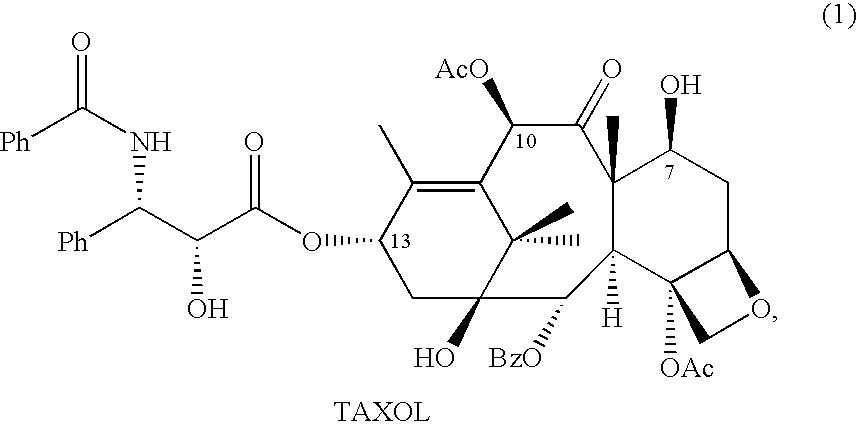
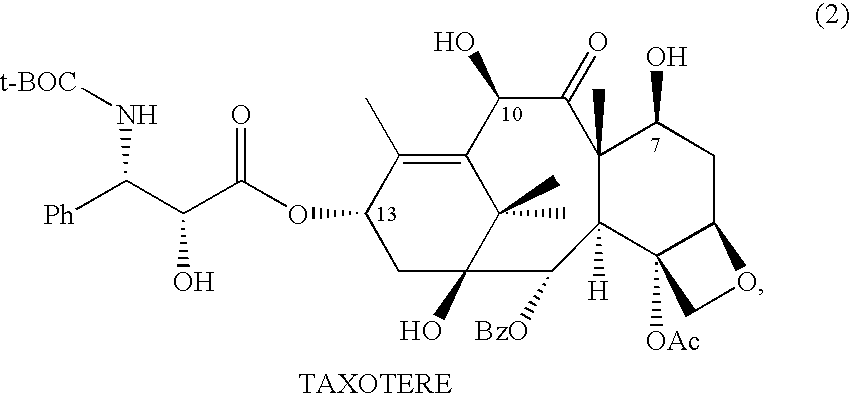
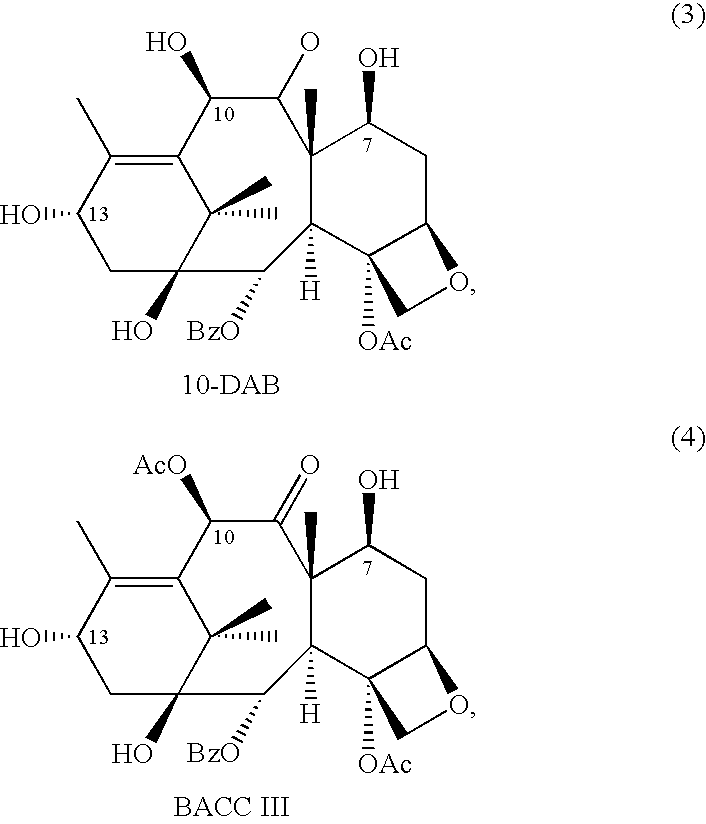
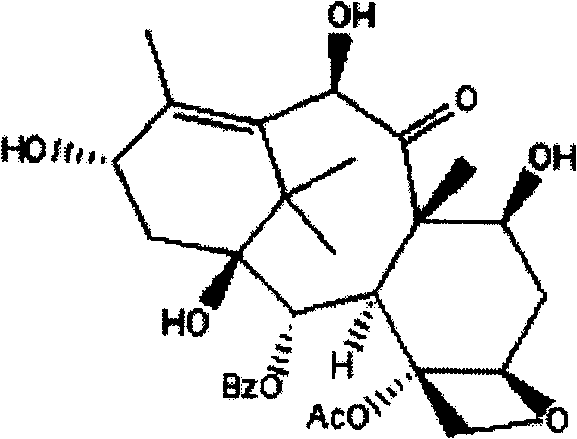
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
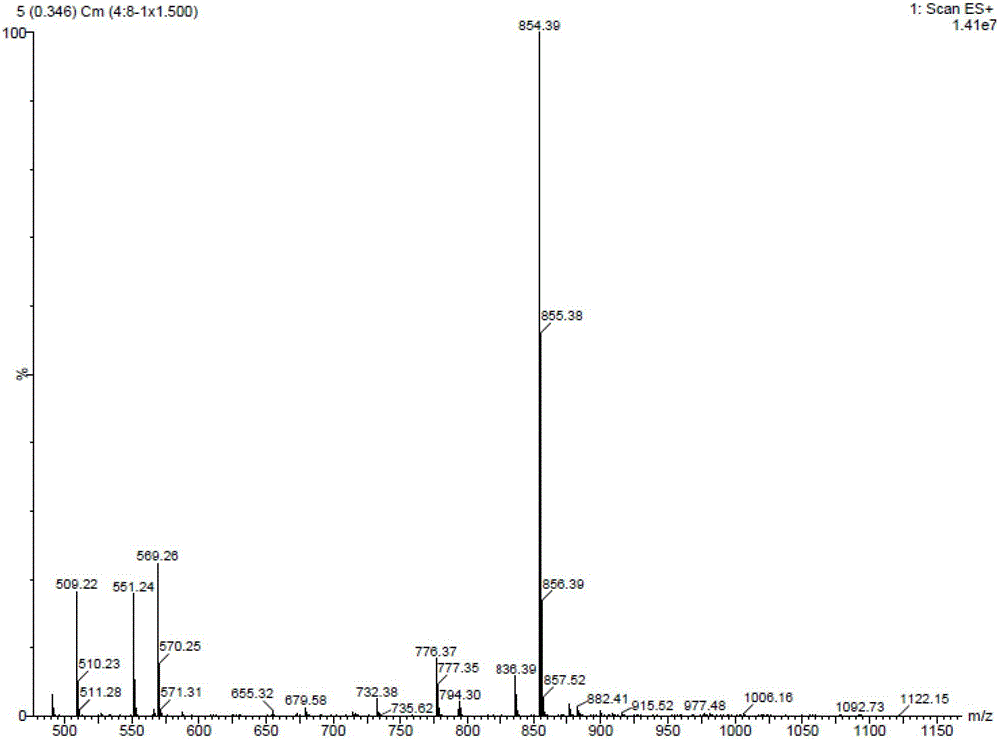
74 results about "10-Deacetylbaccatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
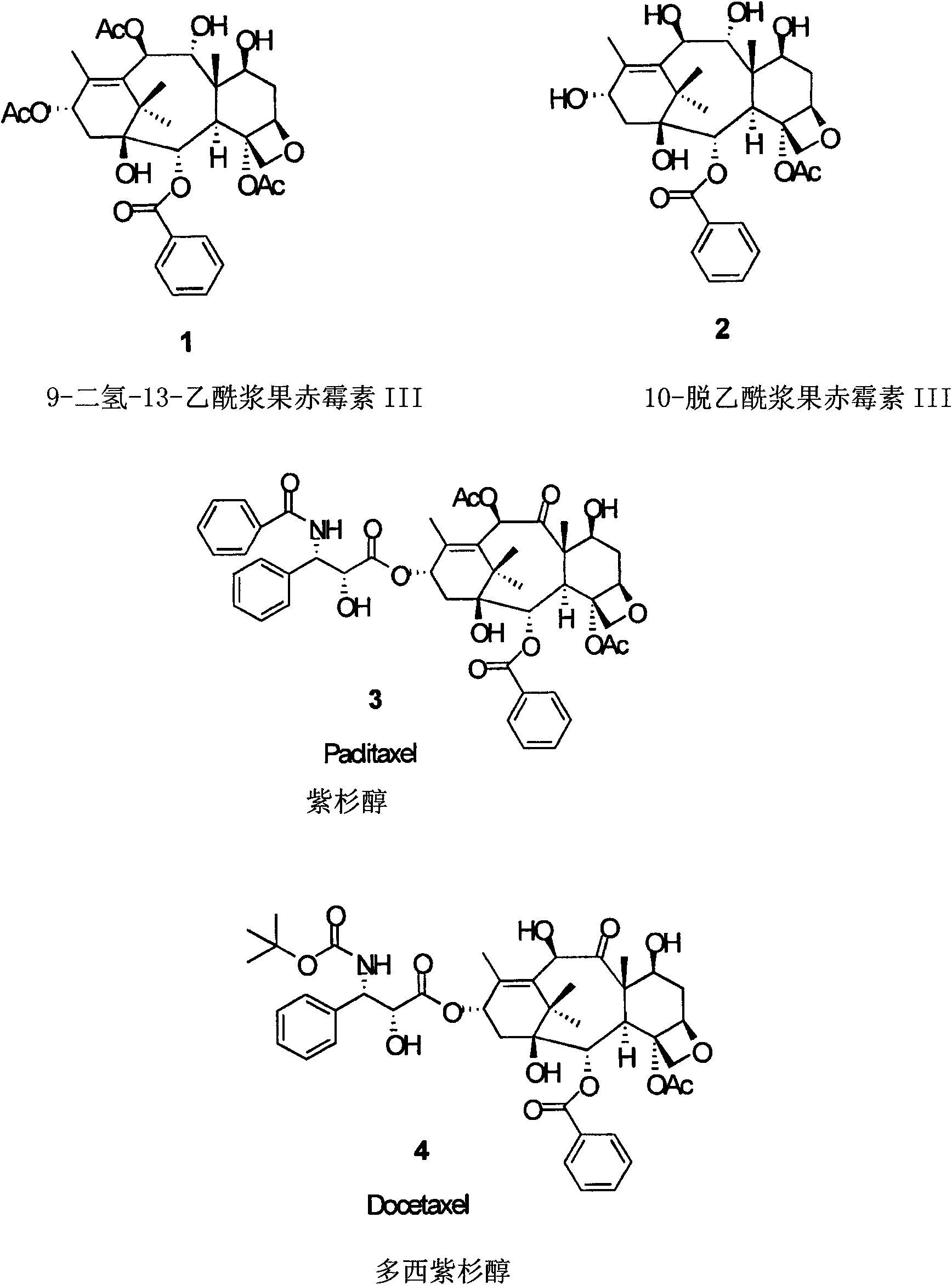
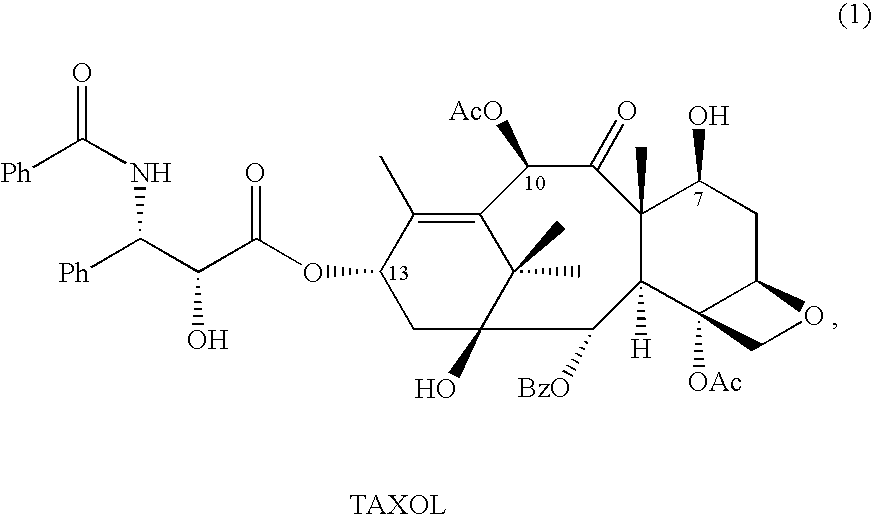
10-Deacetylbaccatins are a series of closely related natural organic compounds isolated from the yew tree (Genera Taxus). 10-Deacetylbaccatin III is a precursor to the anti-cancer drug docetaxel (Taxotere).
