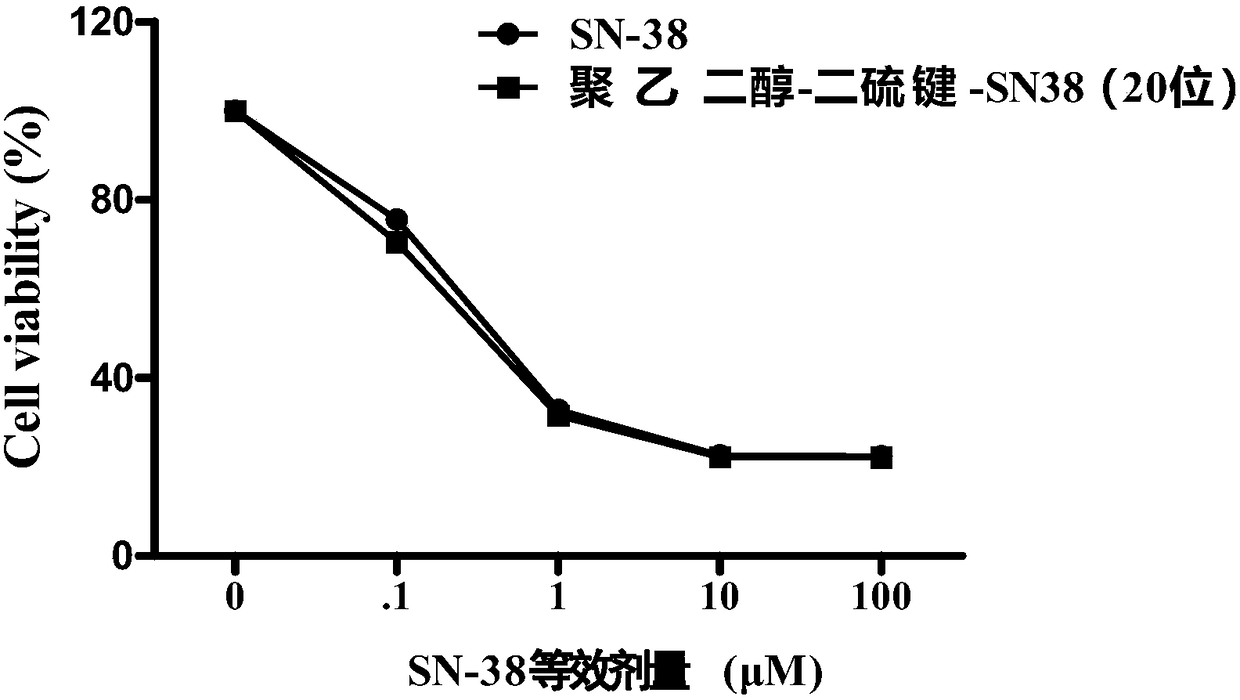
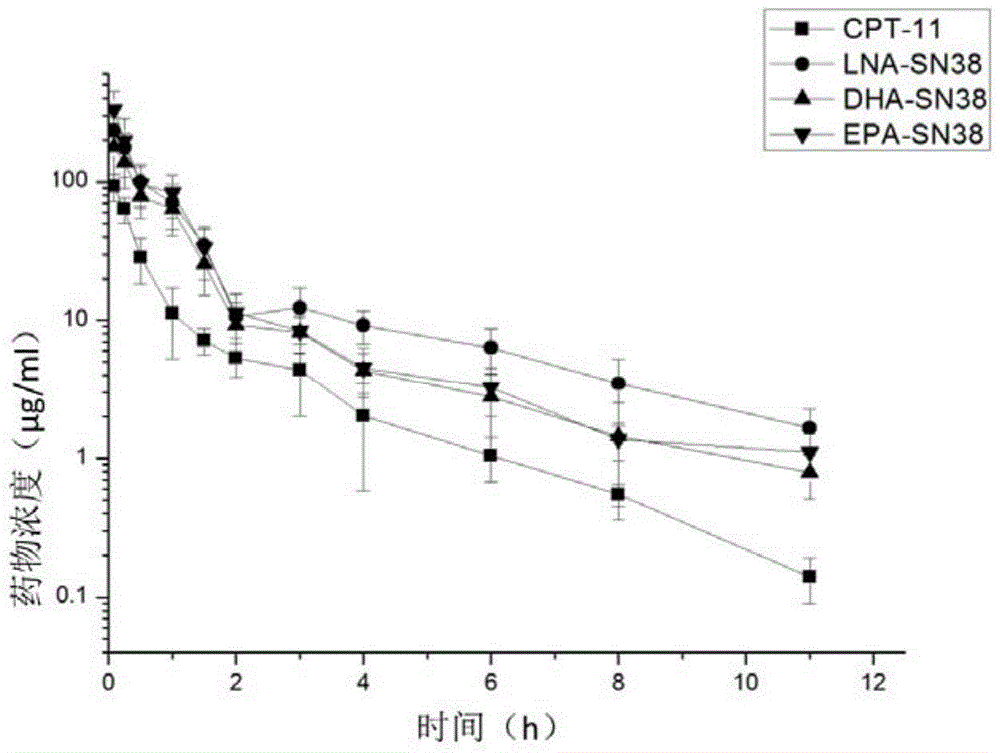
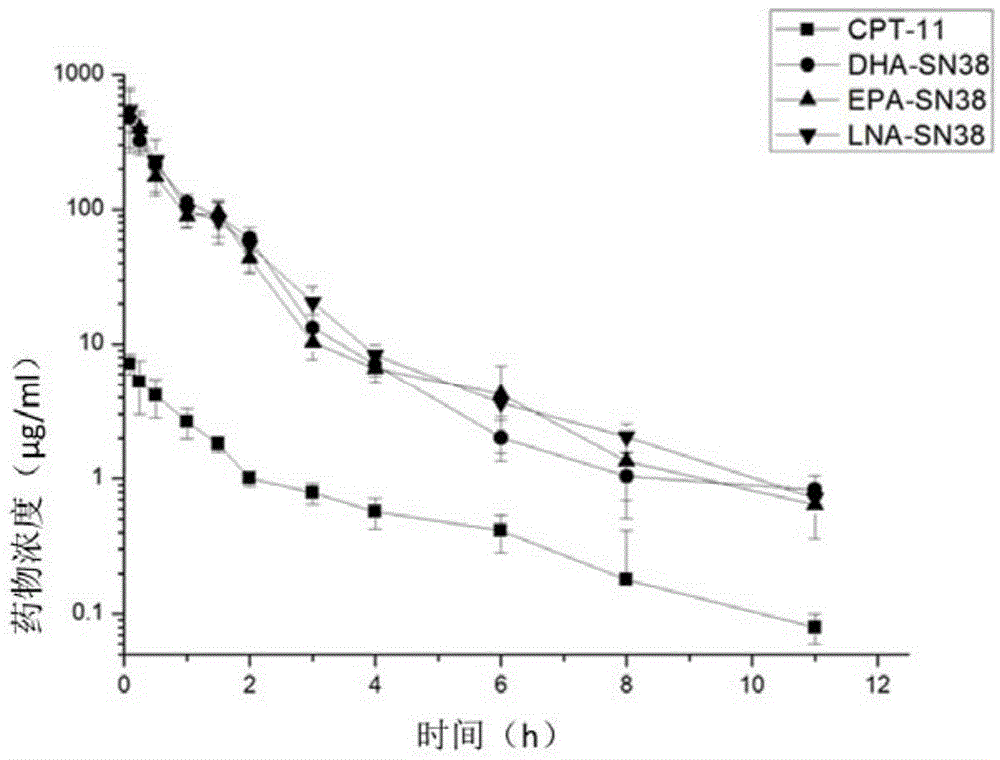
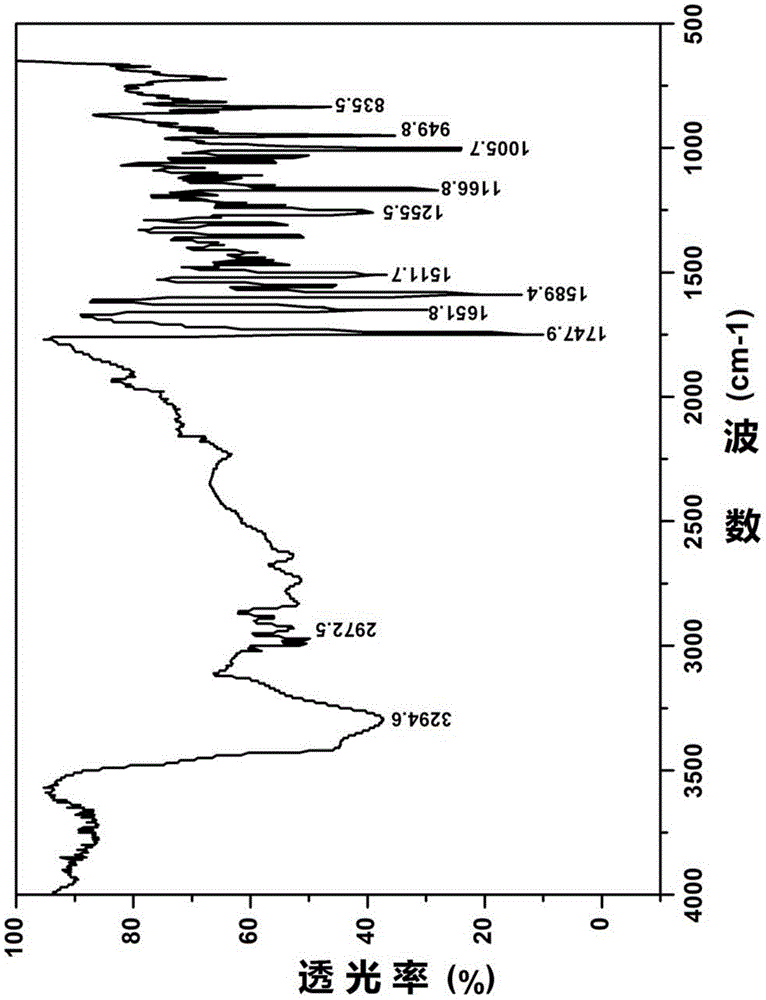
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "7-ethyl-10-hydroxycamptothecin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
7-Ethyl-10-hydroxycamptothecin ≥98% (HPLC), powder Synonym: SN-38 CAS Number 86639-52-3. Empirical Formula (Hill Notation) C 22 H 20 N 2 O 5. Molecular Weight 392.40 . MDL number MFCD00871873. PubChem Substance ID 329815105
