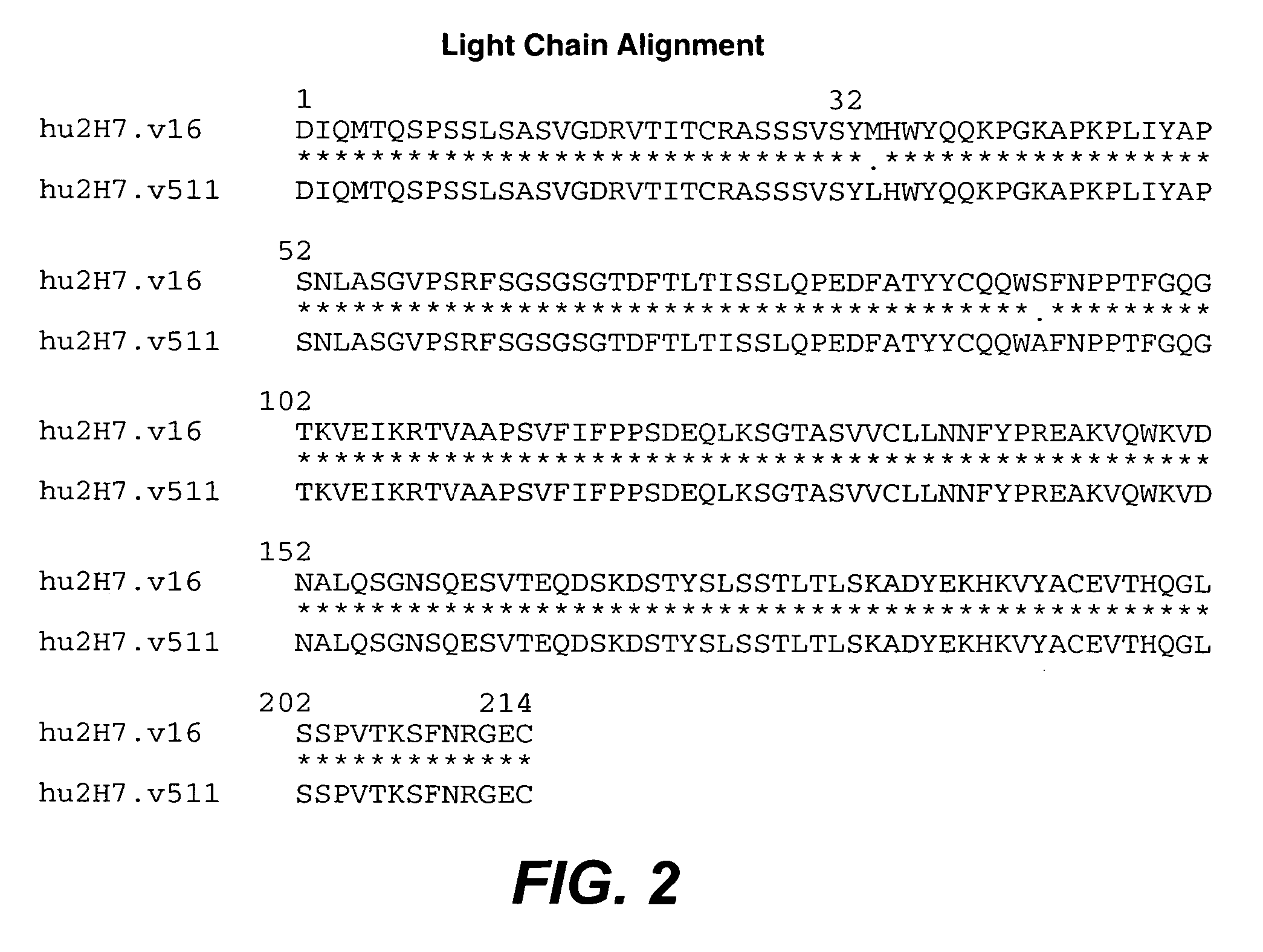
Treatment of inflammatory bowel disease (IBD)
The present invention concerns treatment of IBD, especially ulcerative colitis (UC), with an antibody that binds to CD20.
Owner:GENENTECH INC
Methods of suppressing or treating an inflammatory bowel disease by administering an antibody or portion thereof that binds to AILIM
InactiveUS7465444B2Good treatment effectOrganic active ingredientsPeptide/protein ingredientsCrohn's diseaseUlcerative colitis
Antibodies against AILIM (also called ICOS and 8F4) were found to significantly suppress the onset of inflammatory bowel diseases (especially Crohn's disease and colitis (ulcerative colitis and such)), and exhibit a significant therapeutic effect against inflammatory bowel diseases.