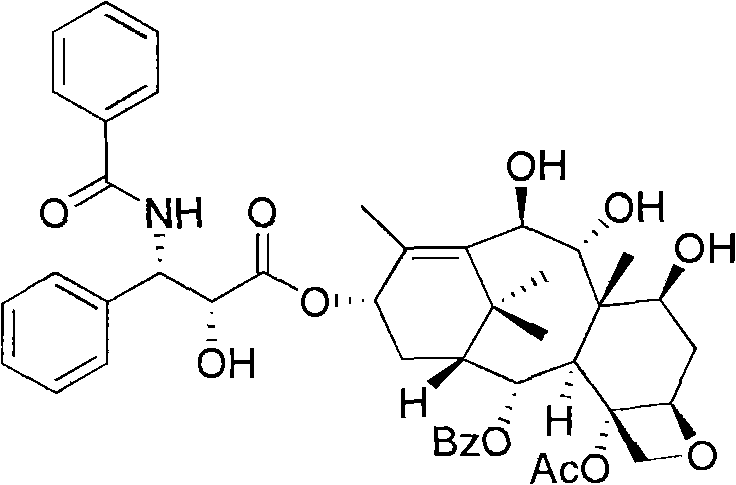
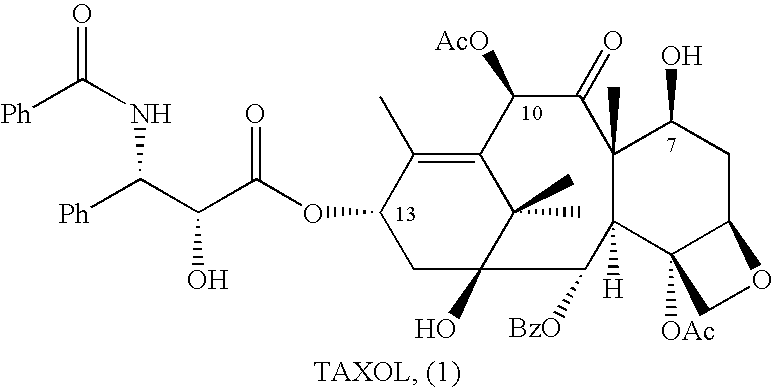
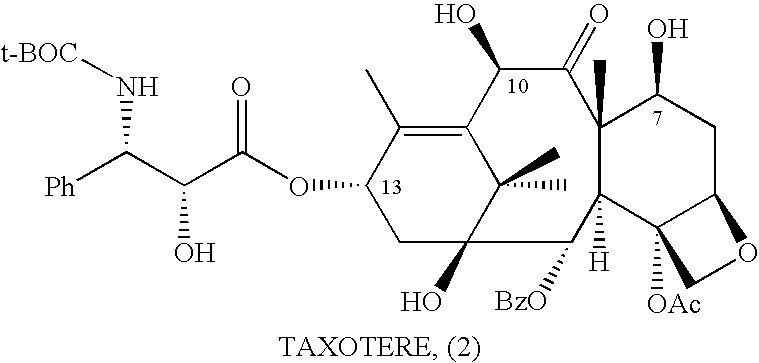
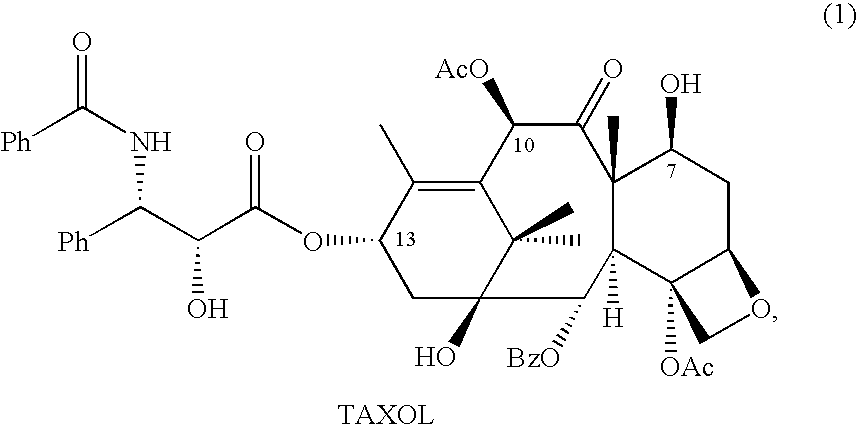
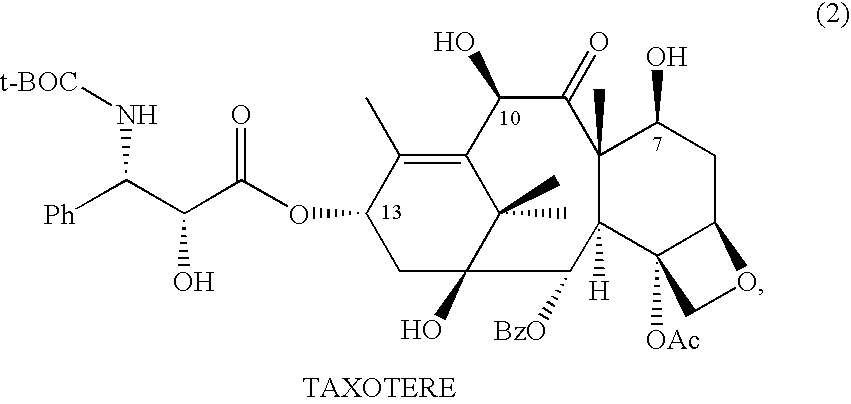
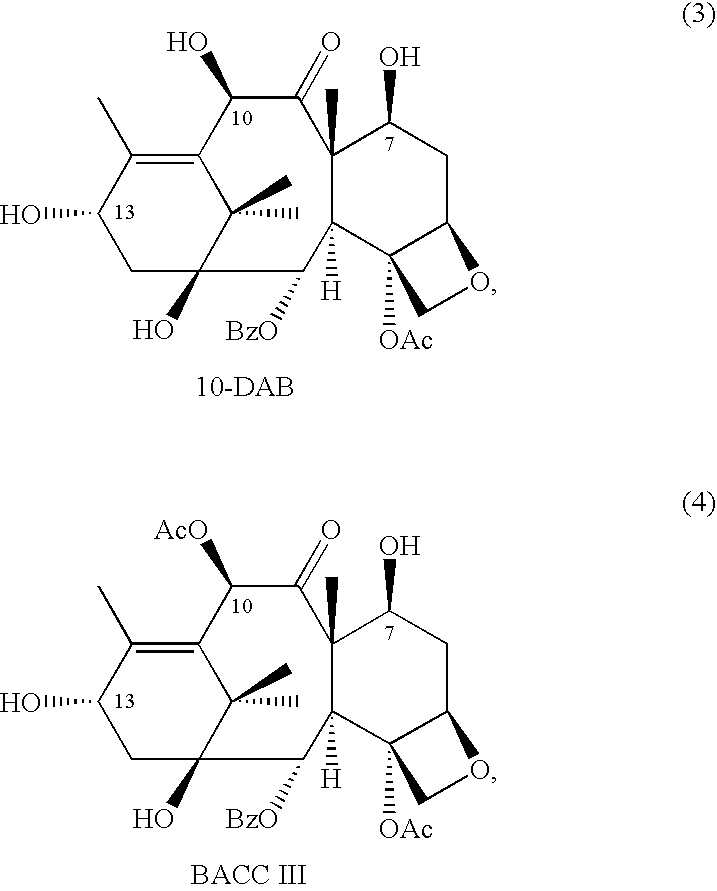
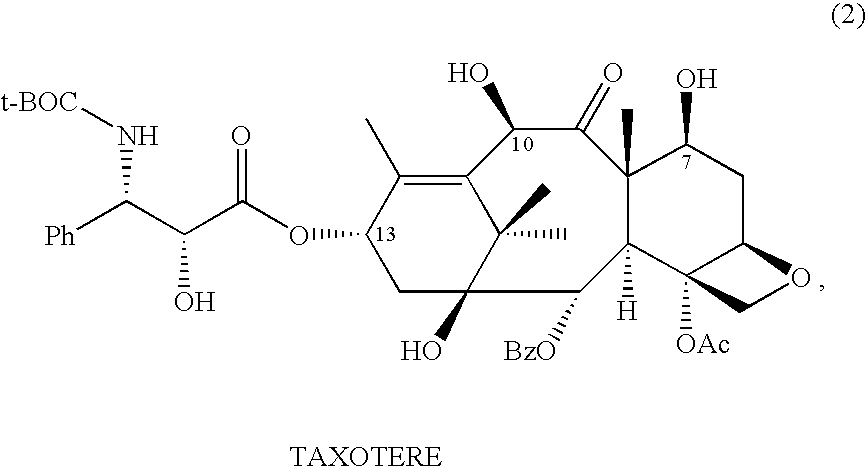
Anticancer taxanes such as paclitaxel, docetaxel and their structural analogs, and a method for the preparation thereof
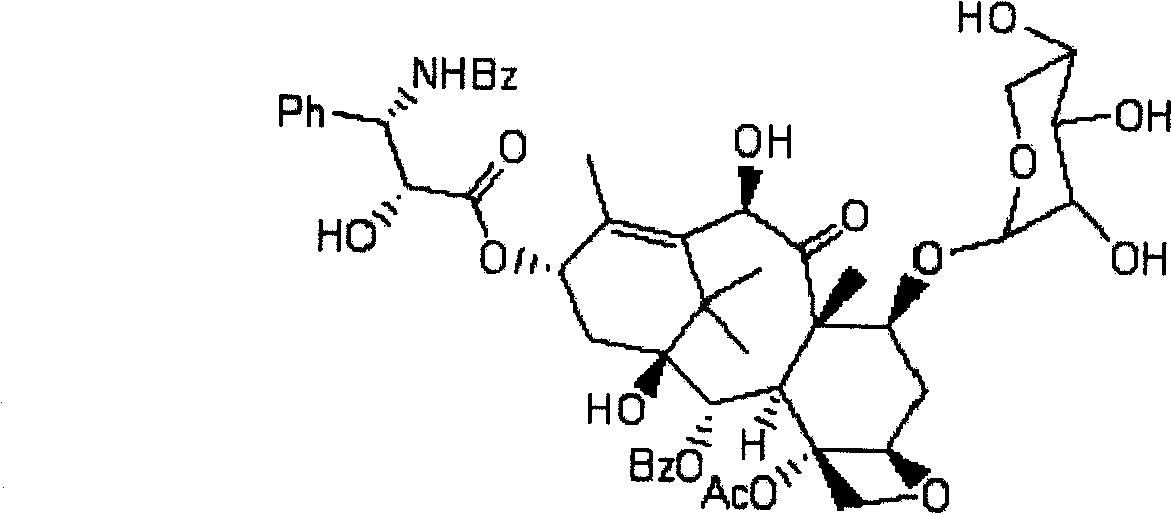
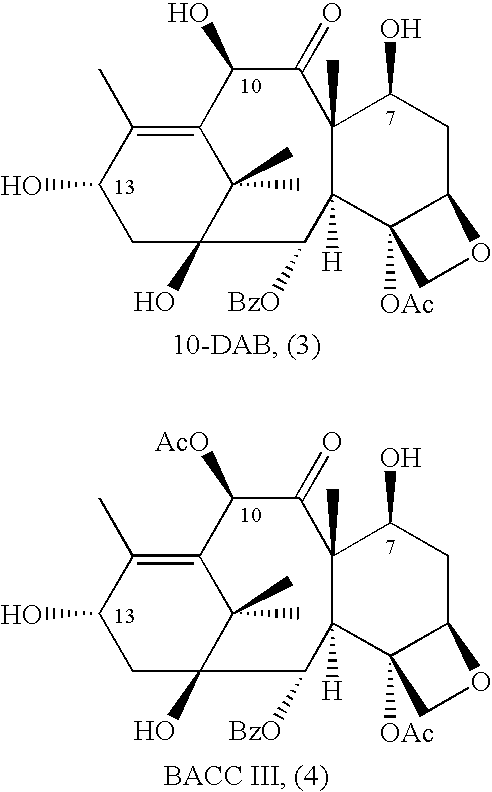
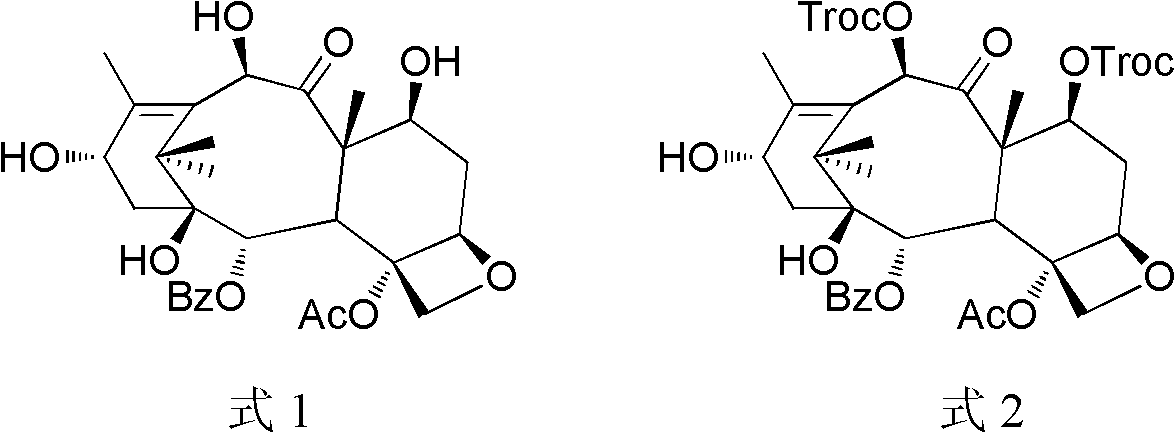
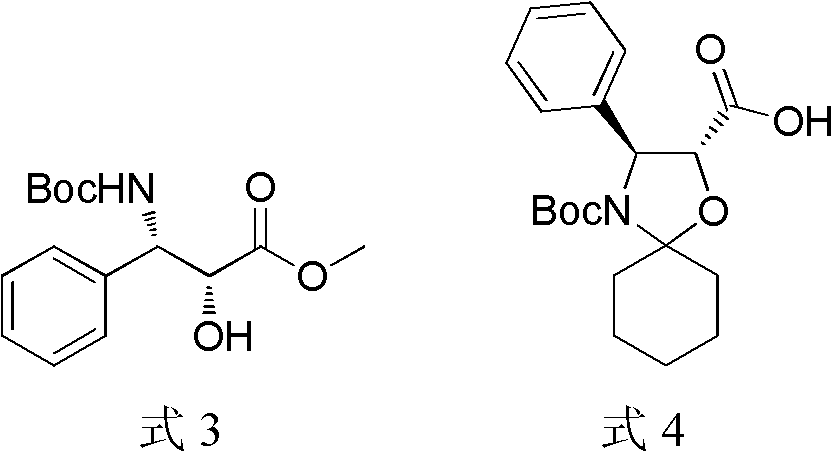
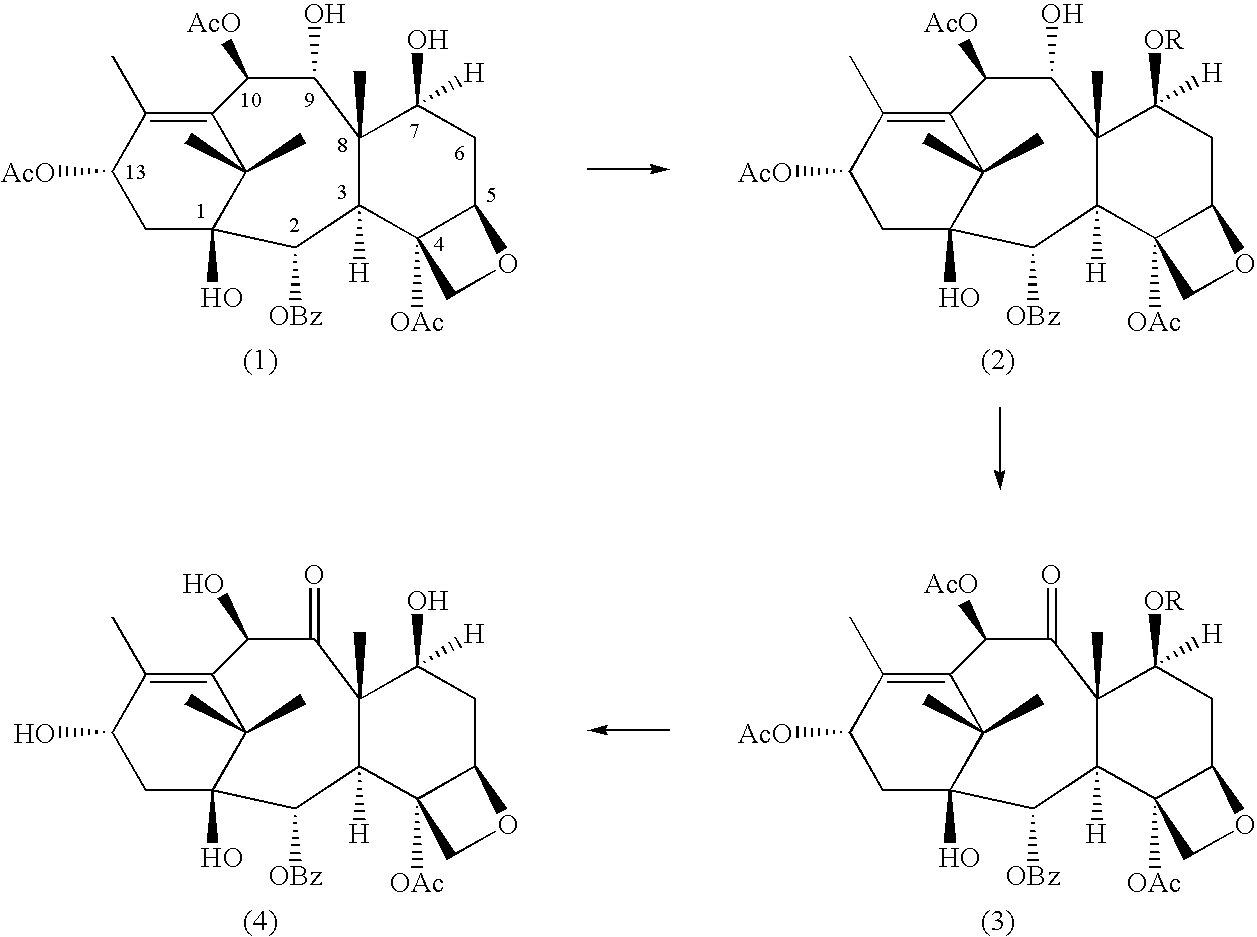
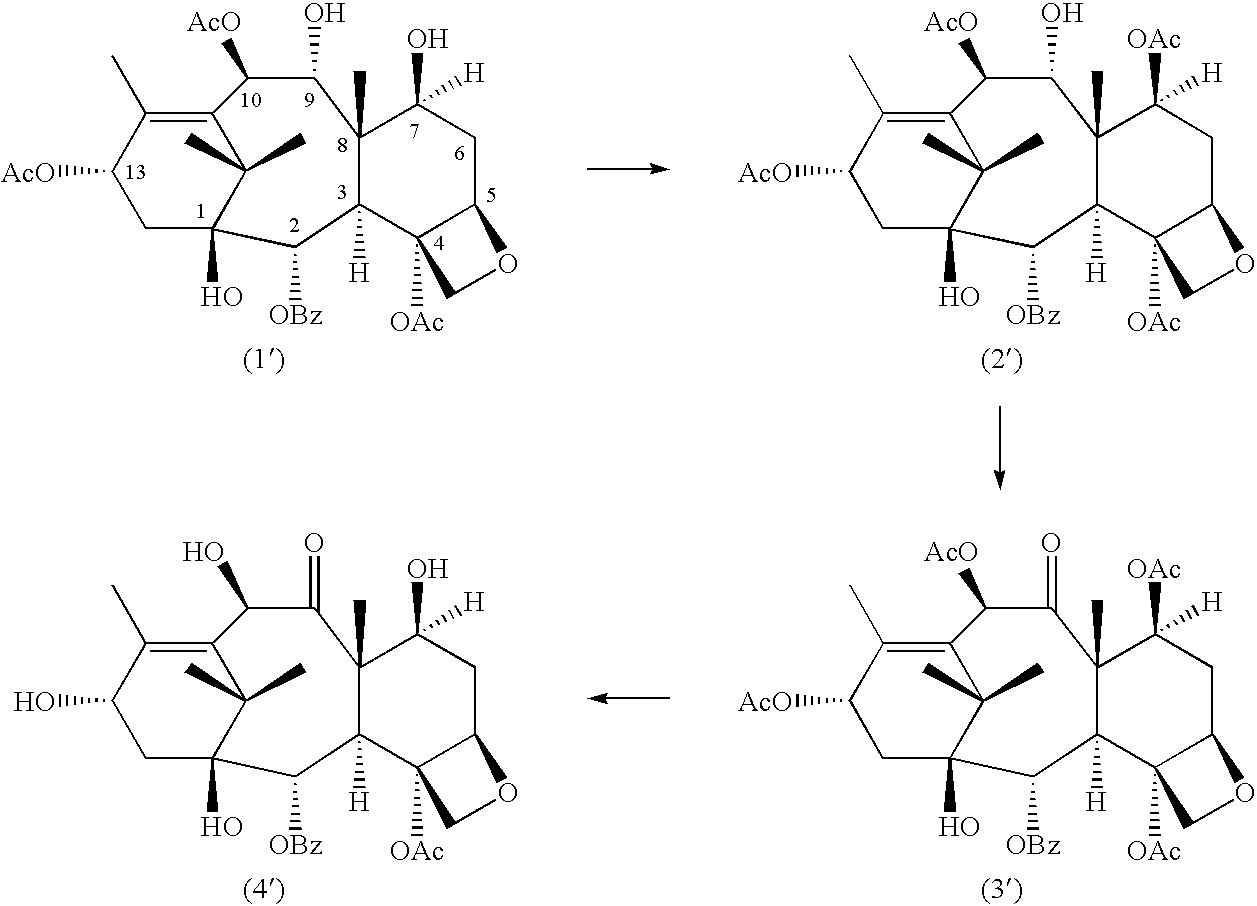
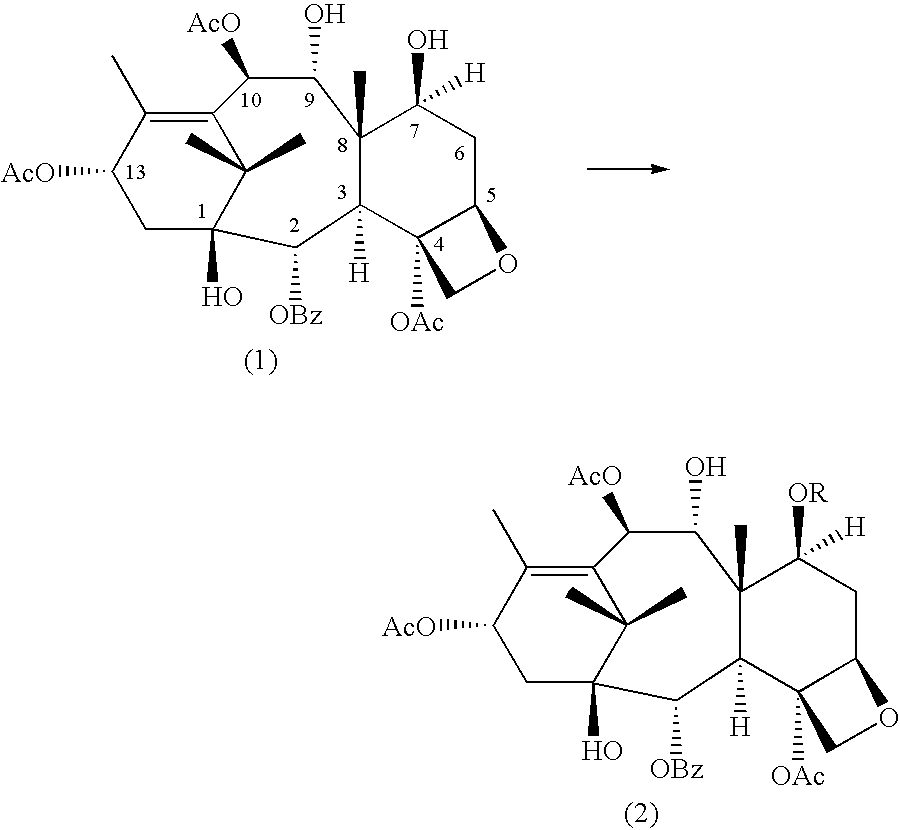
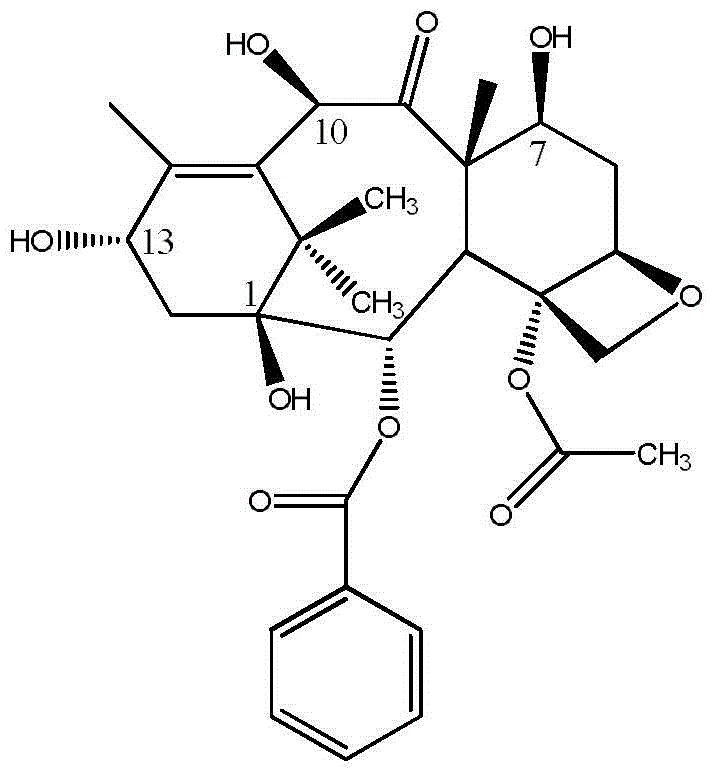
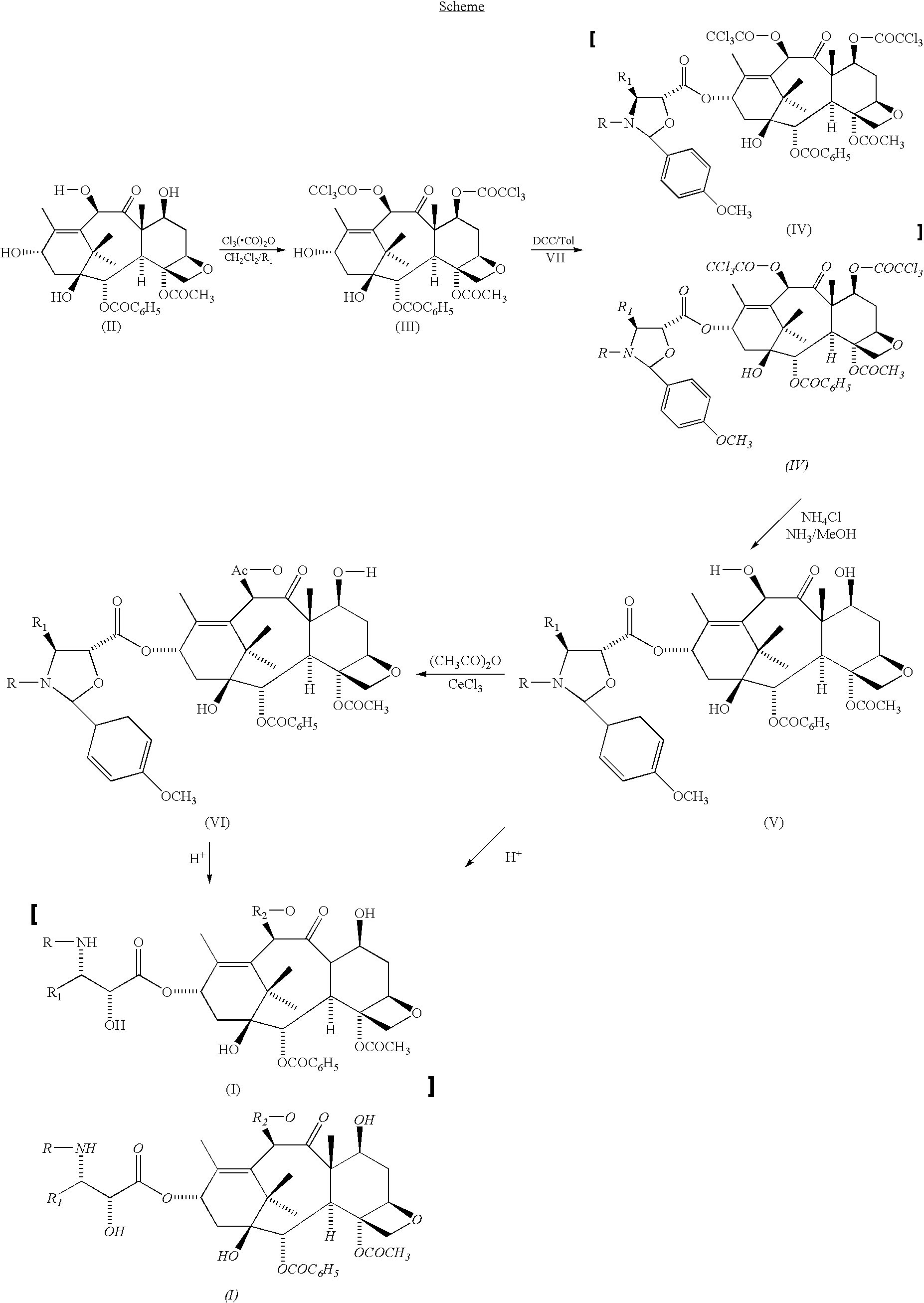
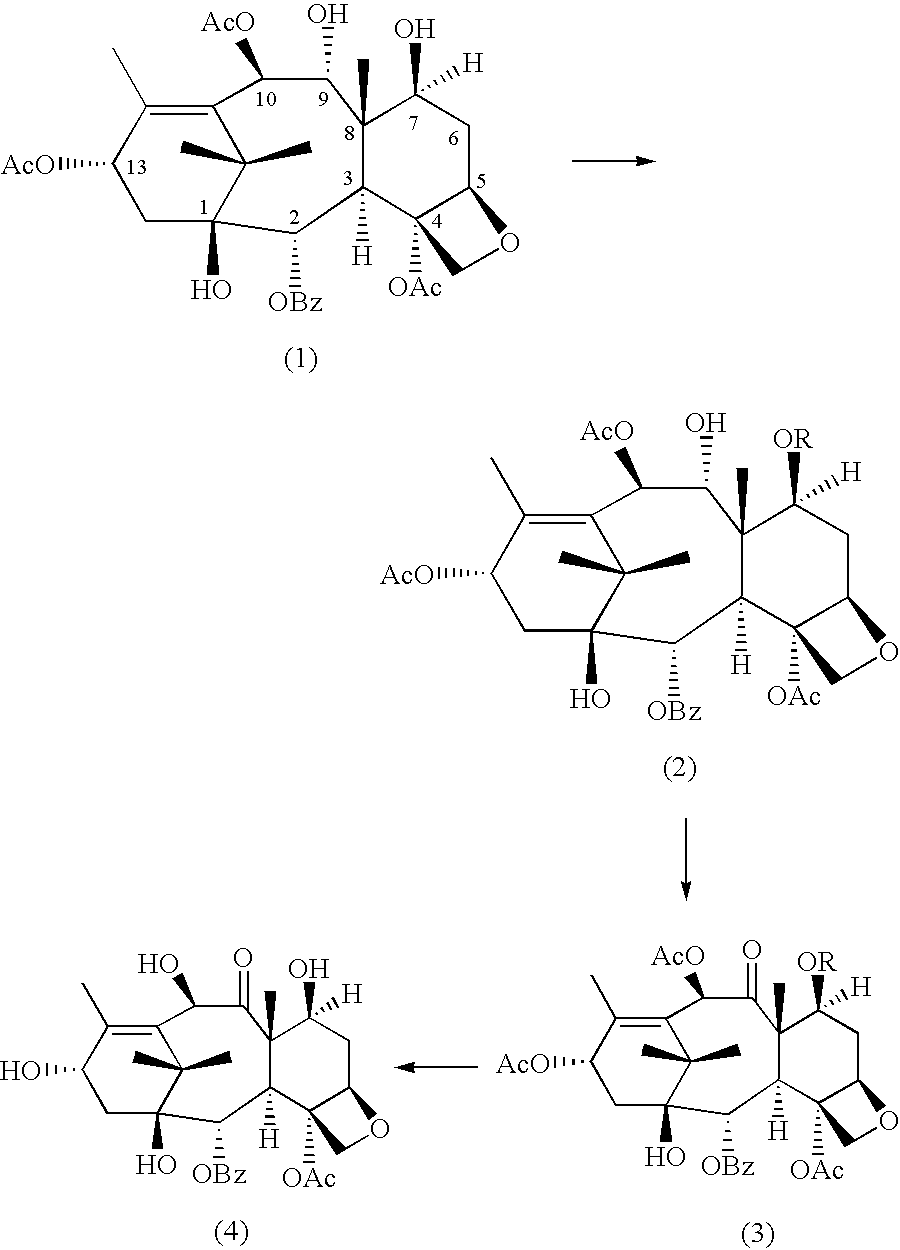
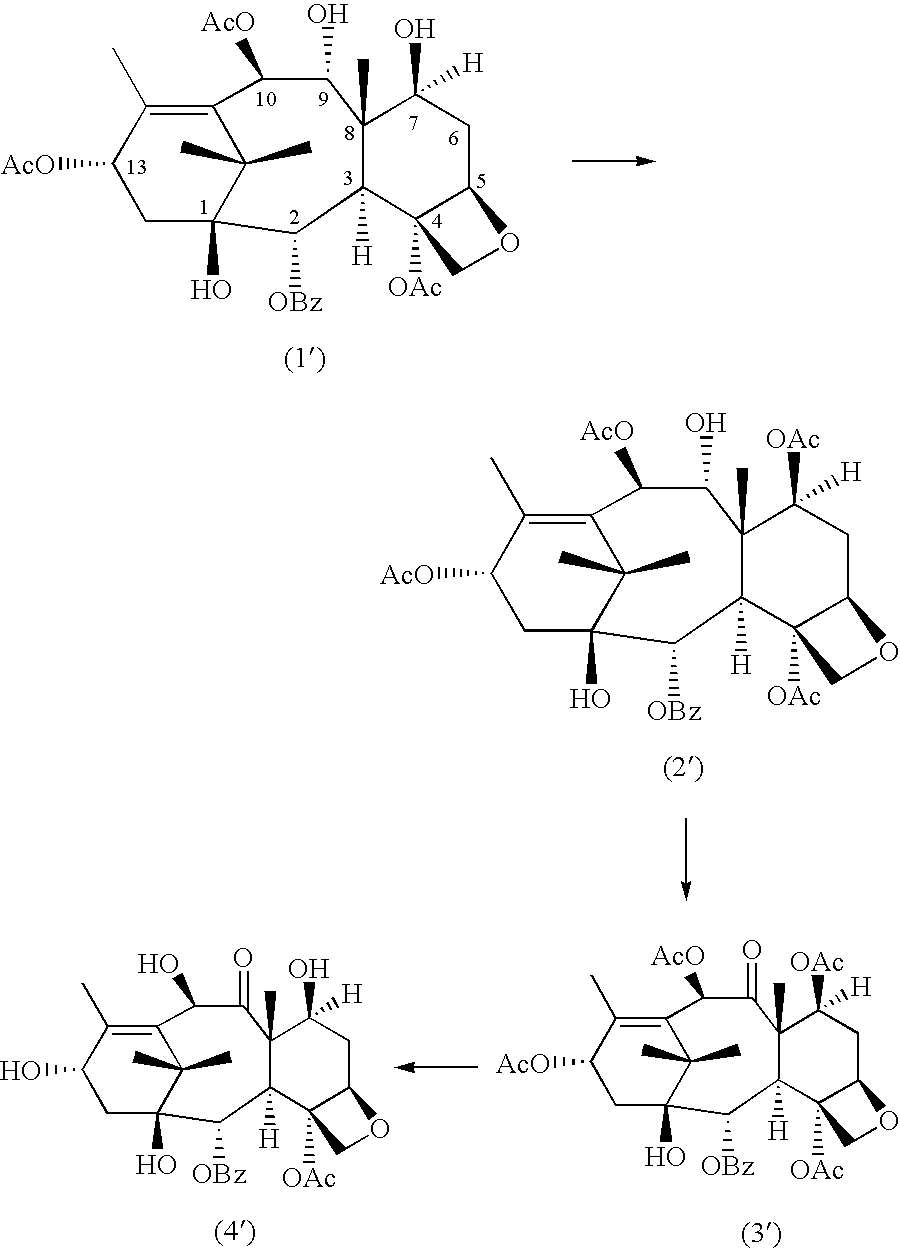
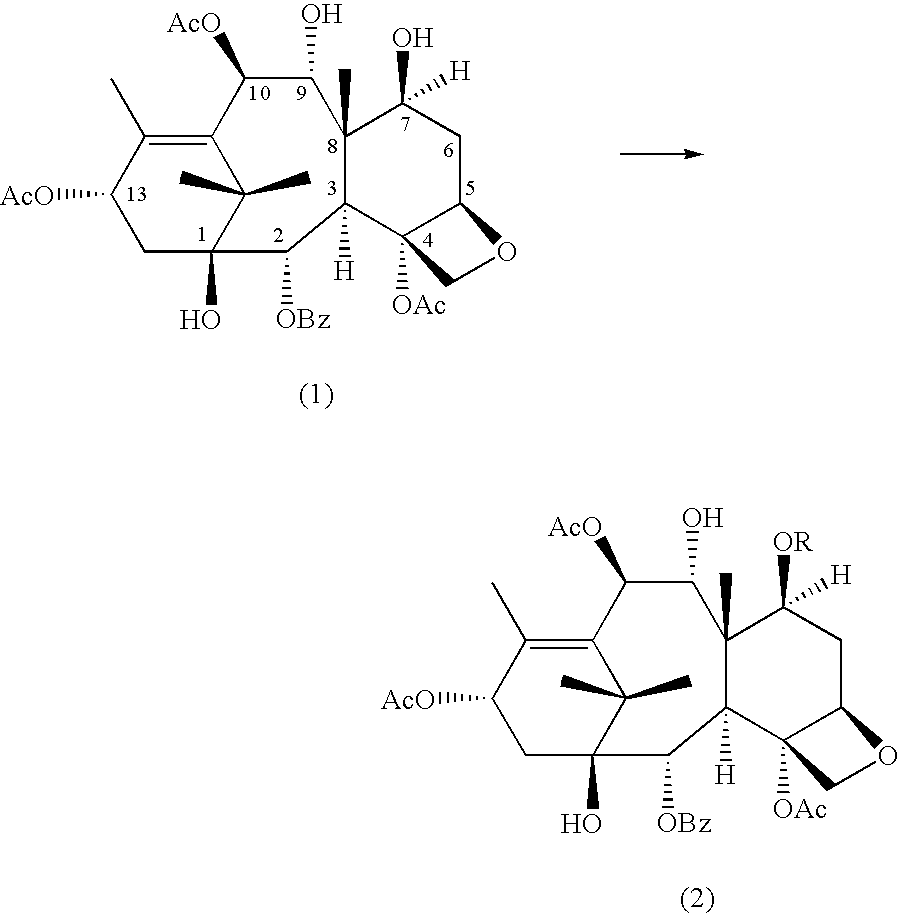
A process for the preparation of taxanes comprising wherein R is a tert. butoxycarbonyl or benzoyl group; PMP is p-methoxyphenyl group; G1 is acetyl group; G2 is haloacetyl group comprisinga) protecting the C-7 hydroxyl group of 10-deacetylbaccatin III with haloacetyl chlorides and then acetylating the C-10 hydroxyl group with acetyl chloride to obtain a protected 10-deacetylbaccatin III (1);b) subjecting the protected 10-deacetylbaccatin III (1) to coupling with an oxazolidine-5-caboxylic acid of formula 2 wherein R is tert. butoxycarbonyl or benzoyl; PMP is p-methoxyphenyl group in the presence of a condensation agent and an activating agent to obtain C-13 esters of formula 3;c) treating the coupled products 3 