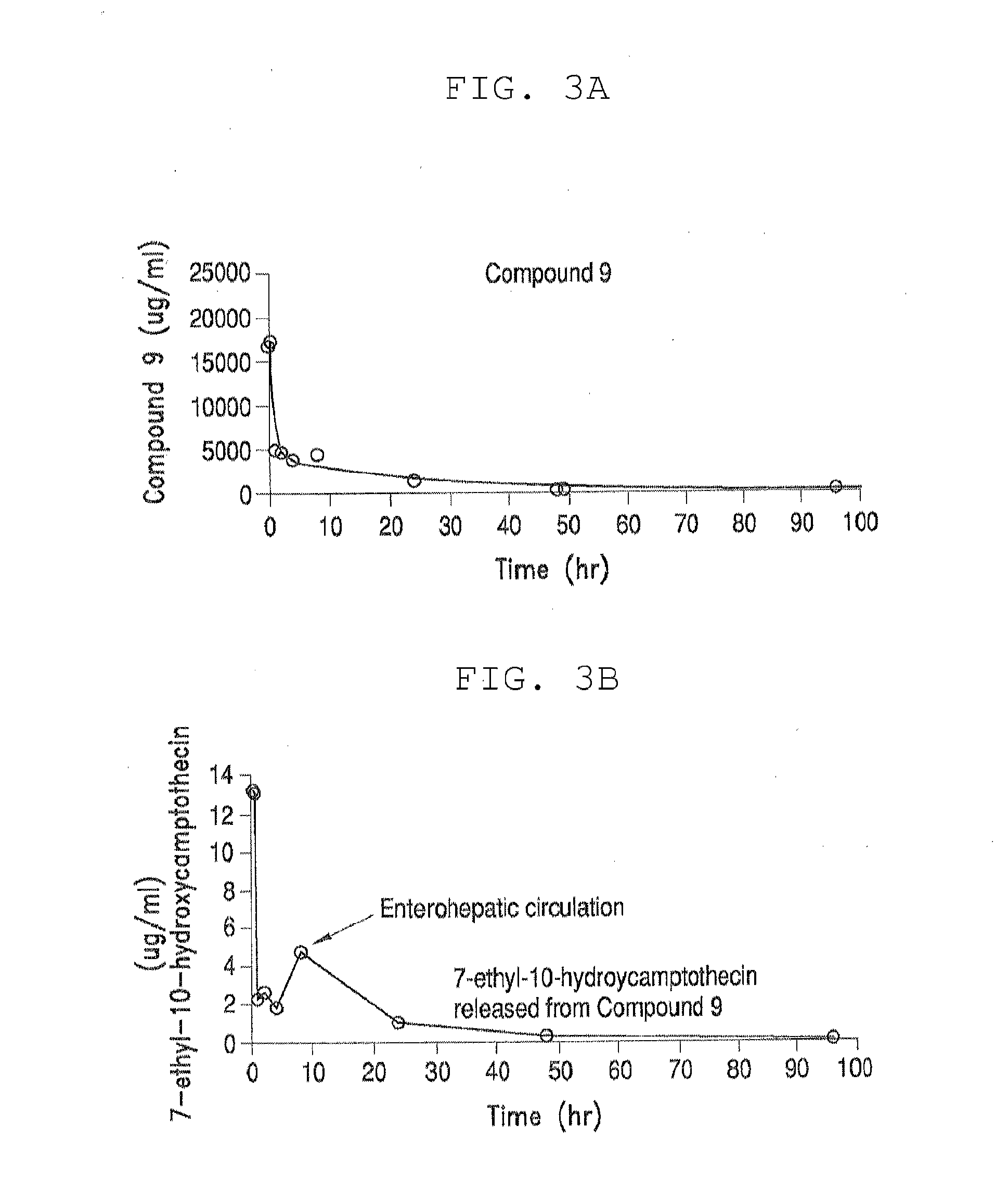
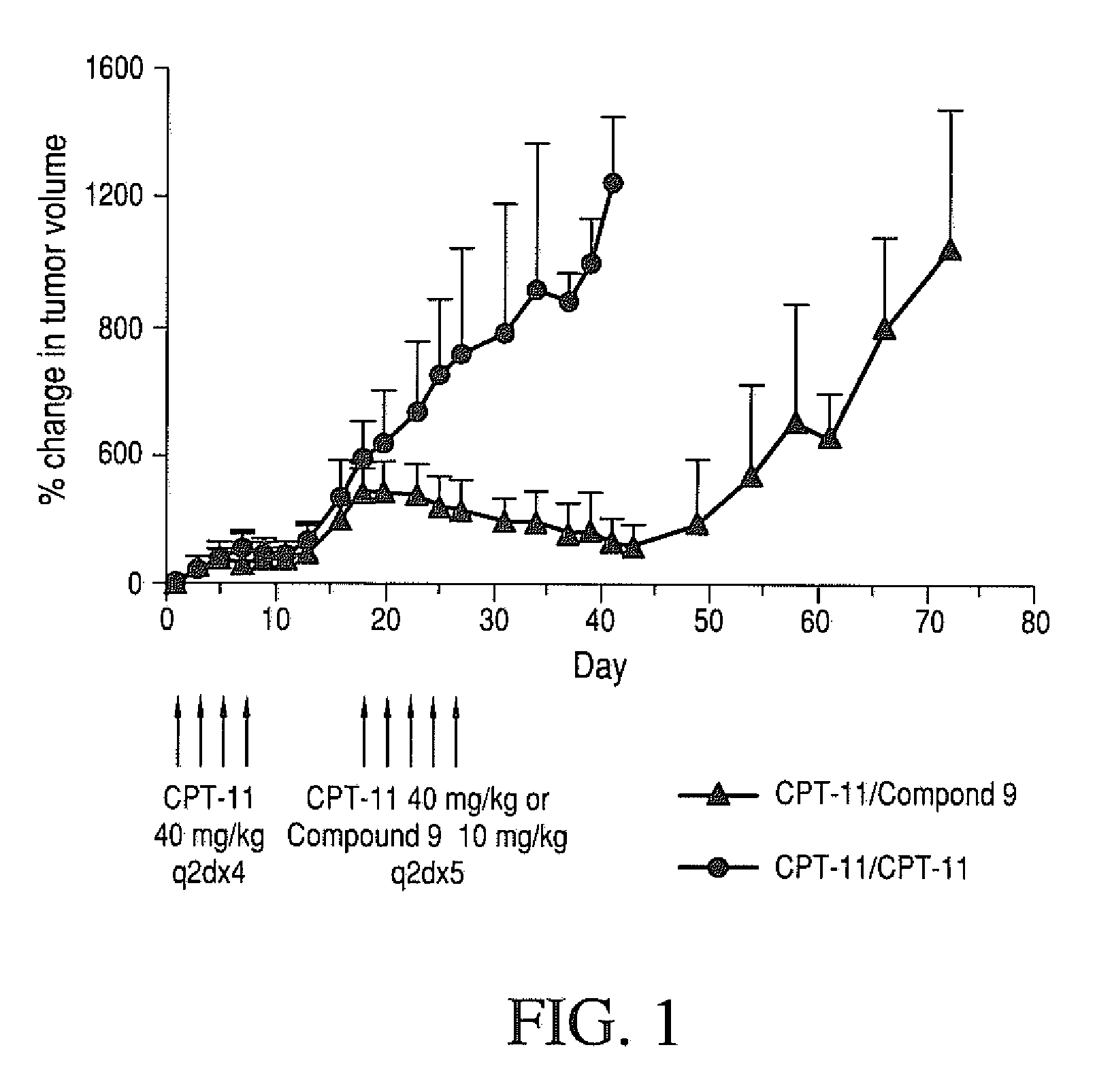
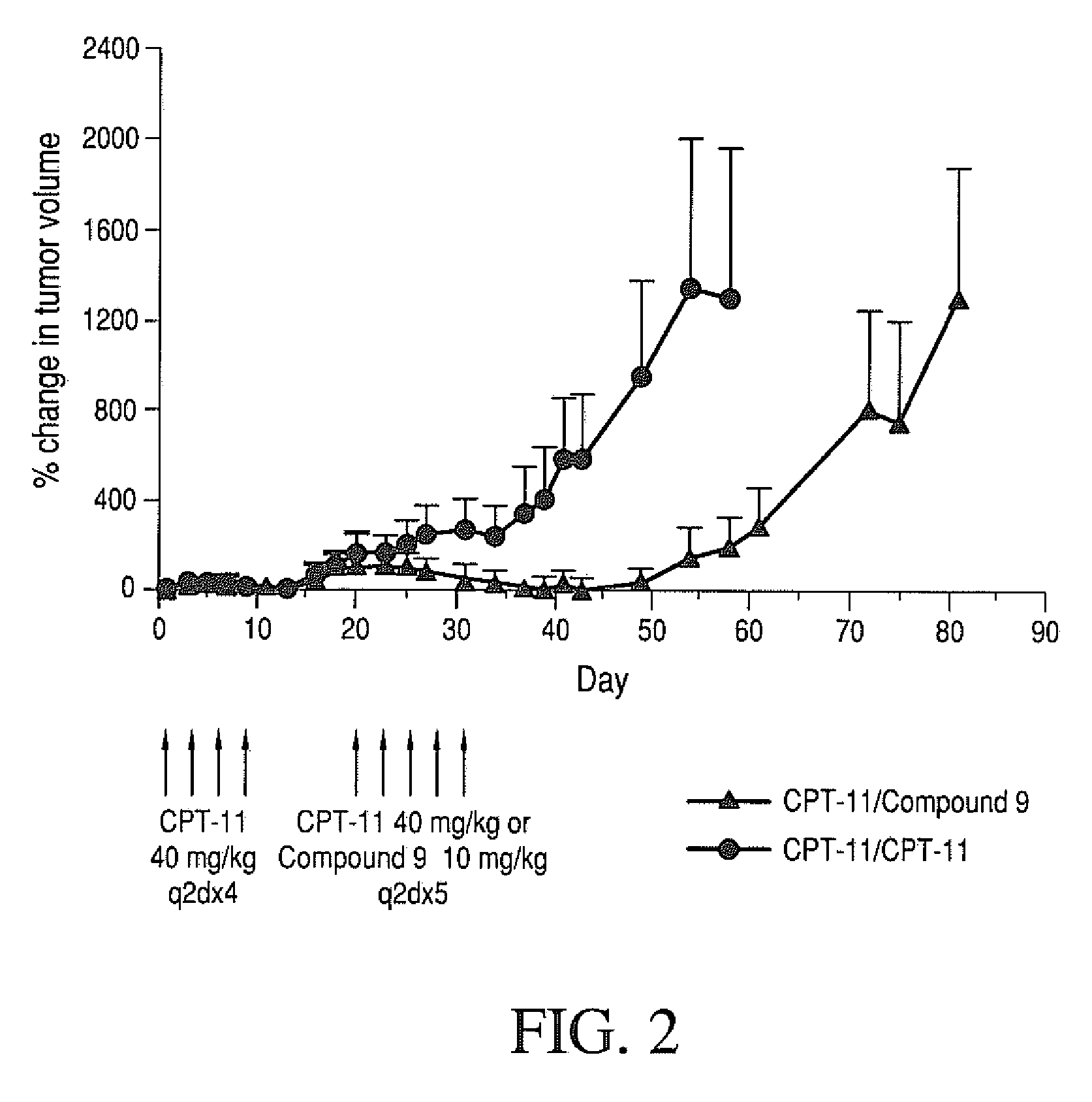
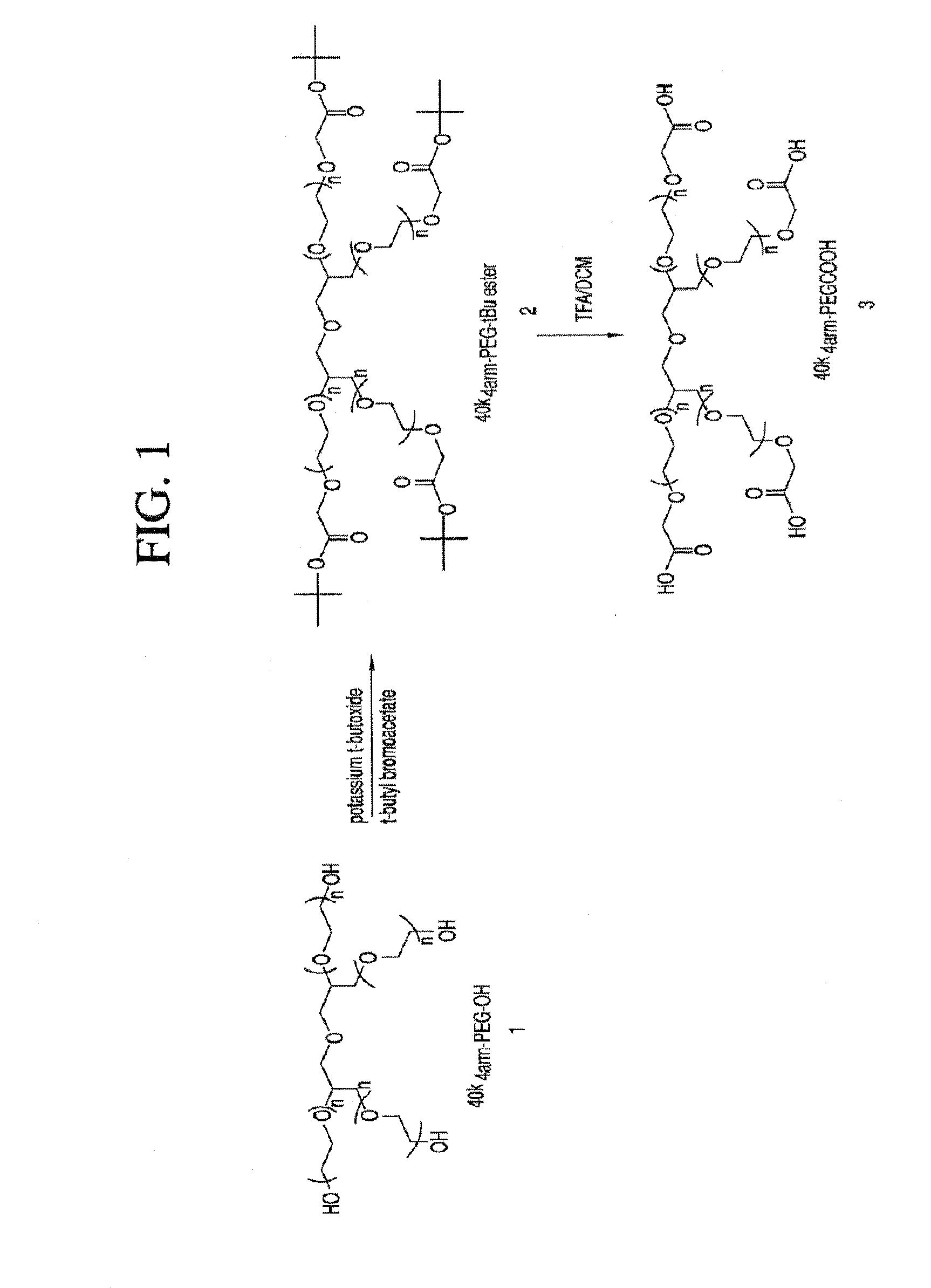
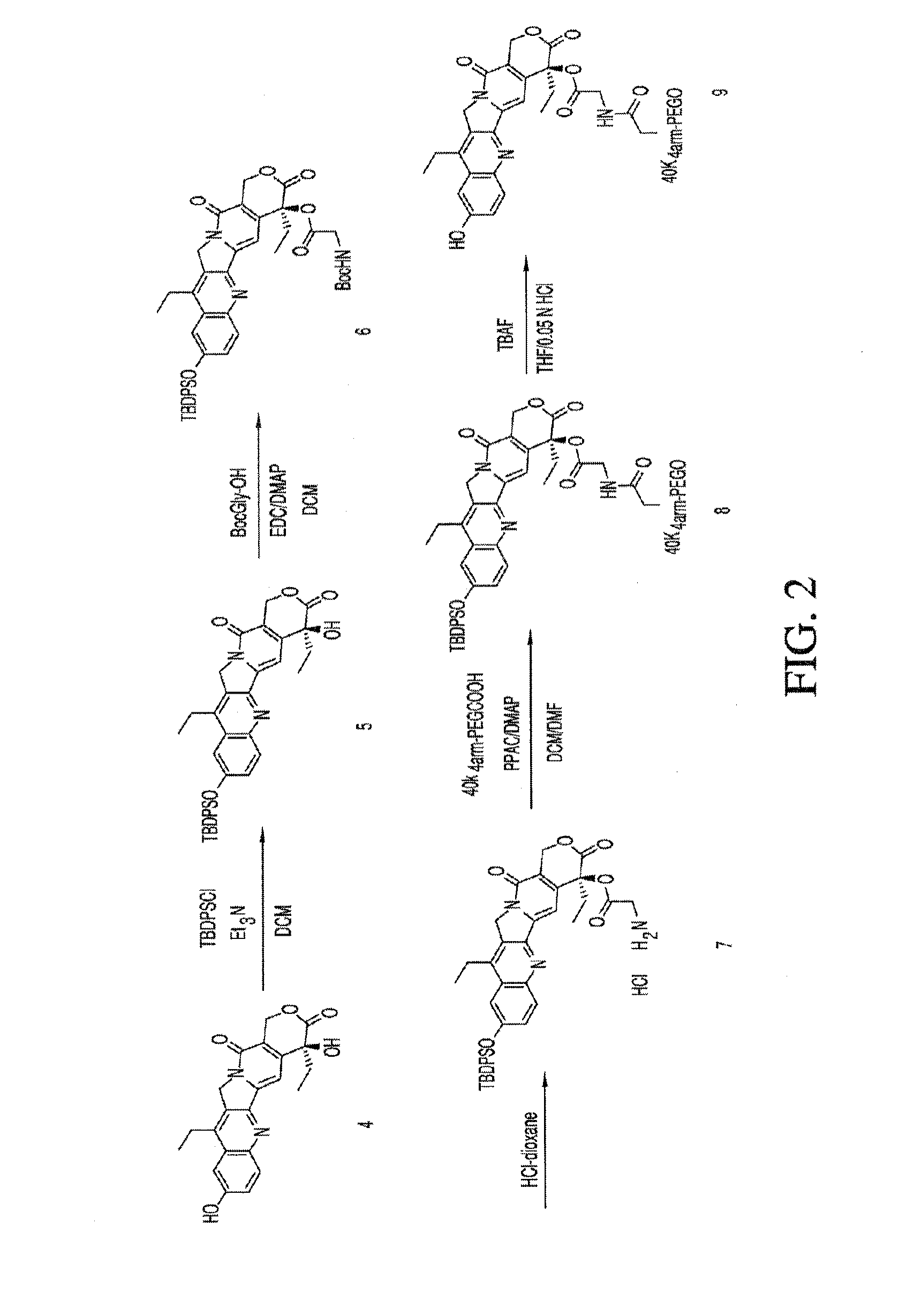
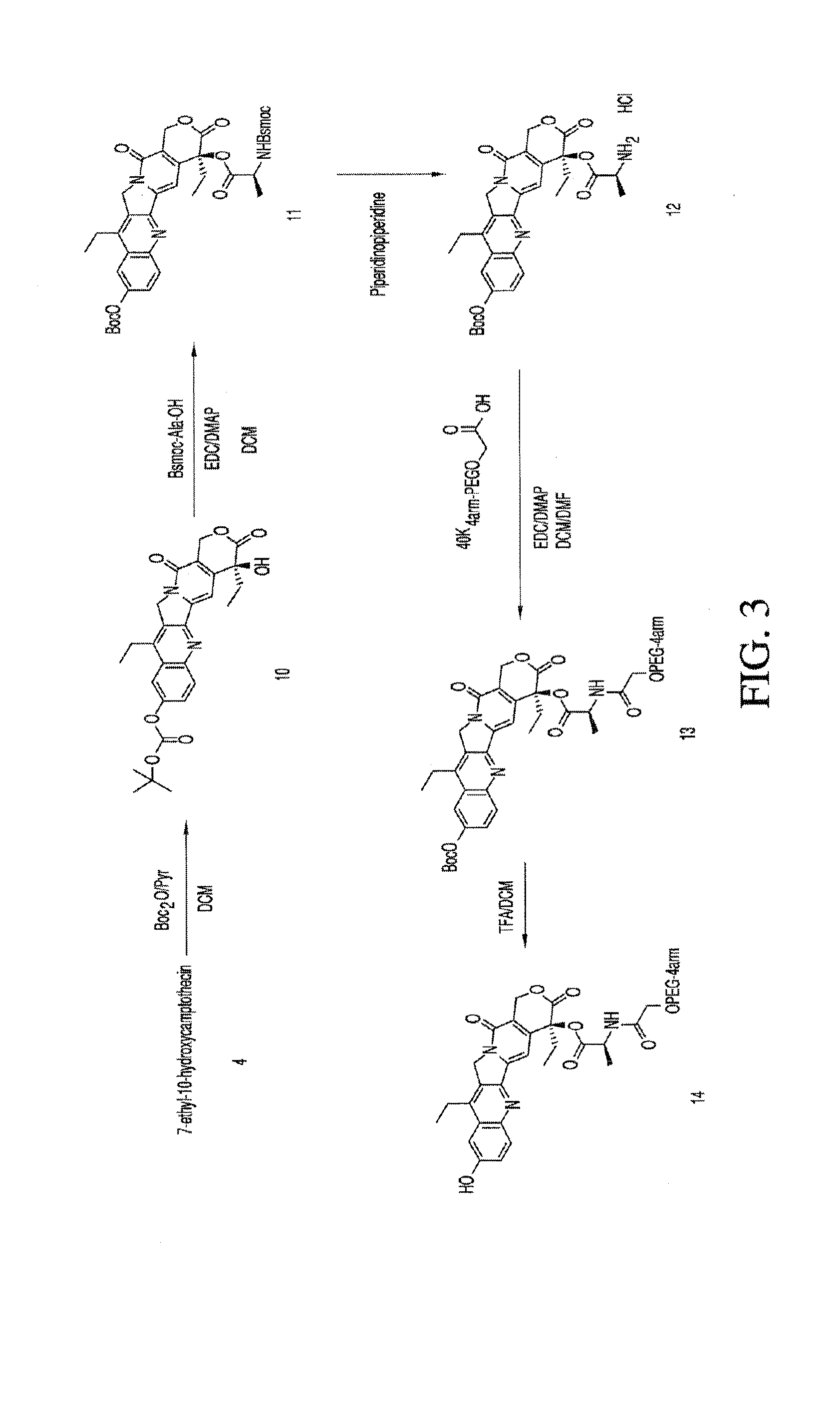
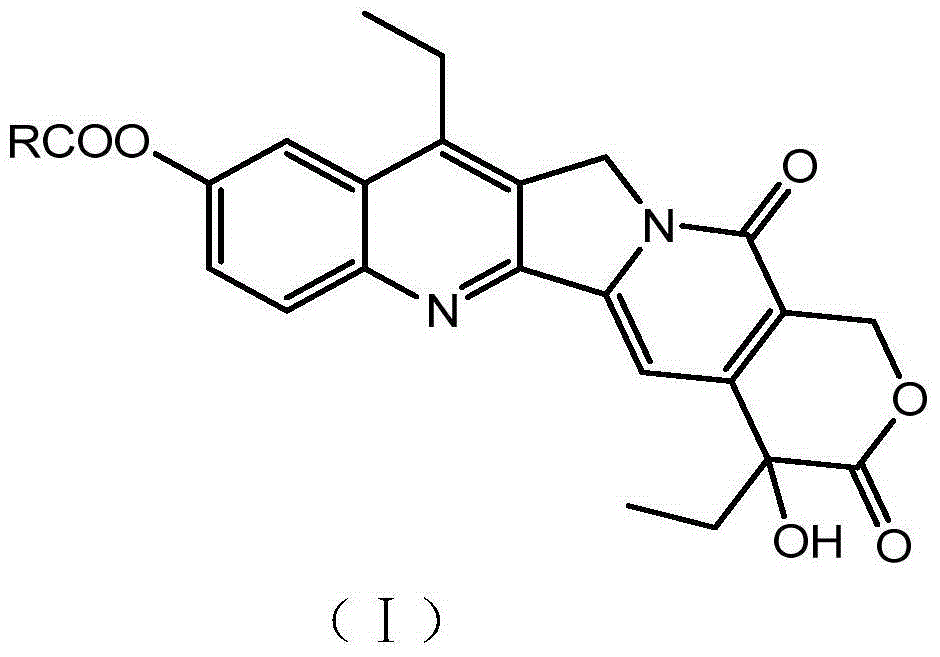
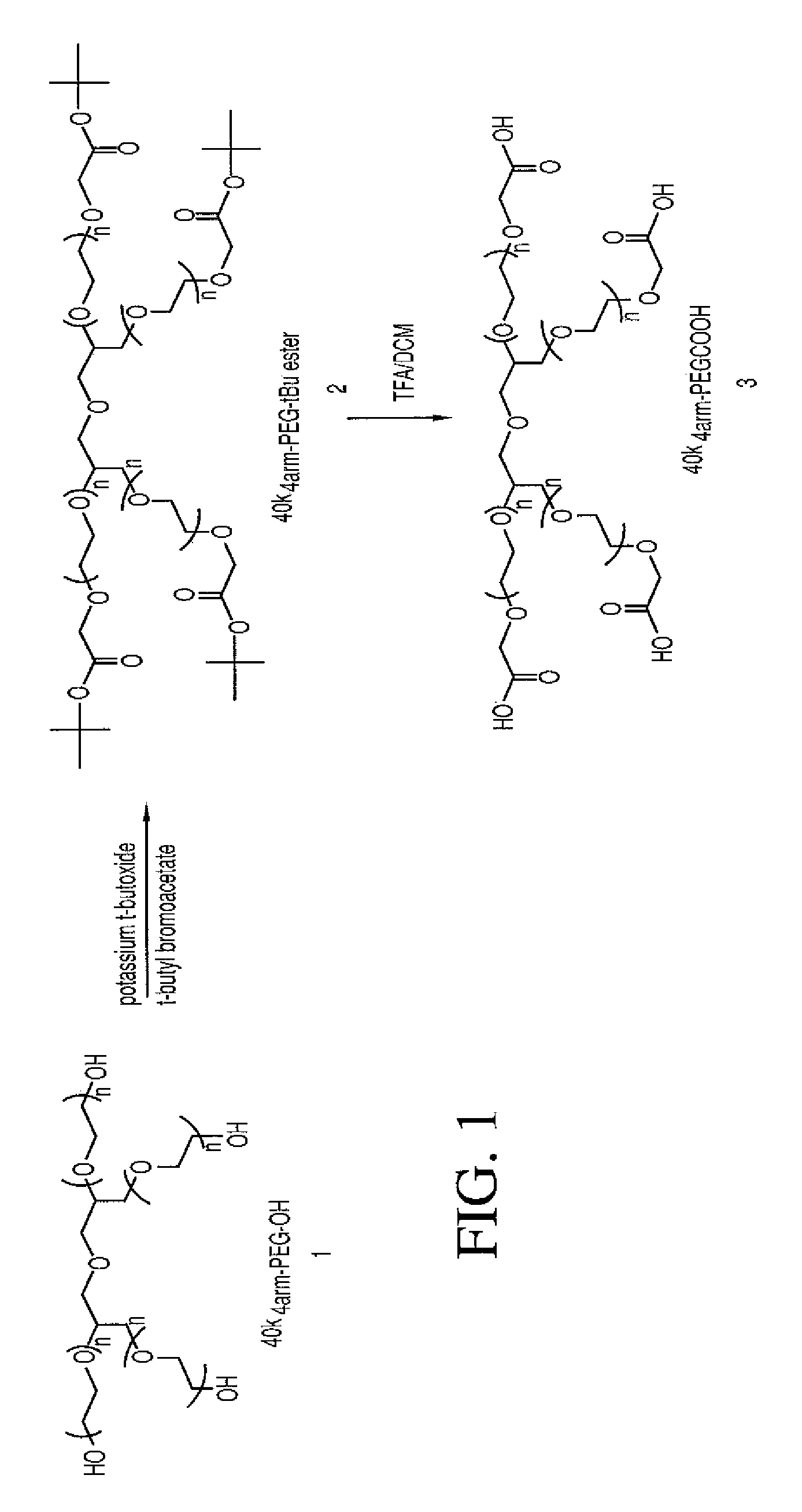
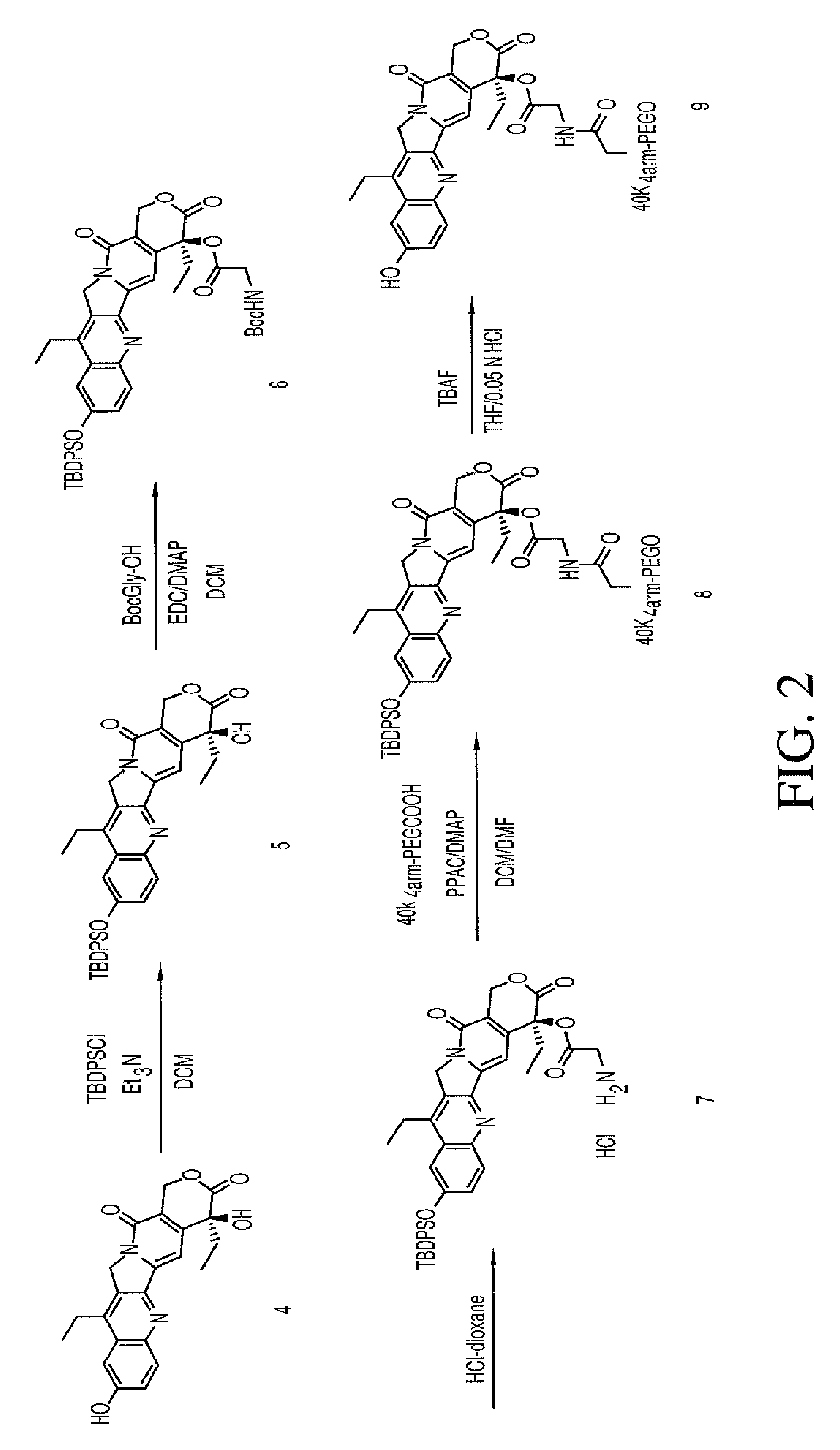
Methods of treating her2 positive cancer with her2 receptor antagonist in combination with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
InactiveUS20120171201A1Reduce resistanceTherapeutic utilityAntibody ingredientsImmunoglobulinsPolymeric prodrugMedicine
The present invention relates to methods of treating a HER2 positive cancer in mammals. The present invention includes administering a HER2 antagonist in combination with a polymeric prodrug of 7-ethyl-10-hydroxycamptothecin to the mammals in need thereof.