Owner:JAPAN TOBACCO INC
Pharmaceutical compositions and dosage forms of thalidomide
Pharmaceutical compositions and single unit dosage forms of thalidomide and pharmaceutically acceptable prodrugs, salts, solvates, hydrates, or clathrates are disclosed. Also disclosed are methods of treating and preventing diseases and conditions such as, but not limited to, leprosy, chronic graft-vs-host disease, rheumatoid arthritis, sarcoidosis, an inflammatory condition, inflammatory bowel disease, and cancer using the novel dosage forms disclosed herein.
Owner:CELGENE CORP
Method for treating congestive heart failure and other disorders
ActiveUS7494979B2Increase gastrointestinal motilityReduce complicationsPeptide/protein ingredientsMetabolism disorderGastroparesisInflammatory Bowel Diseases
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
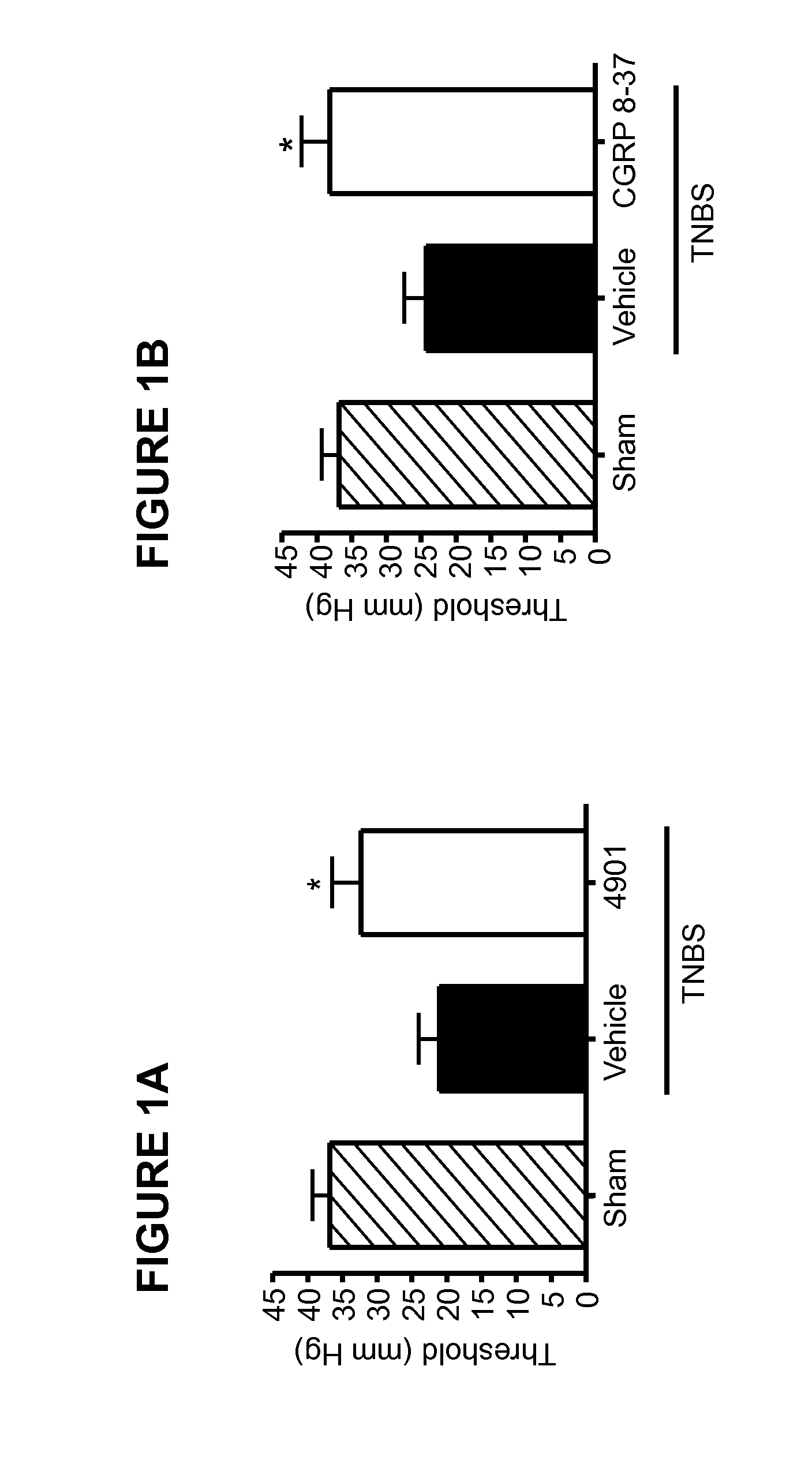
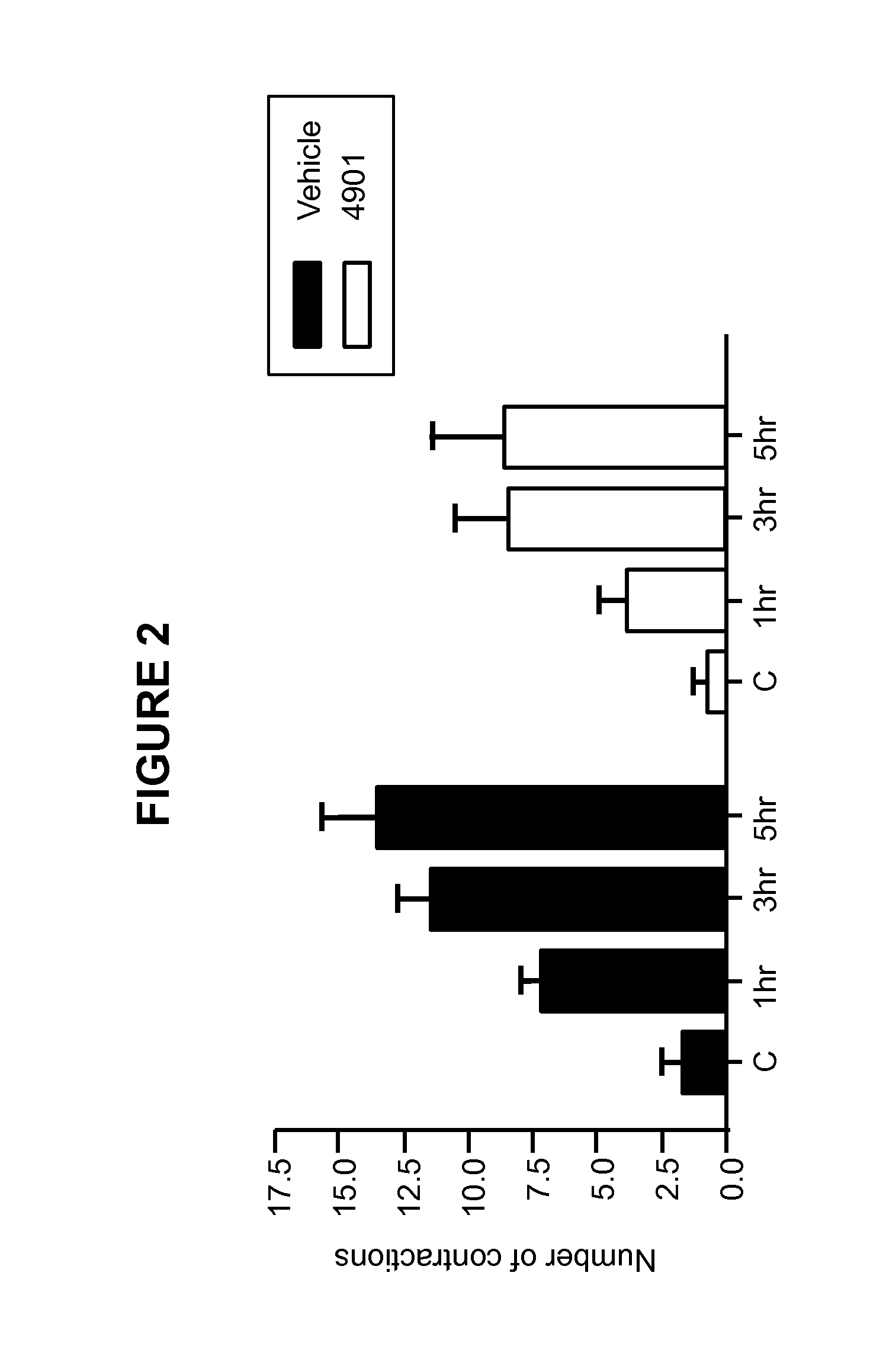
Methods for treating visceral pain by administering antagonist antibodies directed against calcitonin gene-related peptide
The invention features methods for preventing or treating visceral pain, including pain associated with functional bowel disorder, inflammatory bowel disease and interstitial cystitis, by administering an anti-CGRP antagonist antibody.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com