with weak acidic medium to open the oxazolidine ring to obtain intermediates of formula 4; wherein R is a tert. butoxycarbonyl or benzoyl group; G1 is acetyl group; G2 is haloacetyl groupd) subjecting the intermediates of compound 4 to selective deprotection of haloacyl group in the presence of acetyl group under mild alkaline condition at −20 to +40° C. for 6-24 h in the presence of ammonia or aliphatic amine or aromatic amines or their combination to obtain paclitaxel or docetaxel.
Owner:DABUR PHARM LTD
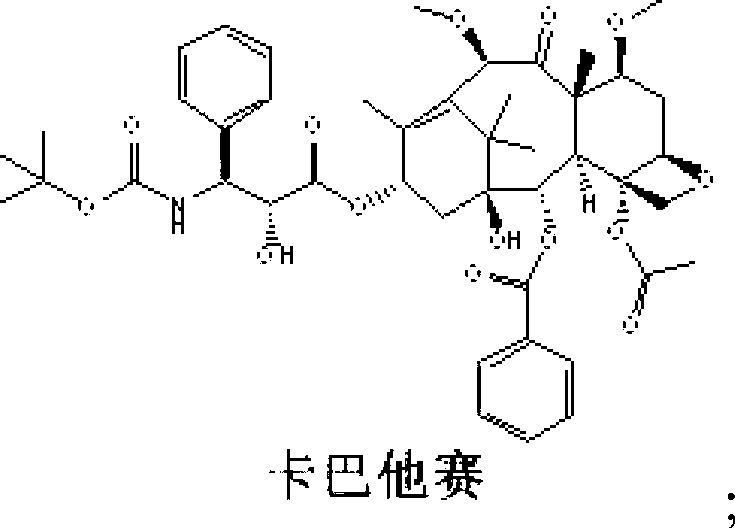
Synthetic method for cabazitaxel
InactiveCN102675256AHigh yieldHigh purityOrganic chemistryBulk chemical productionCompound aState of art
The invention discloses a synthetic method for cabazitaxel, and the synthetic method comprises the following steps: performing double methylation on the C7 site and C10 site of a compound A so as to obtain a compound B, and hydrolyzing the comound B under the acidity condition so as to obtain the cabazitaxel. The cabazitaxel has a reaction formula shown in the specification. Compared with the prior art, the synthetic method has the advantage that the compound A of which the C13-site hydroxyl is connected with a protecting group is used as a raw material, and a method of obtaining the cabazitaxel by virtue of double methylation of the C7-site and C10-site hydroxyl and hydrolysis, so that a step of generating by-products by the methylation of the C13-site hydroxyl of 10-Deacetylbaccatin III and a complex purification step are omitted. The synthetic method has the advantages of simple technology and moderate reaction condition, and can be used for improving the yield and purity of the cabazitaxel.