Owner:BELROSE PHARMA
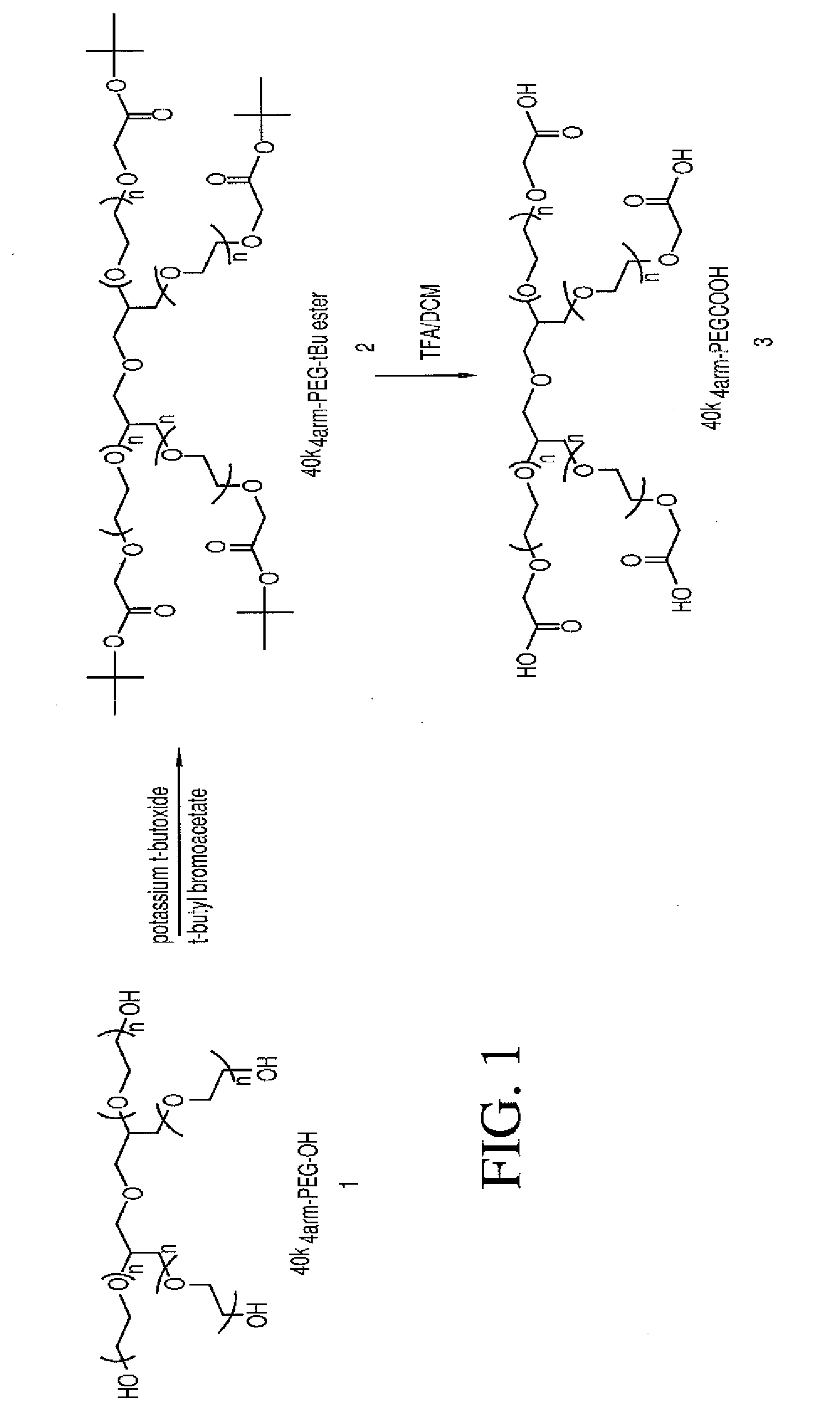
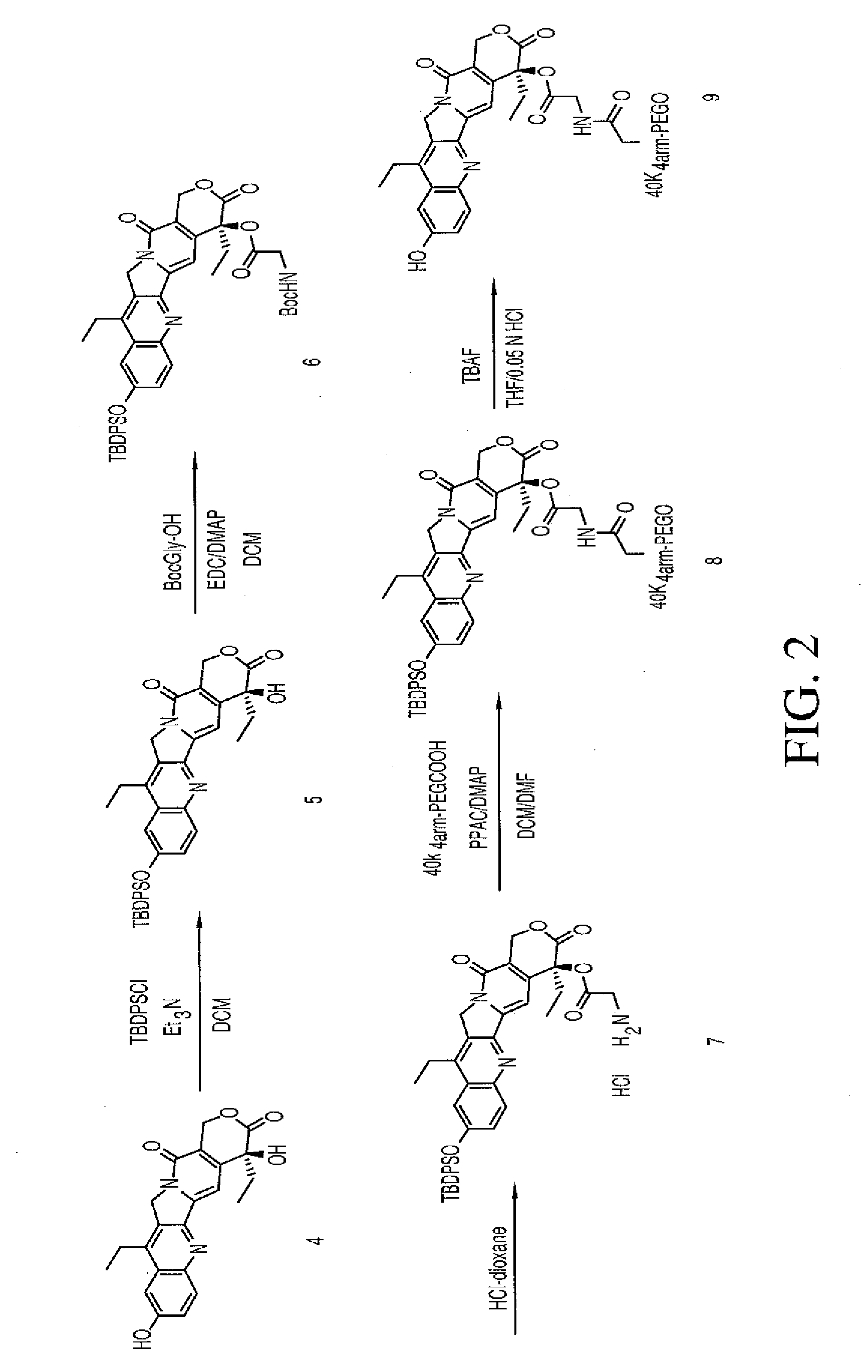
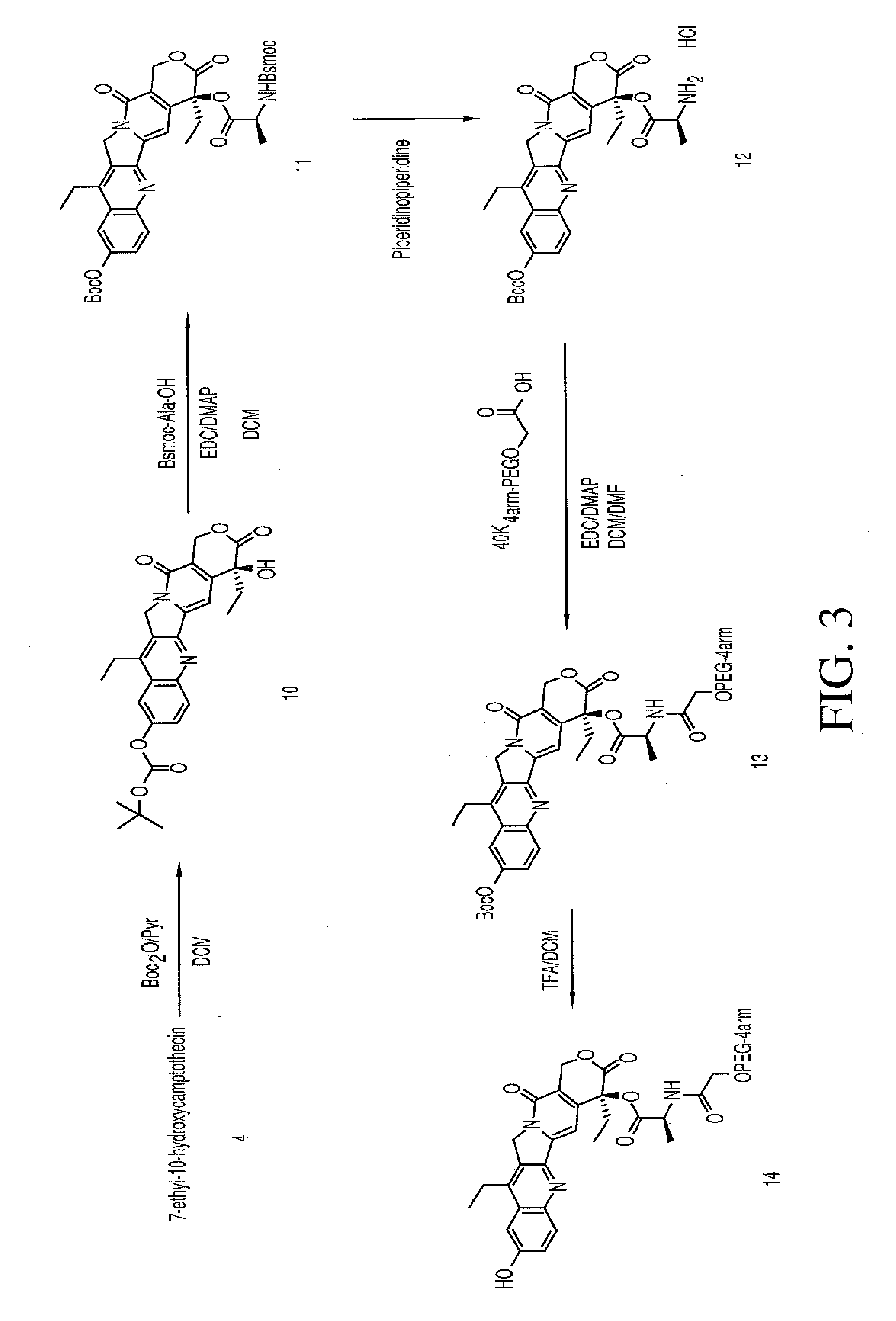
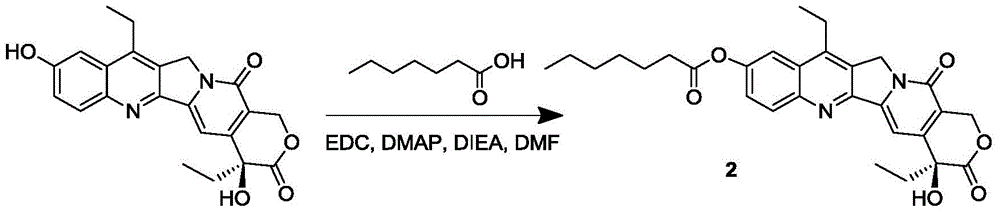
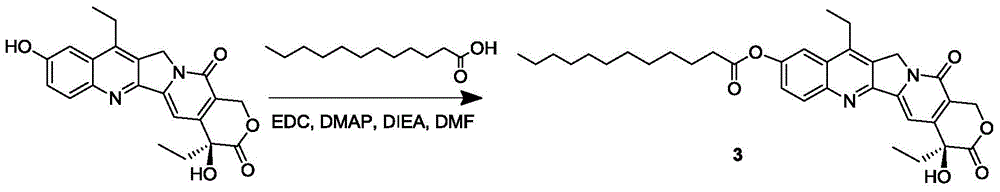
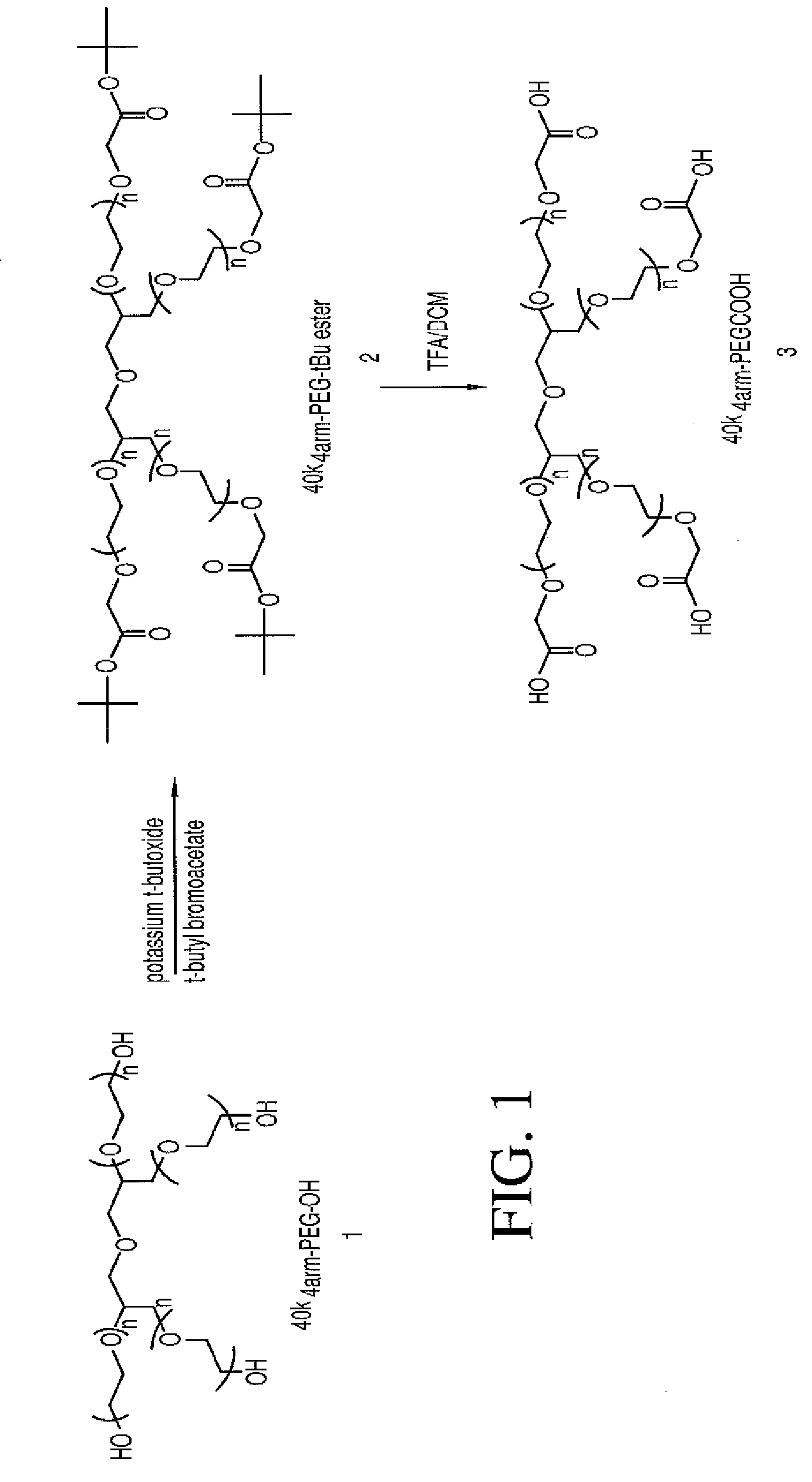
Multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin for treatment of breast, colorectal, pancreatic, ovarian and lung cancers
InactiveUS20070197575A1Advanced technologyGood conjugateBiocideOrganic chemistryMedicinePolyethylene glycol
A four arm-polyethylene glycol-7-ethyl-10-hydroxycamptothecin conjugate, such as, is disclosed. Methods of making the conjugates and methods of treating mammals using the same are also disclosed.
Owner:BELROSE PHARMA
Amphiphilic prodrug of 7- ethyl-10-hydroxycamptothecin and preparation method thereof
InactiveCN102060991AControl release speedPromote degradationOrganic chemistryPharmaceutical delivery mechanismSolubility7-ethyl-10-hydroxycamptothecin
The invention discloses an amphiphilic prodrug of 7- ethyl-10-hydroxycamptothecin, which is prepared by connecting hydroxyl at the site 10 and / or 20 site of the 7-ethyl-10- hydroxycamptothecin and a hydrophilic group through a scissionable chemical bond. The amphiphilic prodrug is self-assembled in water to form a micelle or vesicle structure. Therefore, on one hand, the solubility of SN-38 in water is greatly increased, and the stability of SN-38 lactone rings is improved; and on the other hand, the drug loading rate is high and the SN-38 can be quickly released in cells, consequently the defect of low drug loading rate of traditional drugs is overcame. Moreover, with the nano-micelles or the nano-vesicles of the prodrug, EPR (enhanced permeability and retention) effect targeted cancer tissues of tumors can be effectively utilized.
Owner:ZHEJIANG UNIV
Methods of treating her2 positive cancer with her2 receptor antagonist in combination with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
The present invention relates to methods of treating a HER2 positive cancer in mammals. The present invention includes administering a HER2 antagonist in combination with a polymeric prodrug of 7-ethyl-10-hydroxycamptothecin to the mammals in need thereof.
Owner:ENZON PHARM INC
7-ethyl-10-hydroxycamptothecine drug precursor, preparation method and application thereof
InactiveCN105315294AGood antitumor activityImprove bioavailabilityOrganic active ingredientsOrganic chemistrySolubility7-ethyl-10-hydroxycamptothecin
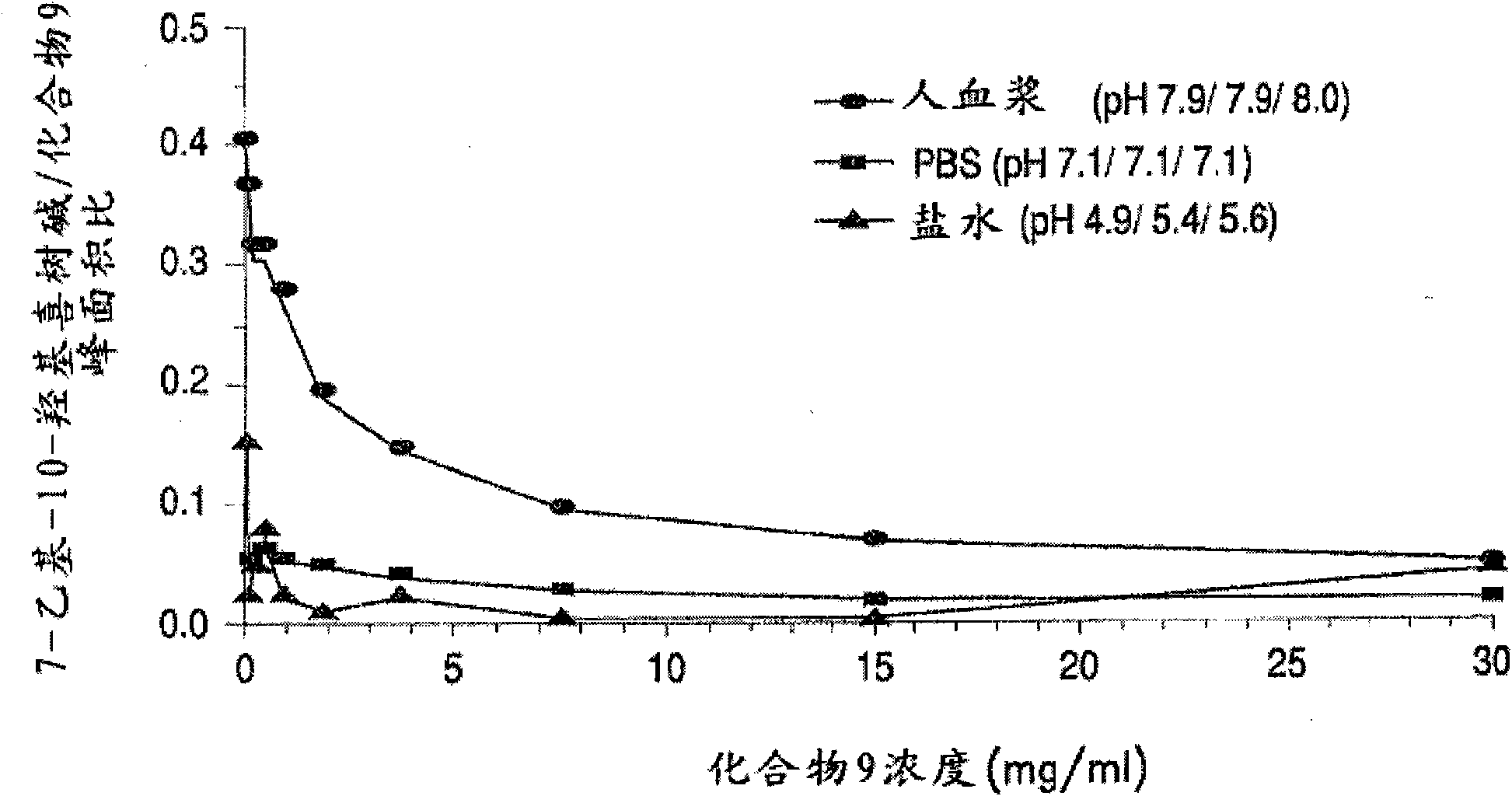
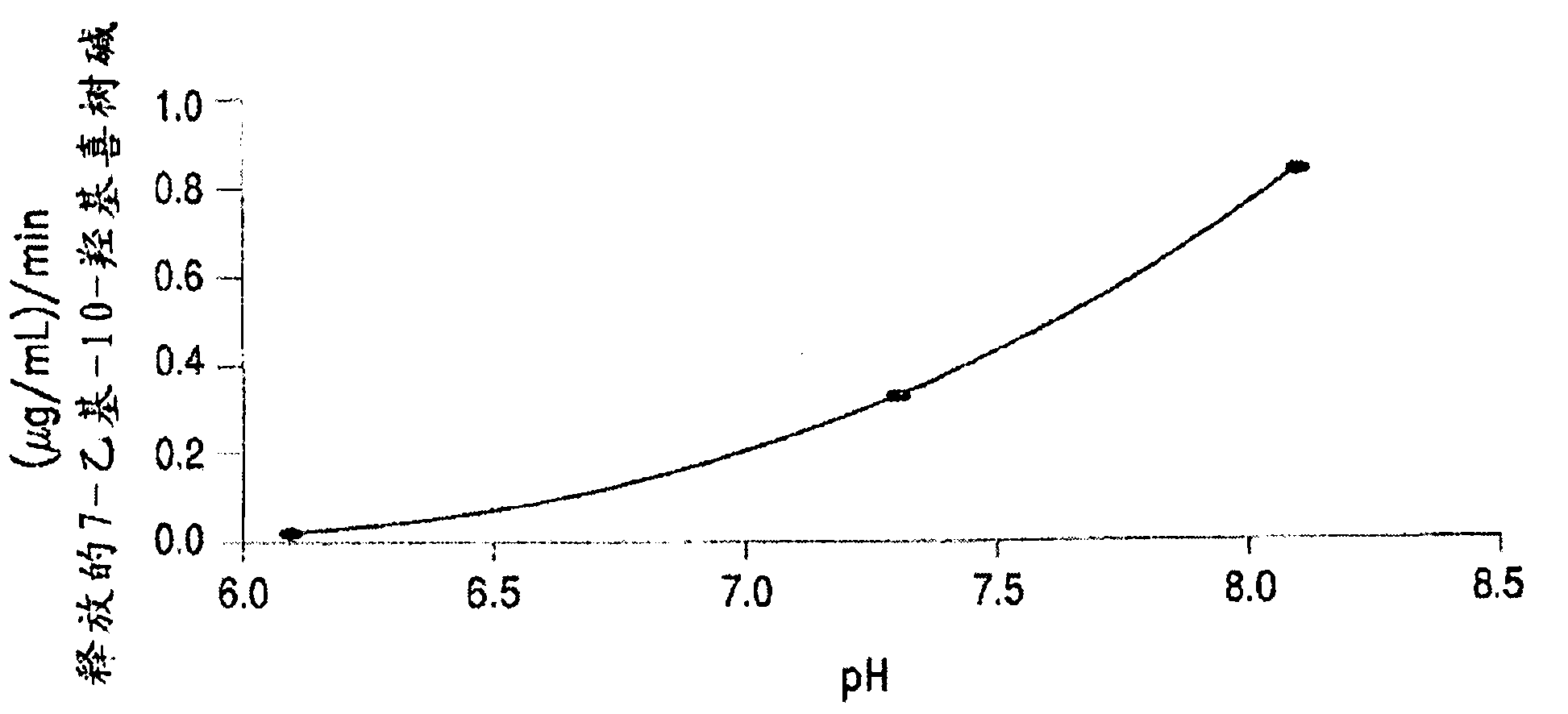
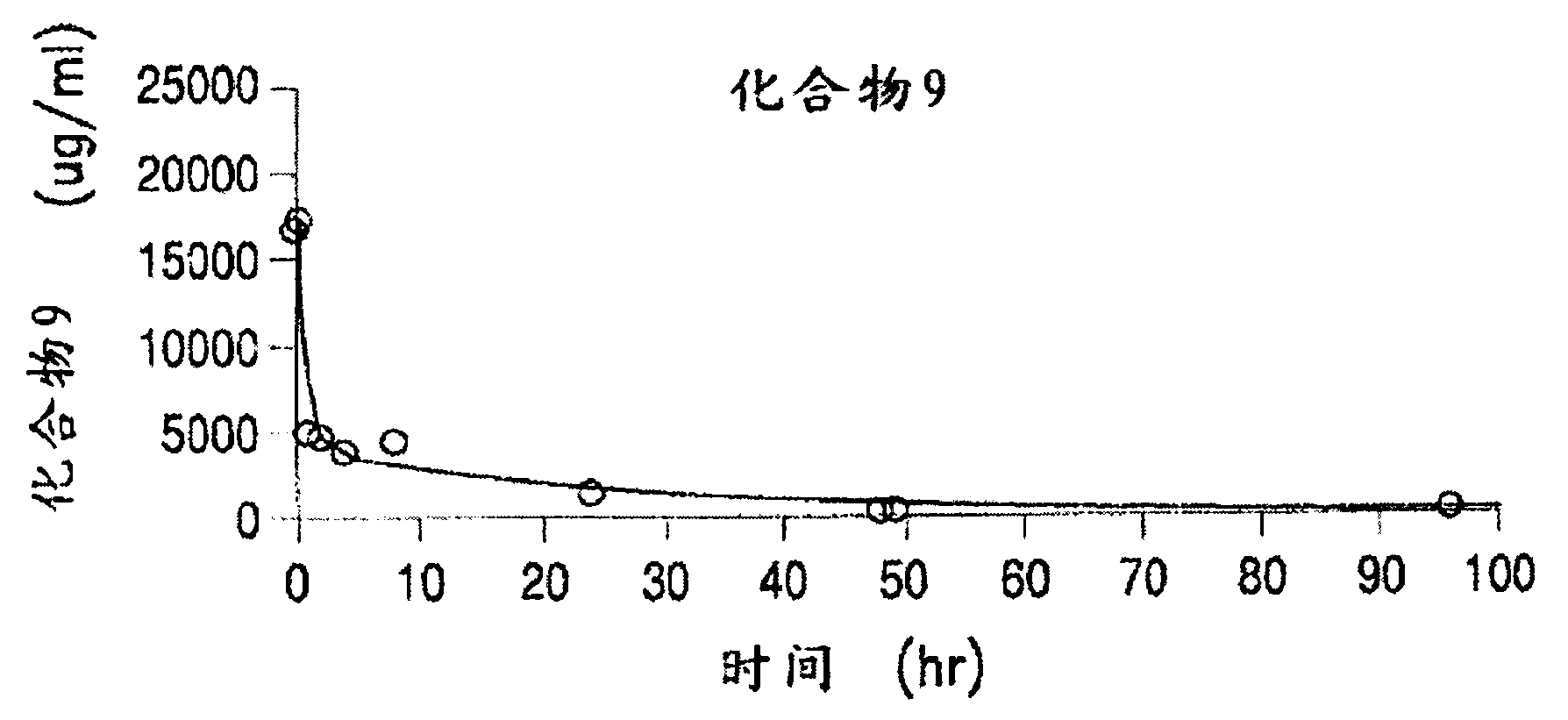
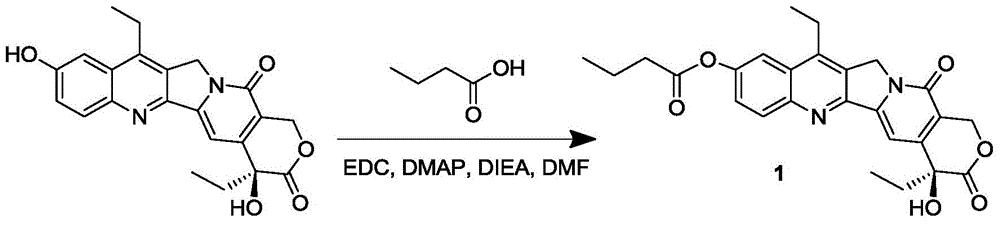
The invention discloses a 7-ethyl-10-hydroxycamptothecine drug precursor, a preparation method and an application thereof. A structure formula of the drug precursor is represented as the formula I or II. The drug precursor is prepared through an esterification reaction between a C-10 hydroxyl group or a C-20 hydroxyl group of 7-ethyl-10-hydroxycamptothecine and a hydrophobic molecule. The drug precursor has excellent anti-tumor activity and can directly release active components in vivo in a hydrolysis manner without catalytic hydrolysis of carboxylesterase, thereby achieving a high bioavailability. The drug precursor not only has excellent solubility in water but also has great solubility in amphipathic surfactants, such as tween-80 and the like, wherein the solubility can reach more than 30 mg / ml, and a high stability is achieved even that the drug precursor is diluted in water. The drug precursor can be prepared just through a one-step esterification method, is high in yield and low in preparation cost, is high in stability and good in safety, satisfies requirements in clinical medication and in large-scale industrial production, and has excellent market prospect and clinical application value.
