Methods of treating her2 positive cancer with her2 receptor antagonist in combination with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
a technology of her2 receptor and her2 positive cancer, which is applied in the field of her2 positive cancer treatment, can solve the problems of inability to treat her2 positive cancer, adverse effects of trastuzumab treatment, and dangerous patients to receive trastuzumab in combination with anthracycline-based chemotherapy. , to achieve the effect of inhibiting tumor growth and/or proliferation, reducing resistance, and poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0345]Toxicity Data
[0346]A maximum tolerated dose (“MTD”) of 4arm-PEG-Gly-(7-ethyl-10-hydroxycamptothecin) (compound 9) was studied using nude mice. Mice were monitored for 14 days for mortality and signs of illness and sacrificed when body weight loss was >20% of the pretreatment body weight.
[0347]Table 2, below, shows the maximum tolerated dose of each compound for both single dose and multiple dose administration. Each dose for multiple dose administration was given mice every other day for 10 days and the mice were observed for another 4 days, thus for total 14 days.
TABLE 2MTD Data in Nude MiceDose LevelSurvival / Compound(mg / kg)TotalCommentsCompound 9255 / 5Single dose305 / 5354 / 5Mouse euthanized due to >20% body weight lossCompound 9105 / 5Multiple dose*153 / 5Mice euthanized due to >20% body weight loss200 / 5Mice euthanized due to >20% body weight loss
[0348]The MTD found for 4arm-PEG-Gly-(7-ethyl-10-hydroxycamptothecin) (compound 9) was 30 mg / kg when given as single dose, and 10 mg / kg w...
example 2
[0349]Properties of PEG Conjugates
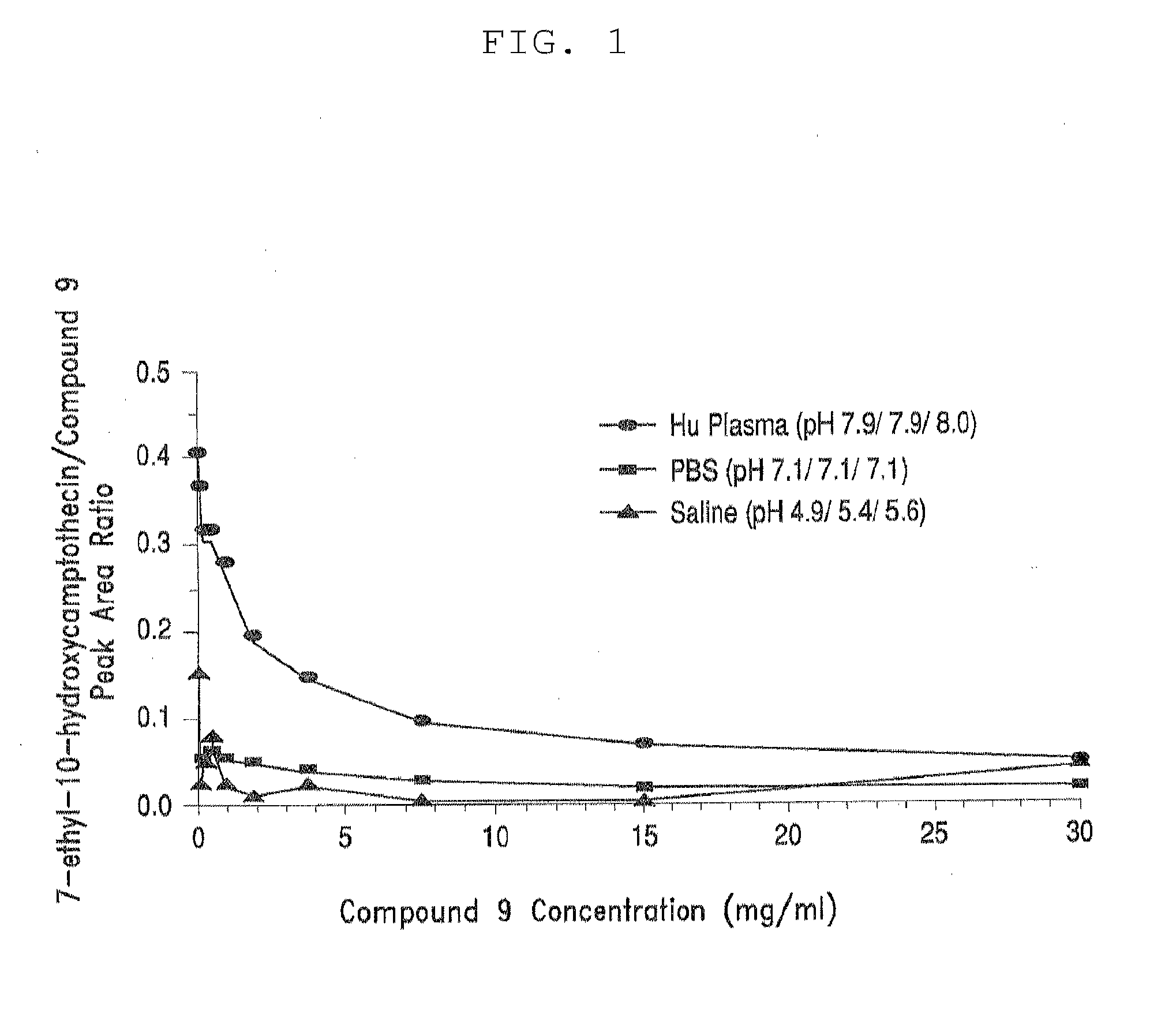
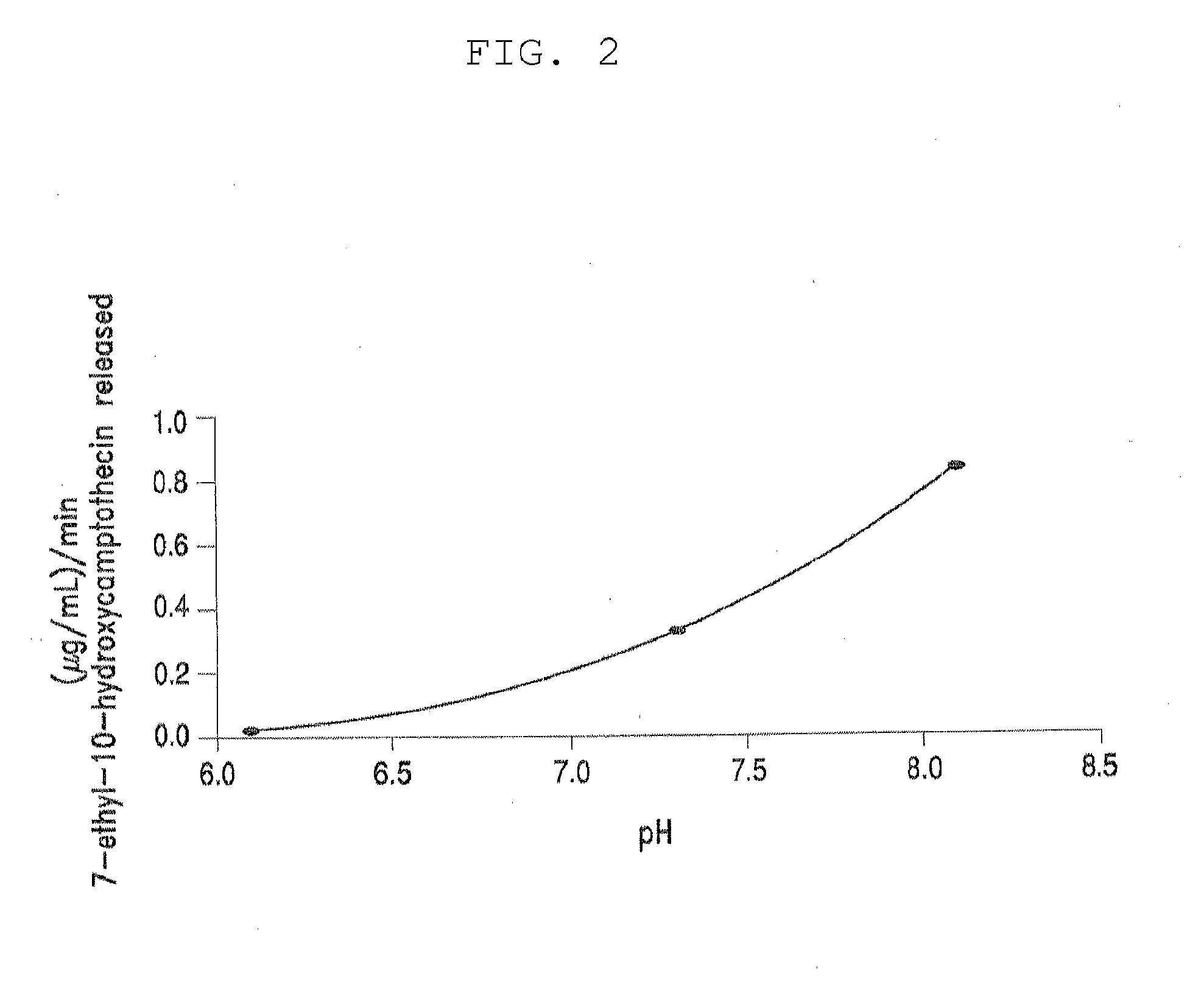
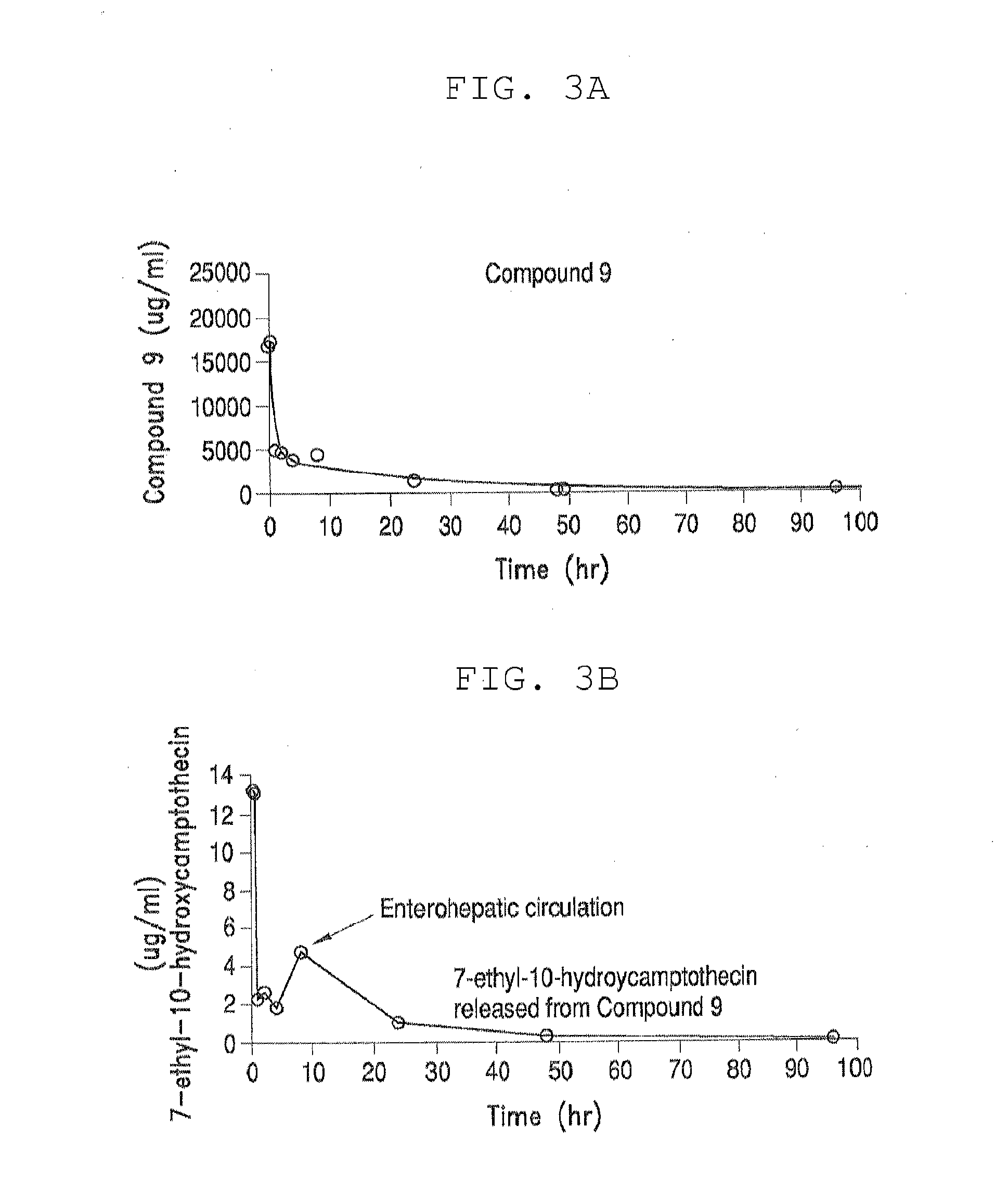
[0350]Table 3, below, shows solubility of four different PEG-(7-ethyl-10-hydroxycamptothecin) conjugates in aqueous saline solution. All four PEG-(7-ethyl-10-hydroxycamptothecin) conjugates showed good solubility of up to 4 mg / mL equivalent of 7-ethyl-10-hydroxycamptothecin. In human plasma, 7-ethyl-10-hydroxycamptothecin was steadily released from the PEG conjugates with a doubling time of 22 to 52 minutes and the release appeared to be pH and concentration dependent as described in the following
example 3
[0351]
TABLE 3Properties of PEG-7-ethyl-10-hydroxycamptothecin ConjugatesSolubility t 1 / 2(min) in Doubling Time in SalineHumanin Plasma (min)cCompound(mg / mL)aPlasmabHumanMouseRatCompound 918012.331.449.5570(Gly)Compound 1212112.551.945.8753(Ala)Compound 23ND19.028.843.4481(Sar)Compound 1814226.822.241.91920(Met)a7-ethyl-10-hydroxycamptothecin is not soluble in saline.bPEG conjugate half life.c7-ethyl-10-hydroxycamptothecin formation rate from conjugates.
[0352]PEG-7-ethyl-10-hydroxycamptothecin conjugates show good stability in saline and other aqueous medium for up to 24 hours at room temperature.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| elimination half life | aaaaa | aaaaa |
| apparent elimination half life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com