Owner:CHONGQING BEISHENG PHARMA TECH CO LTD
Method for extracting and purifying two kinds of taxane compound from yew branches and leaves
The invention relates to a method for using specific resin and silicon gel column chromatography purification via negative pressure cavitation water extraction to high-efficiency produce 10-deacetylbaccatin III and 7-xylose-10-deacetylpaclitaxel, which uses taxus branch leaves as raw material, dries and breaks to process negative pressure cavitation water extraction, filters and uses AB-8 resin to process dynamic absorption on filter liquor to concentrate 10-deacetylbaccatin III and 7-xylose-10-deacetylpaclitaxel and process further purification via middle pressure silicon gel column chromatography, to obtain the 10-deacetylbaccatin III of at least 60% purity and 7-xylose-10-deacetylpaclitaxel of at least 67% purity. The invention can use renewable taxus branch leaves to protect ecological resource and effectively extract effective component and biological semi synthesis precursor materials, with simple operation, support for industrial application and important industrial production value.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Method for extracting four-taxone compounds with high-efficiency abduction
InactiveCN101220384AEfficient use ofHigh yieldOrganic chemistryFermentation10-DeacetylbaccatinOxygen
The invention relates to a method for utilizing enzyme, ultraviolet radiation and oxygen as inductors for the high efficient production of 10-deacetylbaccatin III, baccatin III, cephalomannine and paclitaxel in taxus chinensis branches and leaves by the method of fresh grinding homogenization. The invention takes the fresh branches and leaves of taxus chinensis as raw materials for fresh grinding homogenization, then the enzyme is added, the oxygen is introduced, and a solid is treated with 80 percent ethanol ultrasonic extraction under ultraviolet irradiation. The contents of the 10-deacetylbaccatin III, the baccatin III, the cephalomannine and the paclitaxel in the branches and leaves of the taxus chinensis can be averagely improved by more than 17 percent, 23 percent, 74 percent and 23 percent respectively by the biological induction method. The raw materials used by the invention are the renewable fresh branches and leaves of the taxus chinensis, which can not only ensure no damage to ecological resources, but also can extract the effective components of taxus chinensis and the biological semisynthetic precursor substances with high efficiency. Furthermore, the method has simple and easy operation, which is applicable to industrial application and has great significance for industrial production.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
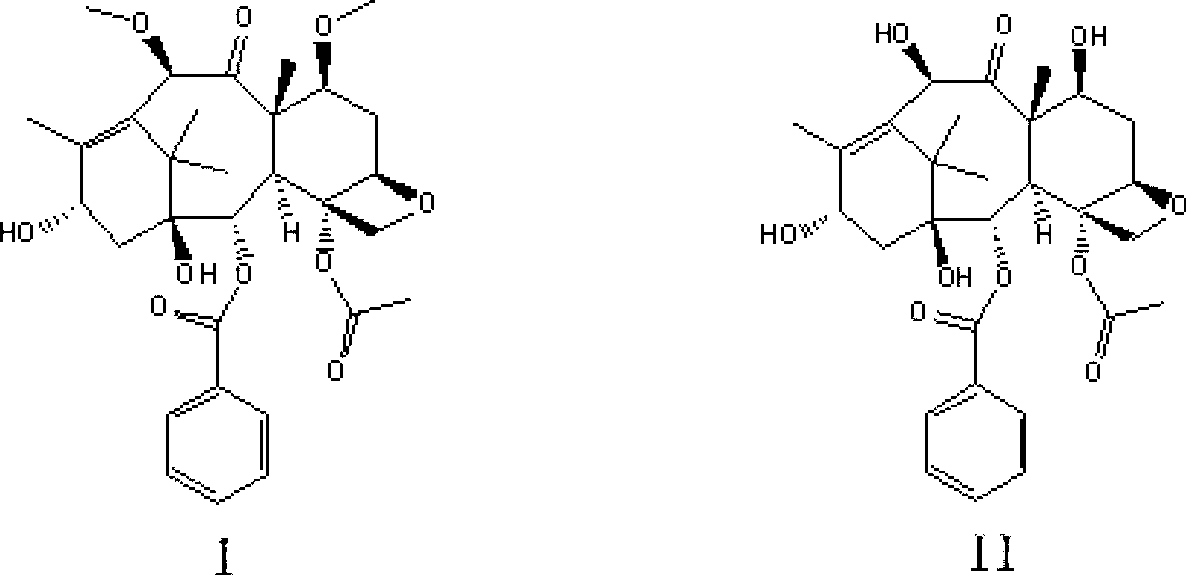
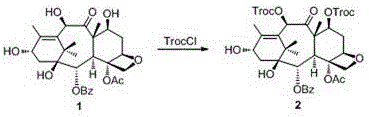
Preparation method of cabazitaxel and intermediate thereof
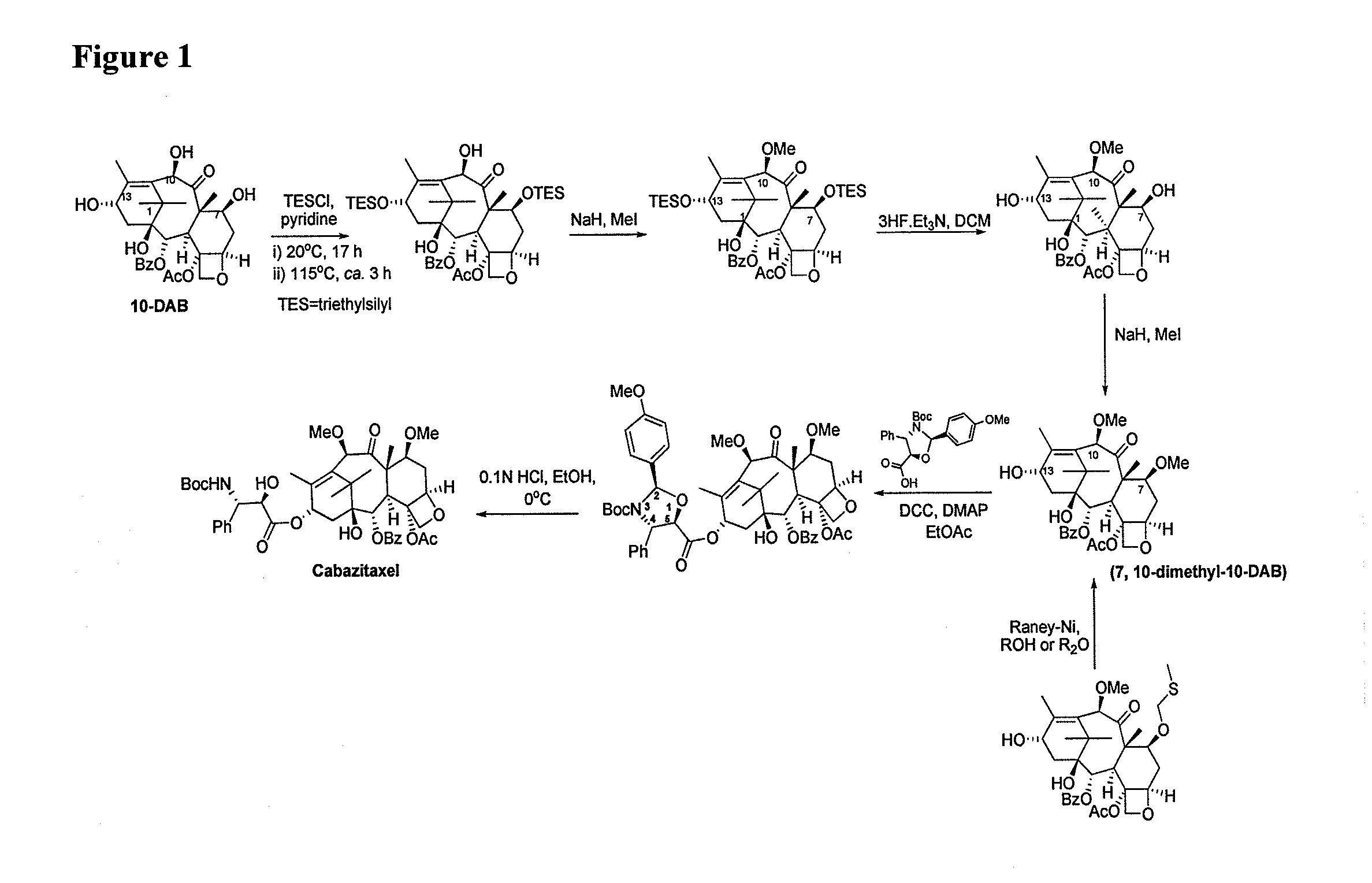
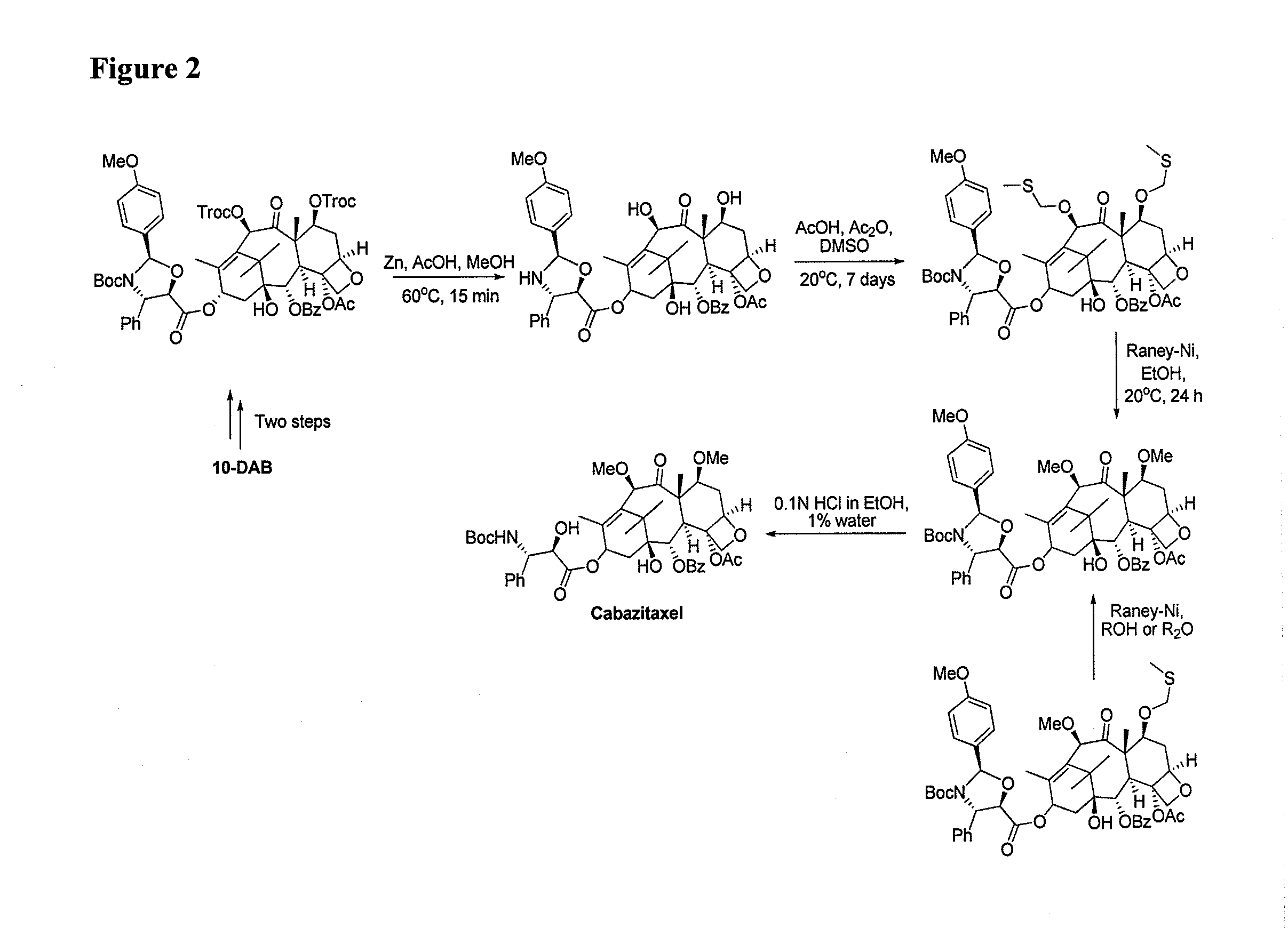