Owner:王杭祥
Multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin for treatment of breast, colorectal, pancreatic, ovarian and lung cancers
A four arm-polyethylene glycol-7-ethyl-10-hydroxycamptothecin conjugate, such as,is disclosed. Methods of making the conjugates and methods of treating mammals using the same are also disclosed.
Owner:BELROSE PHARMA
7-ethyl-10-hydroxycamptothecin amphiphilic polymer prodrug as well as preparation method and nano-particles thereof
InactiveCN103251596AImprove solubilityHigh drug loadingOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityChemical Linkage
The invention discloses a 7-ethyl-10-hydroxycamptothecin amphiphilic polymer prodrug comprising a segment of polyethylene glycols and a segment of poly(7-ethyl-10-hydroxycamptothecin); and the poly(7-ethyl-10-hydroxycamptothecin) is formed in the way that hydroxyls at the tenth position or / and twentieth position on 7-ethyl-10-hydroxycamptothecin are connected with a polymer framework through degradable chemical bonds. Polyethylene glycols with different lengths are introduced, so that the prodrug is self-assembled in water to form nano particles, and the solubility of the 7-ethyl-10-hydroxycamptothecin in water is greatly increased. The nanometer size of the polymer prodrug is adjustable so that the 7-ethyl-10-hydroxycamptothecin amphiphilic polymer prodrug can effectively meet the requirement for sizes under different tumor systems and target different intracorporeal organs, and cancers of different organs are possibly treated.
Owner:ZHEJIANG UNIV
Treatment of resistant or refractory cancers with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
A method of treating a resistant or refractory cancer in a mammal includes administering an effective amount of a compound ofto the mammal. In preferred aspects, the cancer is resistant or refractory to CPT-11 or CPT therapy.
Owner:BELROSE PHARMA
Multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin for treatment of breast, colorectal, pancreatic, ovarian and lung cancers
The present invention discloses a four-arm- polyethylene glycol-7-ethyl-10-hydroxy-camptothecin conjugate (formula I), method for preparing the conjugate and method for treating mammal using the conjuagate.
Owner:ENZON PHARM INC
Process for the Manufacturing of 7-Ethyl-10-Hydroxy Camptothecin
The invention discloses the preparation method of 7-ethyl-10-hydroxycamptothecin from 4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione and 1-(2-amino-5 -hydroxyphenyl)-propan-1-one using higher reaction temperature and faster heating to that temperature.
Owner:FERMION
Process for preparing irinotecan
InactiveUS20070208050A1Easy to processBiocideOrganic chemistry7-ethyl-10-hydroxycamptothecinMedicinal chemistry
The present invention relates to a process for the preparation of pure irinotecan or salts thereof, and a process for the preparation of intermediate compound 7-ethyl-10-hydroxycamptothecin.
Owner:DR REDDYS LAB LTD +1