The invention discloses a preparation method of a cabazitaxel intermediate 7,10-dimethoxy-10-baccatin III. In the presence of alkali, 10-deacetylbaccatin III and a specific methylation reagent are subjected to selective methylation reaction at low temperature to obtain the cabazitaxel intermediate 7,10-dimethoxy-10-baccatin III. In the preparation method, the methylation reaction has high selectivity for C-7 and C-10 hydroxy sites on the 10-deacetylbaccatin III, so the yield is high. The invention also discloses a preparation method of cabazitaxel, which comprises the following steps: preparing the cabazitaxel intermediate according to the preparation method above; condensing the cabazitaxel intermediate with one side chain of docetaxel; and hydrolyzing the obtained condensation product under acidic conditions to obtain the cabazitaxel. The preparation method of cabazitaxel has the advantage of high total yield and is suitable for commercialized production.
Owner:BEIJING COLLAB PHARMA
10-deacetylbaccatin iii and method for methoxylation of its derivative
The invention relates to 10-deacetylbaccatin III and a method for methoxylation of its derivative, which belongs to the medicine synthesis technical field. The invention is characterized in that the under the condition that 10-DAB, the 7 position and the 10 position of its derivative are hydroxy or one of them is hydroxyl, a phase transfer catalyst quaternary ammonium salt N<+>R4X<-> is added under the existence of a methylating agent, and dissolved according to a weight ratio of 1:10-100 of solute to organic solvent, and reacted with low temperature, a lower layer water phase is separated after finishing the reaction, an organic phase is washed by a saturated salt solution, and the organic phase is concentrated by concentrated acid, a petroleum ether is added for completely deposing, filtered, deposed and dried to obtain the product. The invention provides a simple and easy method for preparing the 10-DAB and a methoxy compound of the 7 position and the 10 position hydroxy of its derivative. The method for large scale intermediate preparation enables possibility of preparation of docetaxel with large dosage, and is a key step for preparing cabazitaxel.
Owner:无锡紫杉药业股份有限公司
10- deacetylate-9(R)-hydrogenization-1-deoxypaclitaxel analogue and preparation thereof
InactiveCN101353333ABiologically activeAddress resource shortagesOrganic active ingredientsOrganic chemistrySide effect10-Deacetylbaccatin
The invention relates to a 10-deacetylbaccatin-9(R)-hydrogenation-1-deoxidized paclitaxel analog and a preparation method thereof. The structural formula of the analog is as the right; in the method of the invention, 1-deoxidized baccatin VI is taken as a material for synthesizing the 1-deoxidized paclitaxel analog. The method of the invention maintains the ring skeleton and the necessary functional group of taxanes and selects the taxanes as a semi-synthetic precursor or carries out structure decoration on the taxanes; the obtained output 10-deacetylbaccatin-9(R)-hydrogenation-1-deoxidized paclitaxel analog has the biological activity of antitumor of the natural paclitaxel and has a certain application prospect in the aspects of reducing the multidrug resistance and the side effects of the natural paclitaxel. The method has the advantages of easily obtained materials, simple and convenient operation, good selectivity and high yield.