Treatment of neuroblastoma with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
InactiveUS20100098654A1Difficulty of therapyEliminate and significantly reduce immune responseNervous disorderPharmaceutical non-active ingredientsPolymeric prodrugMedicine
The present invention relates to methods of treatment of neuroblastoma. The present invention includes administering polymeric prodrugs of 7-ethyl-10-hydroxycamptothecin to patients in need thereof.
Owner:ENZON PHARM INC
Saturated long-chain fatty acid-modified 7-ethyl-10-hydroxycamptothecin compound and long-circulating liposome thereof
InactiveCN105777770AStable in natureNot easily oxidizedOrganic active ingredientsOrganic chemistrySaturated fatty acid esterLong chain fatty acid
The invention relates to the technical field of medicine, and relates to a saturated long-chain fatty acid-modified 7-ethyl-10-hydroxycamptothecin (SN-38) compound and a long-circulating liposome thereof. Under substitution reaction conditions and the existence of an acid-binding agent, SN-38 is subjected to saturated long-chain fatty acid chloride, such that a SN-38 monounsaturated fatty acid ester compound is obtained. The compound has a following general formula (I). The long-circulating liposome prepared with the compound provided by the invention has the advantages of improving drug anti-tumor effect, enhancing drug stability, reducing drug toxic and side effects, and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Long-circulated thermal sensitive liposome containing 7-ethyl-10-hydroxycamptothecin and preparation method thereof
InactiveCN102670507AImprove lipophilicityExtended stayOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityAntioxidant
The invention belongs to the technical field of medicine, and relates to a lyophilized powder for injection of a long-circulated thermal sensitive liposome containing 7-ethyl-10-hydroxycamptothecin and a preparation method thereof. The lyophilized powder for injection comprises 1%-30% of 7-ethyl-10-hydroxycamptothecin, 10%-90% of phosphatide, 0%-10% of octadecylamine, 0%-70% of a lyophilizing protective agent, 0%-50% of accessory G, 0.001%-50% of an antioxidant, 0%-30% of cholesterol, 0%-50% of thermal sensitive accessory H, and a balanced amount of a buffer solution. The long-circulated thermal sensitive liposome containing 7-ethyl-10-hydroxycamptothecin has improved water-solubility and stability, and has reduced toxicity.
Owner:SUZHOU FAMO BIOLOGICAL TECH
Method of manufacturing of 7-ethyl-10-hydroxycamptothecin
InactiveUS7544801B2Organic chemistryAntineoplastic agents7-ethyl-10-hydroxycamptothecinCarboxylic acid
The method of manufacturing of 7-ethyl-10-hydroxycamptothecin of formula I characterized in that 7-ethyl-1,2,6,7-tetrahydrocampotothecin of formula IV is oxidized with an oxidizing agent selected from the group comprising iodosobenzene, an ester of iodosobenzene, sodium periodate, potassium periodate, potassium peroxodisulfate and ammonium peroxodisulfate, in a solvent formed by a saturated aliphatic monocarboxylic acid containing 1 to 3 carbon atoms, and in the presence of water.
Owner:PLUS CHEMICAL S A
Treatment of non-hodgkin's lymphomas with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamtothecin
The present invention relates to methods of treatment of non-Hodgkin's lymphomas. The present invention includes administering polymeric prodrugs of 7-ethyl-10-hydroxy-camptothecin to patients in need thereof.
Owner:BELROSE PHARMA
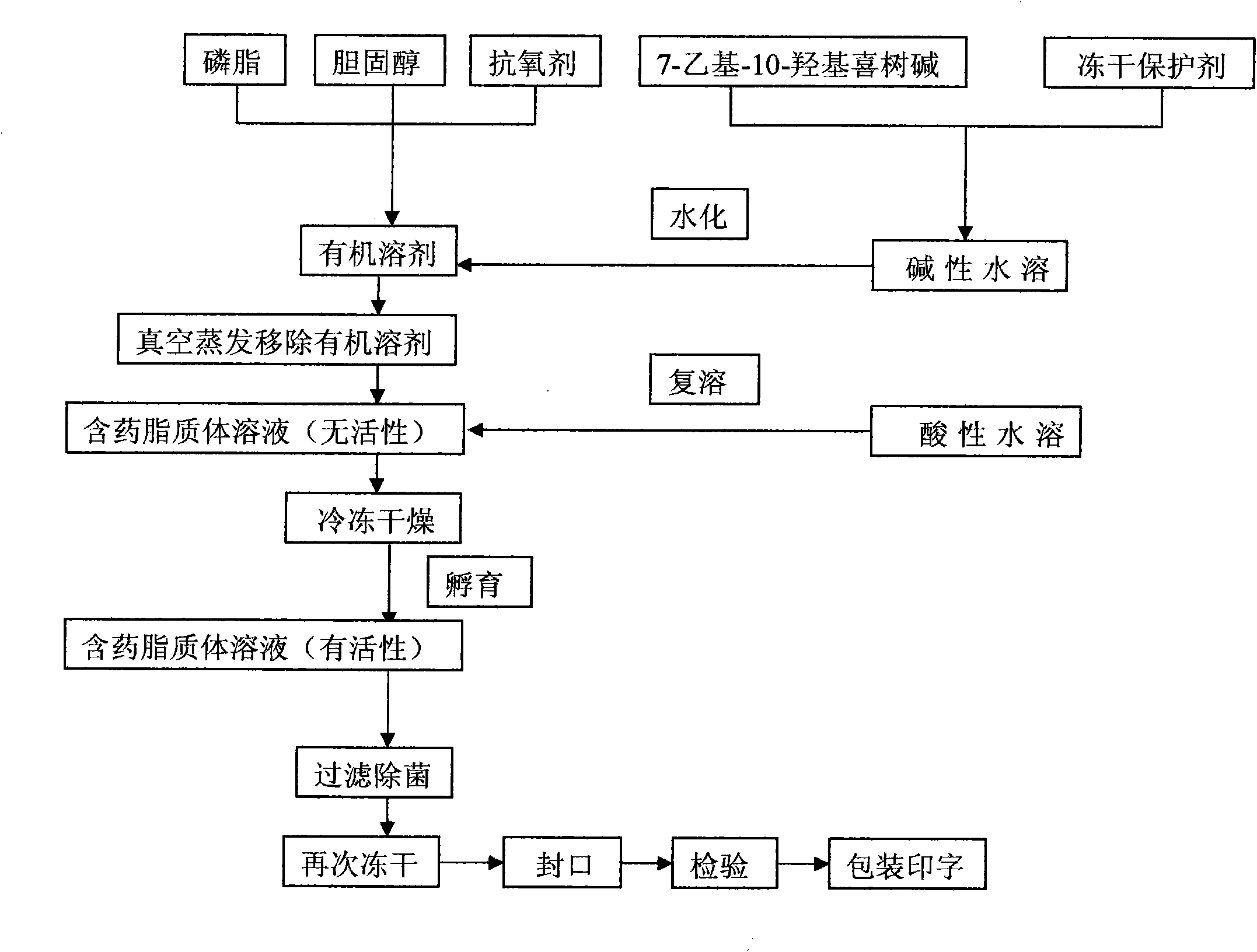
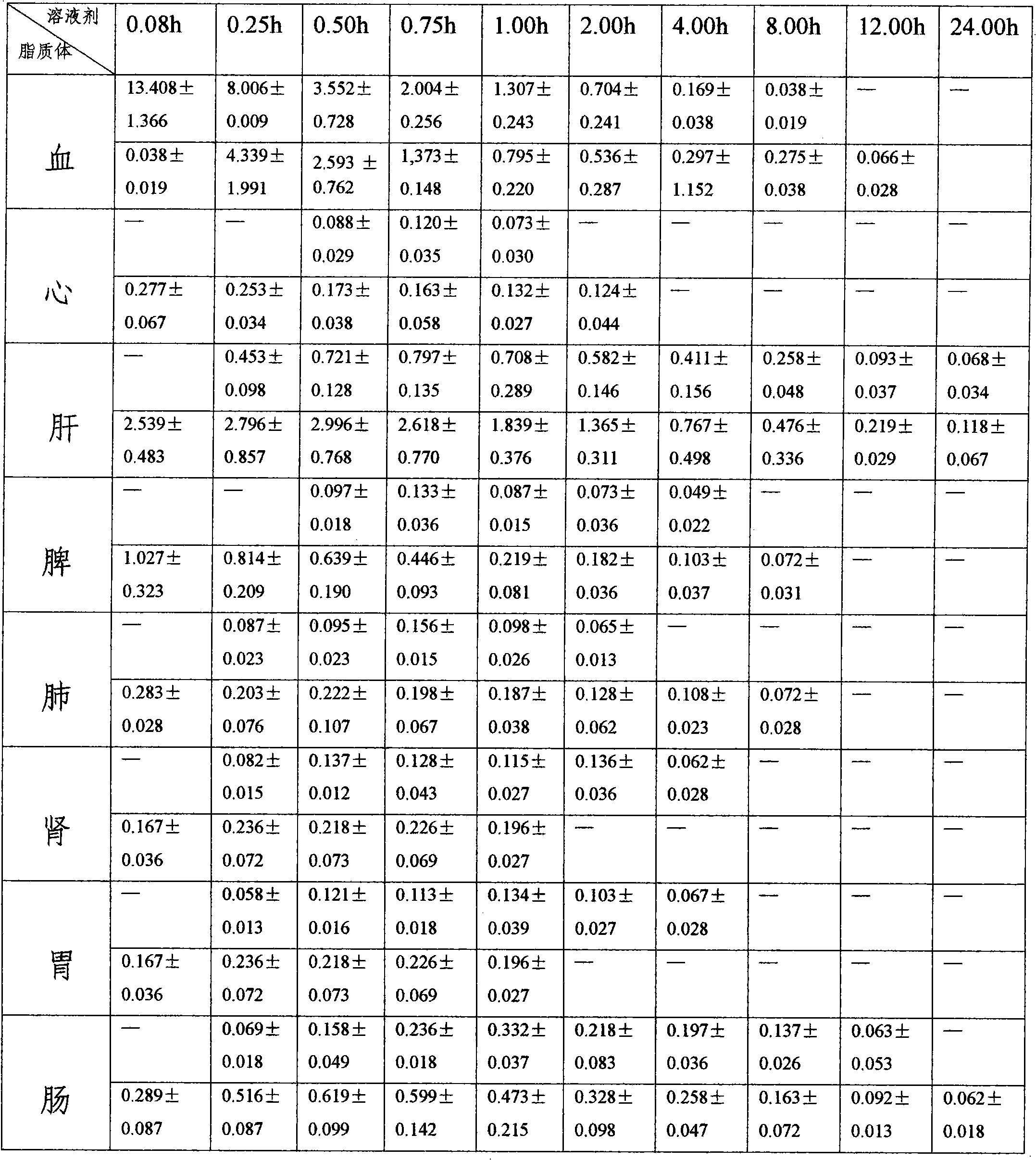
7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and preparation method thereof
InactiveCN101874788AImprove stabilityImprove in vivo stabilityPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention belongs to the medical technical field, and discloses 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and a preparation method thereof. The 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection comprises the following components: 1-10g of 7-ethyl-10-hydroxycamptothecine, 30-60g of phospholipids, 10-40g of cholesterol, 2-8g of VE, 100-300g of a freeze drying protectant, 2000-8000ml of an organic solvent, 1000-4000ml of alkaline buffer salt solution and 1000-4000ml of acid buffer salt solution. The preparation method comprises the following steps: dissolving liposoluble components in the organic solvent and water-soluble components in the alkaline buffer salt; transferring the organic solvent, and then adding the alkaline buffer salt for hydration; and carrying freeze drying in vacuum, re-dissolving with the acid buffer salt, incubating, filtering, sterilizing, and carrying out freeze drying again to obtain the 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection for injection. The invention solves the problems of low solubility and fast in-vivo metabolism of the 7-ethyl-10-hydroxycamptothecine, thus lowering toxic reaction, eliminating side reaction, having higher target distribution characteristics, prolonging metabolism time and improving solubility and bioavailability.
Owner:SHENYANG PHARMA UNIVERSITY
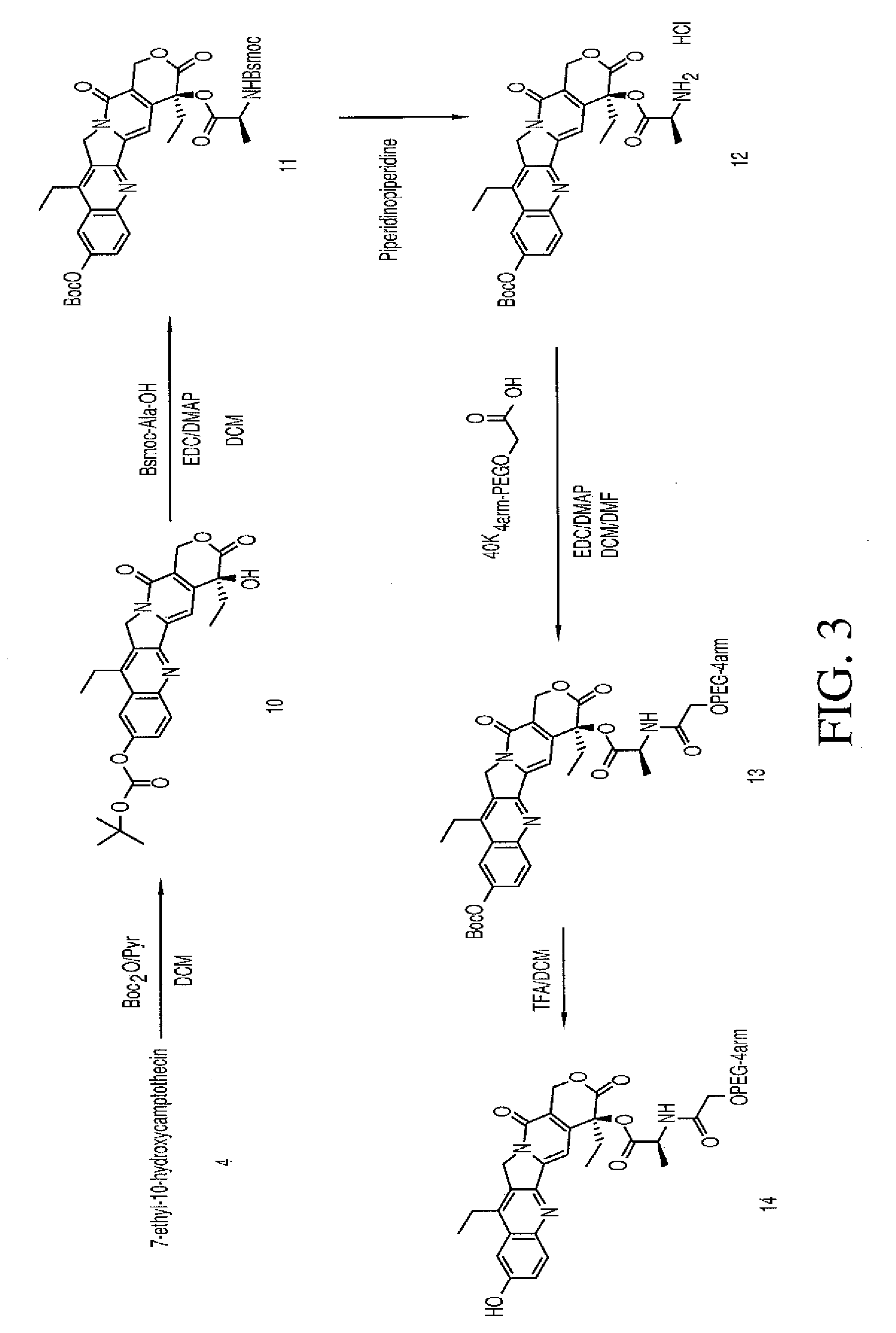
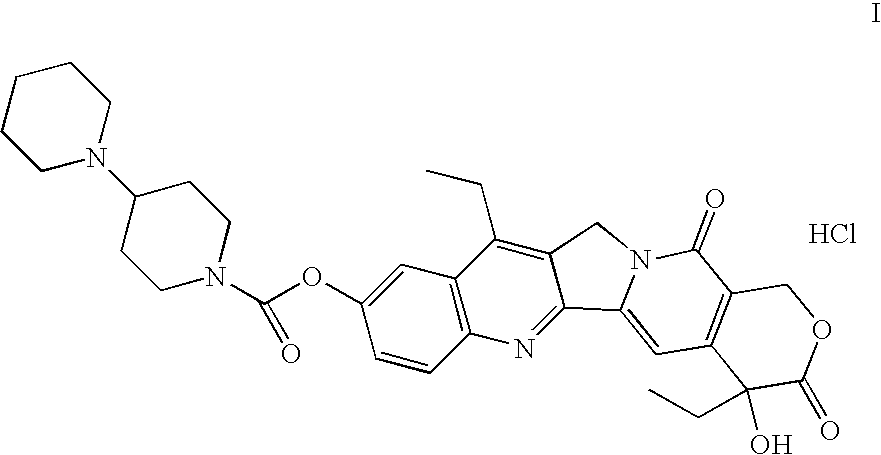
Method of manufacturing of 7-ethyl-10-[4-(1-piperidino)-1- piperidino]- carbonyloxy- camptothecin
InactiveUS20060199961A1Negligible lossOrganic chemistryAntineoplastic agents7-ethyl-10-hydroxycamptothecinSolvent
The invention relates to the method of manufacturing of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecin by condensation of 7-ethyl-10-hydroxycamptothecin with 1-chlorocarbonyl-4-piperidinopiperidine hydrochloride in a polar aprotic solvent in the presence of 4-dimethylaminopyridine.
Owner:PLUS CHEMICAL S A
A kind of method for preparing irinotecan hydrochloride
ActiveCN102260272AAvoid foul smellHigh activityOrganic chemistrySide effect7-ethyl-10-hydroxycamptothecin
The invention provides a method for preparing irinotecan hydrochloride. The method reacts the intermediate 7-ethyl-10-hydroxycamptothecin with 4-piperidinylpiperidine carboxylic acid chloride or its salt in the presence of 4-dimethylaminopyridine or its salt, or its analogue, The final product is obtained through steps such as concentration, washing, and salt formation. The method of the invention avoids the use of malodorous and easily discolored pyridine to participate in the reaction, reduces side reactions, improves the purity and yield of the product, improves the color of the product at the same time, and is easy for large-scale production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
New application of curcumin
ActiveCN102885800ADigestive systemKetone active ingredients7-ethyl-10-hydroxycamptothecinSevere diarrhea
The invention provides application of a curcumin on preparation of a medicine for preventing or / and treating a late diarrhea caused by an anti-tumor medicine, wherein the anti-tumor medicine is irinotecan or an active metabolite 7-thyl-10-hydroxycamptothecine (SN-38), and the late diarrhea is a severe diarrhea capable of lasting for 7 days appeared after the irinotecan is administrated for 24 hours. The curcumin provided by the invention is definite in efficacy; and with the adoption of the curcumin, the late diarrhea can be effectively relieved and the intestinal mucosa is protected.
Owner:CHINA PHARM UNIV
Methods for inhibiting angiogenesis with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
InactiveUS20120122956A1Reduce adverse effectsSure easyBiocidePharmaceutical non-active ingredientsDiseasePolymeric prodrug
The present invention relates to methods of inhibiting angiogenesis in mammals. The present invention includes administering polymeric prodrugs of 7-ethyl-10-hydroxycamptothecin to the mammals in need thereof. The present invention also relates to methods of treating a disease associated with angiogenesis in mammals by administering polymeric prodrugs of 7-ethyl-10-hydroxycamptothecin to the mammals in need thereof.
Owner:BELROSE PHARMA
Amphiphilic polymer prodrug for reducing responsive 7-ethyl-10-hydroxycamptothecine and preparation method thereof
ActiveCN109303780ARetain the advantageExert specific degradationOrganic active ingredientsPharmaceutical non-active ingredients7-ethyl-10-hydroxycamptothecinHydrolysis
The invention relates to an amphiphilic polymer prodrug for reducing responsive 7-ethyl-10-hydroxycamptothecine and a preparation method thereof. The amphiphilic polymer prodrug has the molecular structure as shown in the specification, wherein the polymerization degree n is 5-1000 and m is 1 or 2. According to the prodrug, targeted drug delivery can be realized, and therefore the advantages of anano drug carrier system are kept, and meanwhile, the characteristic that a disulfide bond is specifically degraded in a tumor site can be achieved. Compared with conventional 2,2'-disulfanediyldiacetic acid, 3,3'-dithiodipropionic acid and other connecting arms, the prodrug and the preparation method thereof have the advantage that an anticancer drug in an active compound molecular form can be obtained without further hydrolysis.
Owner:烟台药物研究所
7-ethyl-10-hydroxycamptothecin derivatives, and preparation and application thereof
InactiveCN105777769AImprove druggabilityIncreased oil-water partition coefficientOrganic chemistryAntineoplastic agentsSolubilitySide effect
The invention relates to the technical field of medicine. According to the invention, omega-3 unsaturated fatty acid is used for modifying the 10-site hydroxyl group of 7-ethyl-10-hydroxycamptothecin (SN-38), such that a type of novel 7-ethyl-10-hydroxycamptothecin derivatives are formed. The invention further provides a preparation method of the type of compounds, and an application thereof in preparing anti-tumor drugs. The compound provided by the invention has relatively high lipid solubility, such that medicine preparation is facilitated. The type of compounds have a targeting effect upon tumor tissues, and have good anti-tumor effects and low toxic and side effects.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Nano-medicine of biogenic macrocyclic molecule and preparation method of nano-medicine
InactiveCN109276570AEfficient transportConducive to maintaining anticancer activityOrganic active ingredientsMacromolecular non-active ingredientsWhole body7-ethyl-10-hydroxycamptothecin
The invention relates to the field of pharmacy, in particular to a nano-medicine of a biogenic macrocyclic molecule and a preparation method of the nano-medicine. The nano-medicine of the biogenic macrocyclic molecule comprises 7-ethyl-10-hydroxycamptothecin and a phospholipid nanodisk transporting the 7-ethyl-10-hydroxycamptothecin, and the phospholipid nanodisk comprises a hydrophobic surface facing interior lipid and a hydrophilic surface facing outwards. The nano-medicine has excellent anti-tumor properties, negligible general toxicity and long-term immunotoxicity. The preparation method is simple in operation and mild in reaction conditions, and the nano-medicine can be rapidly prepared and obtained.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
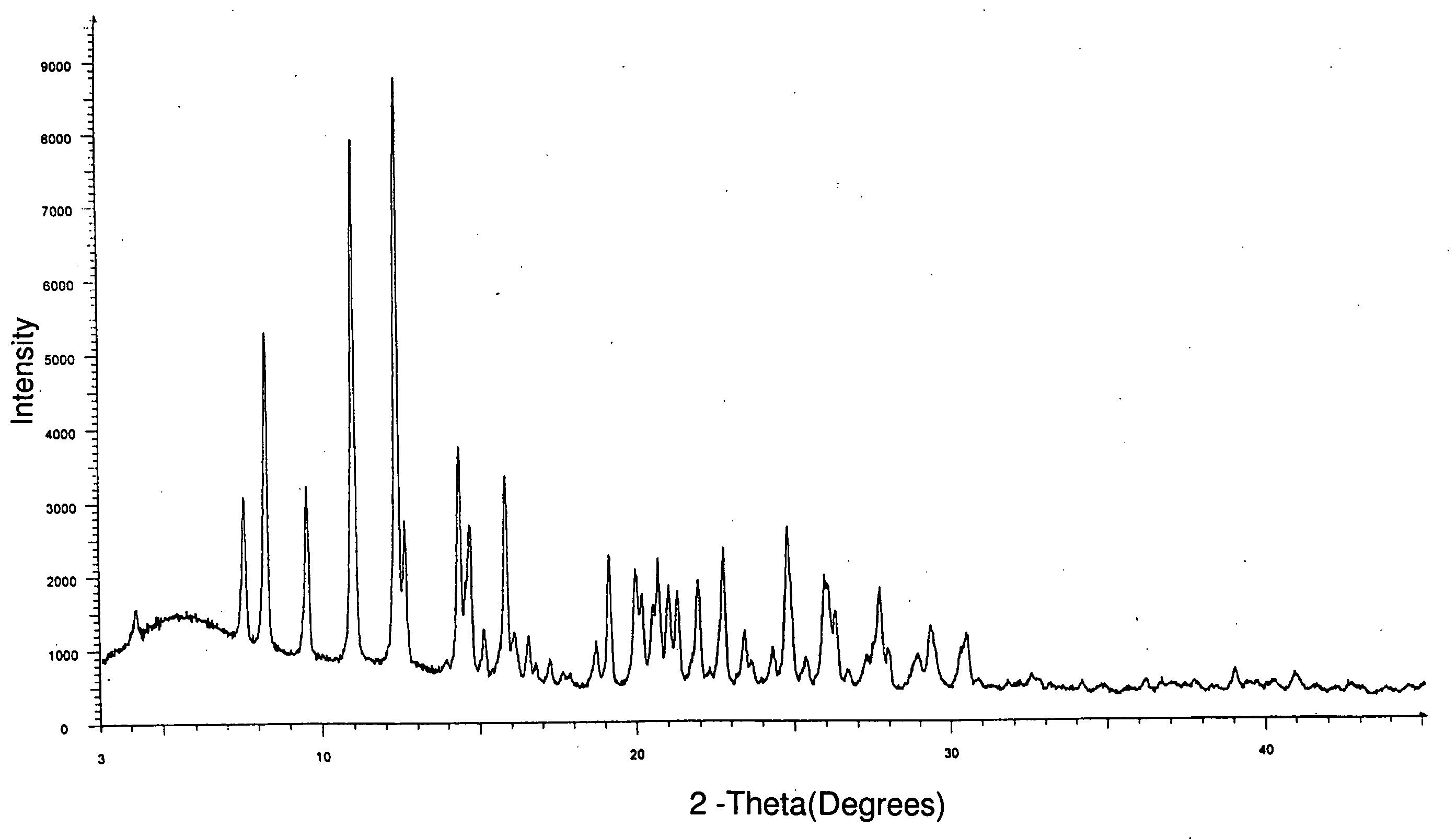
Novel 7-ethyl-10-hydroxycamptothecine crystal and preparation method thereof
ActiveCN104530067ACrystal form with high solubilityEasy to prepareOrganic active ingredientsOrganic chemistrySolubility7-ethyl-10-hydroxycamptothecin
The invention relates to a novel 7-ethyl-10-hydroxycamptothecine crystal and a preparation method thereof. According to the novel 7-ethyl-10-hydroxycamptothecine crystal, X-ray powder which is radiated by using Cu-Ka and expressed through a 2 theta angle has characteristic peaks at diffraction angles of 7.1 degrees, 10.26 degrees, 11.36 degrees, 13.22 degrees, 14.14 degrees, 16.12 degrees, 17.86 degrees, 18.74 degrees, 19.5 degrees, 20.18 degrees, 21.98 degrees, 22.74 degrees, 23.64 degrees, 24.6 degrees, 25.28 degrees, 26.52 degrees and 29.84 degrees, and the error range of 2 theta is + / -0.2 degree. The novel 7-ethyl-10-hydroxycamptothecine crystal disclosed by the invention is high in solubility. The preparation method disclosed by the invention is simple, easy to operate and suitable for technical production.
Owner:SHANDONG UNIV
New application of curcumin
The invention provides application of a curcumin on preparation of a medicine for preventing or / and treating a late diarrhea caused by an anti-tumor medicine, wherein the anti-tumor medicine is irinotecan or an active metabolite 7-thyl-10-hydroxycamptothecine (SN-38), and the late diarrhea is a severe diarrhea capable of lasting for 7 days appeared after the irinotecan is administrated for 24 hours. The curcumin provided by the invention is definite in efficacy; and with the adoption of the curcumin, the late diarrhea can be effectively relieved and the intestinal mucosa is protected.
Owner:CHINA PHARM UNIV
7-ethyl-10-hydroxycamptothecin prodrug and its preparation method and application
InactiveCN105315294BGood antitumor activityImprove bioavailabilityOrganic active ingredientsOrganic chemistrySolubility7-ethyl-10-hydroxycamptothecin
The invention discloses a 7-ethyl-10-hydroxycamptothecine drug precursor, a preparation method and an application thereof. A structure formula of the drug precursor is represented as the formula I or II. The drug precursor is prepared through an esterification reaction between a C-10 hydroxyl group or a C-20 hydroxyl group of 7-ethyl-10-hydroxycamptothecine and a hydrophobic molecule. The drug precursor has excellent anti-tumor activity and can directly release active components in vivo in a hydrolysis manner without catalytic hydrolysis of carboxylesterase, thereby achieving a high bioavailability. The drug precursor not only has excellent solubility in water but also has great solubility in amphipathic surfactants, such as tween-80 and the like, wherein the solubility can reach more than 30 mg / ml, and a high stability is achieved even that the drug precursor is diluted in water. The drug precursor can be prepared just through a one-step esterification method, is high in yield and low in preparation cost, is high in stability and good in safety, satisfies requirements in clinical medication and in large-scale industrial production, and has excellent market prospect and clinical application value.
Owner:王杭祥
7-ethyl-10-hydroxycamptothecine crystal forms, and preparation method and application thereof
ActiveCN104557961ACrystal form with high solubilityEasy to prepareOrganic active ingredientsOrganic chemistrySolubilityX-ray
The invention relates to 7-ethyl-10-hydroxycamptothecine crystal forms II and III, and a preparation method and application thereof. The crystal form II uses Cu-K alpha radiation; the X-ray powder diffraction represented by 2theta angle has characteristic peaks when the diffraction angle is 5.64, 10.80, 12.55, 13.48, 17.09, 17.65, 18.21, 18.79, 24.52 and 26.84 degrees; and the error range of 2theta is + / -0.2 degree. The crystal form III uses Cu-K alpha radiation; the X-ray powder diffraction represented by 2theta angle has characteristic peaks when the diffraction angle is 4.58, 9.34, 10.42, 11.12, 12.30, 13.40, 14.40, 16.34, 16.86, 17.20, 17.70, 18.72, 19.32, 21.48, 22.44, 24.90, 25.40, 25.68 and 27.44 degrees; and the error range of 2theta is + / -0.2 degree. The two crystal forms of 7-ethyl-10-hydroxycamptothecine have high solubility and favorable stability.
Owner:SHANDONG UNIV
Method for preparing high-purity irinotecan
ActiveCN101337966BSimple methodEasy to operateOrganic chemistry7-ethyl-10-hydroxycamptothecinChloride
The invention provides a method for synthesizing and purifying irinotecan. The method prepares irinotecan that is an antineoplastic compound with high purity quotient through the purification of 7-ethide-10-hydroxycamptothecine that is an intermediate for synthesizing irinotecan and crystallization for impurity removal of hydrochloric piperidyl piperidine formyl chloride; secondary reaction is reduced, and the purity quotient as well as the yield of products are increased through improving the reaction condition at the same time, especially through cryoconcentration evolution; the purity quotient of the obtained products is more than 99.5 percent, and the single hetero is less than 0.1 percent. In addition, the method overcomes the disadvantages that the cycle is long, the solvent quantity is large, etc. caused by column chromatography that is used for purifying the products, and large scale production is easy to realize.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Method of manufacturing of 7-ethyl-10-hydroxycamptotecin
The method of manufacturing of 7-ethyl-10-hydroxycamptothecin of formula I characterized in that 7-ethyl-1,2,6,7-tetrahydrocampotothecin of formula IV is oxidized with an oxidizing agent selected from the group coprising iodosobenzene, an ester of iodosobenzene, sodium periodate, potassium periodate, potassium peroxodisulfate and ammonium peroxodisulfate, in a solvent formed by a saturated aliphatic monocarboxylic acid containing 1 to 3 carbon atoms, and in the presence of water.
Owner:PLUS CHEMICAL S A
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




















![Method of manufacturing of 7-ethyl-10-[4-(1-piperidino)-1- piperidino]- carbonyloxy- camptothecin Method of manufacturing of 7-ethyl-10-[4-(1-piperidino)-1- piperidino]- carbonyloxy- camptothecin](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/aeb70bc3-3eb8-4d56-85fa-b2e5a69ff8c7/US20060199961A1-20060907-C00001.png)
![Method of manufacturing of 7-ethyl-10-[4-(1-piperidino)-1- piperidino]- carbonyloxy- camptothecin Method of manufacturing of 7-ethyl-10-[4-(1-piperidino)-1- piperidino]- carbonyloxy- camptothecin](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/aeb70bc3-3eb8-4d56-85fa-b2e5a69ff8c7/US20060199961A1-20060907-C00002.png)
![Method of manufacturing of 7-ethyl-10-[4-(1-piperidino)-1- piperidino]- carbonyloxy- camptothecin Method of manufacturing of 7-ethyl-10-[4-(1-piperidino)-1- piperidino]- carbonyloxy- camptothecin](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/aeb70bc3-3eb8-4d56-85fa-b2e5a69ff8c7/US20060199961A1-20060907-C00003.png)