Owner:SHANGHAI UNIV
Method for extracting 10-deacetylbaccatin III from taxus chinensis
ActiveCN107880001AGuaranteed degradabilityGuaranteed damageOrganic chemistry10-DeacetylbaccatinAqueous solution
The invention discloses a method for extracting 10-deacetylbaccatin III from taxus chinensis. The method comprises the following steps: soaking and extracting 10-deacetylbaccatin III in taxus chinensis by adopting an aqueous solution containing a stability maintainer; and extracting, performing discoloring and flocculating treatment, recrystallizing and the like, thereby obtaining the high-purity10-deacetylbaccatin III. The method disclosed by the invention is clean, low in cost, non-harsh in extraction conditions, less in by-products, less in pigments and high in extraction rate, and is applicable to industrialized production and market popularization and application.
Owner:上海卓鼎生物技术有限公司
Semi-Synthesis and Isolation of Taxane Intermediates from a Mixture of Taxanes
A process is provided for the semi-synthesis and isolation of taxane intermediates useful in the preparation of paclitaxel and docetaxel, in particular, the semi-synthesis and isolation of 10-deacetylbaccatin III, the semi-synthesis of a mixture of 10-deacetylbaccatin III and baccatin III, and protected derivatives thereof, from a mixture of taxanes.
Owner:CHATHAM BIOTEC LTD
Preparation method of docetaxel
ActiveCN102382080ASimple processHigh yieldOrganic chemistryBulk chemical productionNatural productDocetaxel
The invention provides a preparation method of docetaxel. The method comprises the following steps: 1) dissolving 10-deacetylbaccatin III in an solvent, and reacting with a protective agent to selectively protect the 7-hydroxyl group and 10-hydroxyl group in the 10-deacetylbaccatin III; 2) preparing a side chain intermediate; 3) performing an esterification reaction on the products obtained in the step 1) and the step 2); 4) removing the protective groups on the side chain of the esterification product under the acidic condition; 5) protecting amino on the side chain with the protective group under the alkaline condition; and 6) removing the protective groups on the hydroxyl groups to produce docetaxel. The invention provides the new preparation method of docetaxel, wherein the new related side chains are synthesized to prepare docetaxel; and the method has significances to utilize various taxanes which are extracted and separated from natural products and have similar structures with the target compound, optimize the technological process, increase the yield, reduce the cost, reduce the product price, comprehensively utilize resources and the like.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP +1
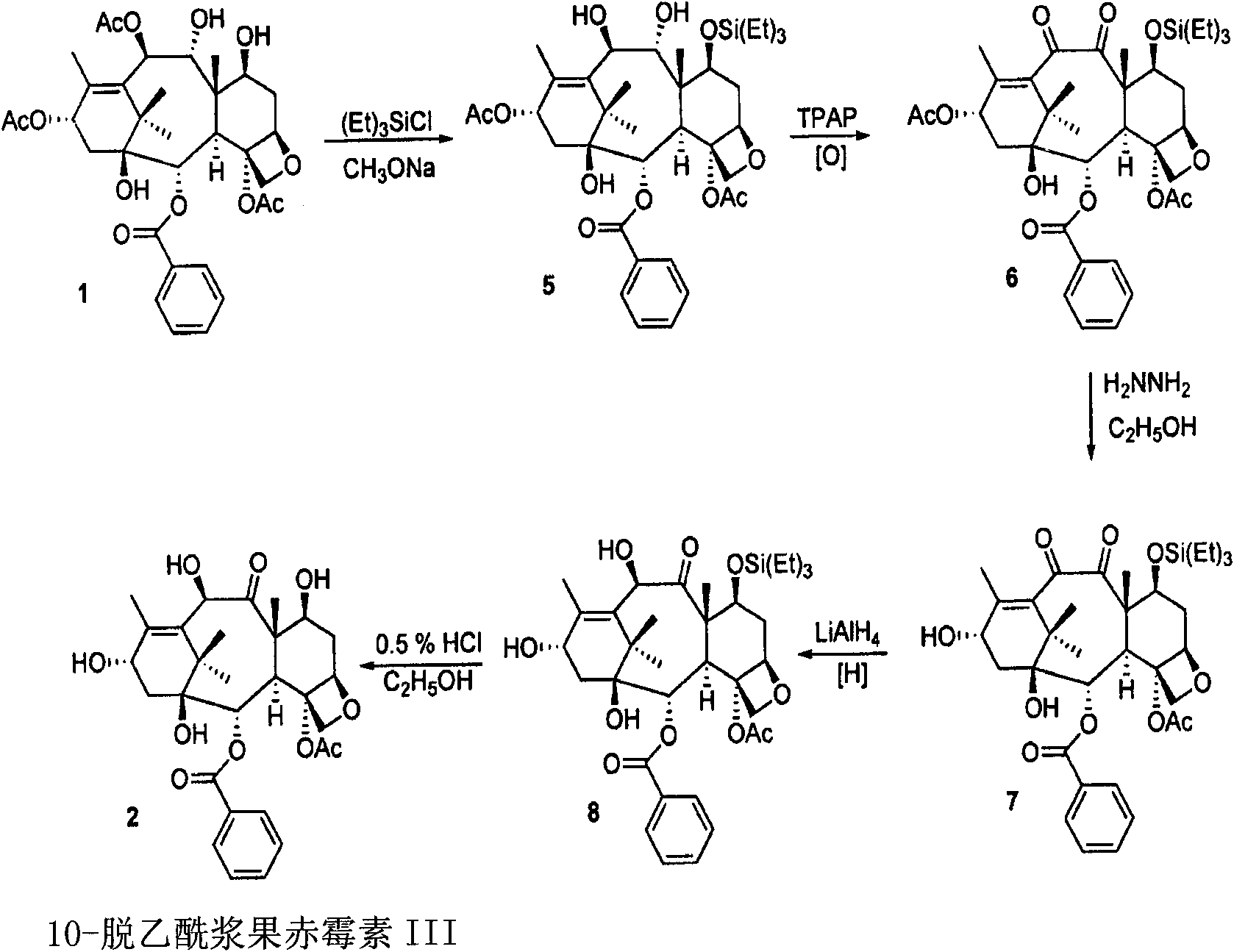
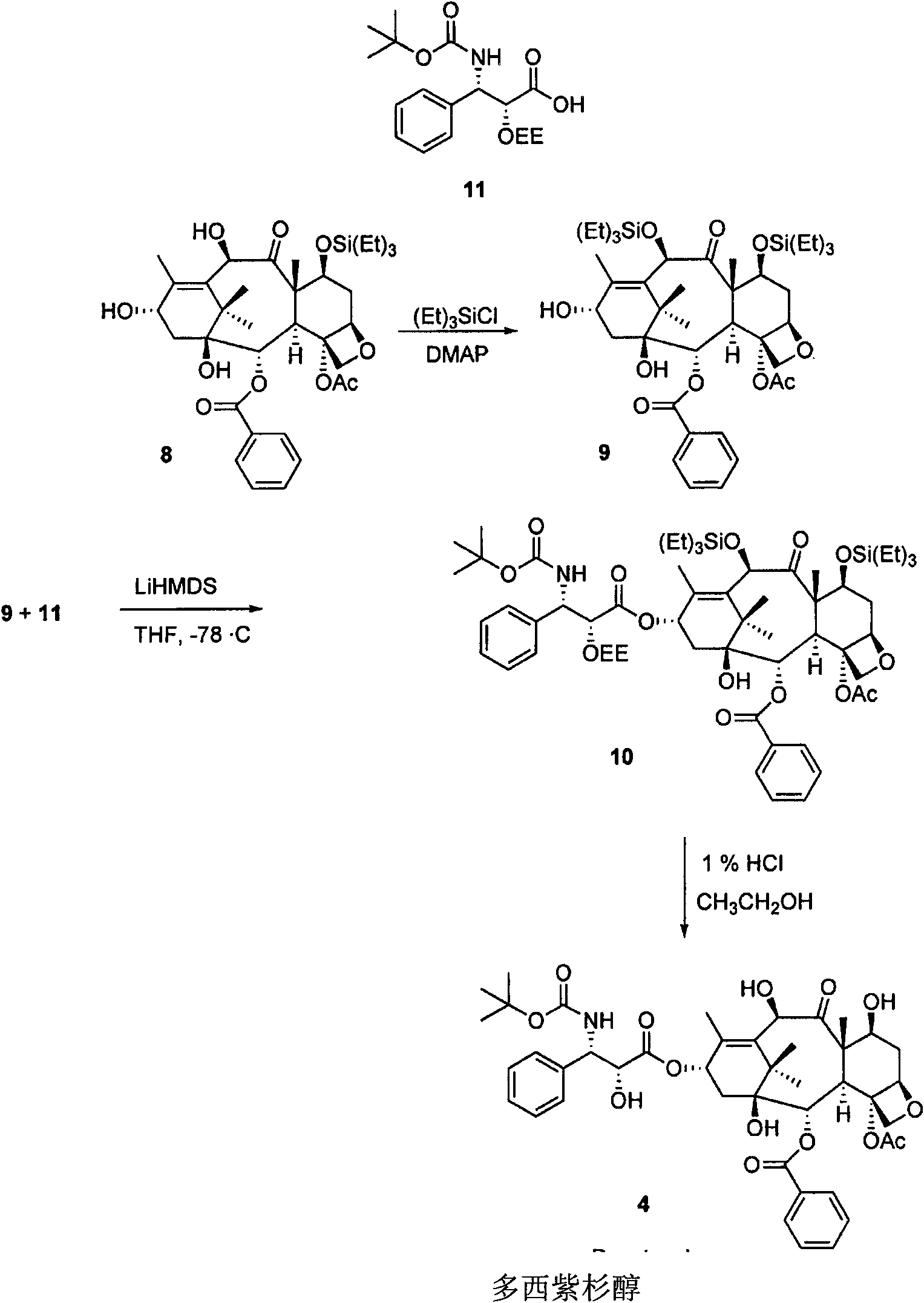
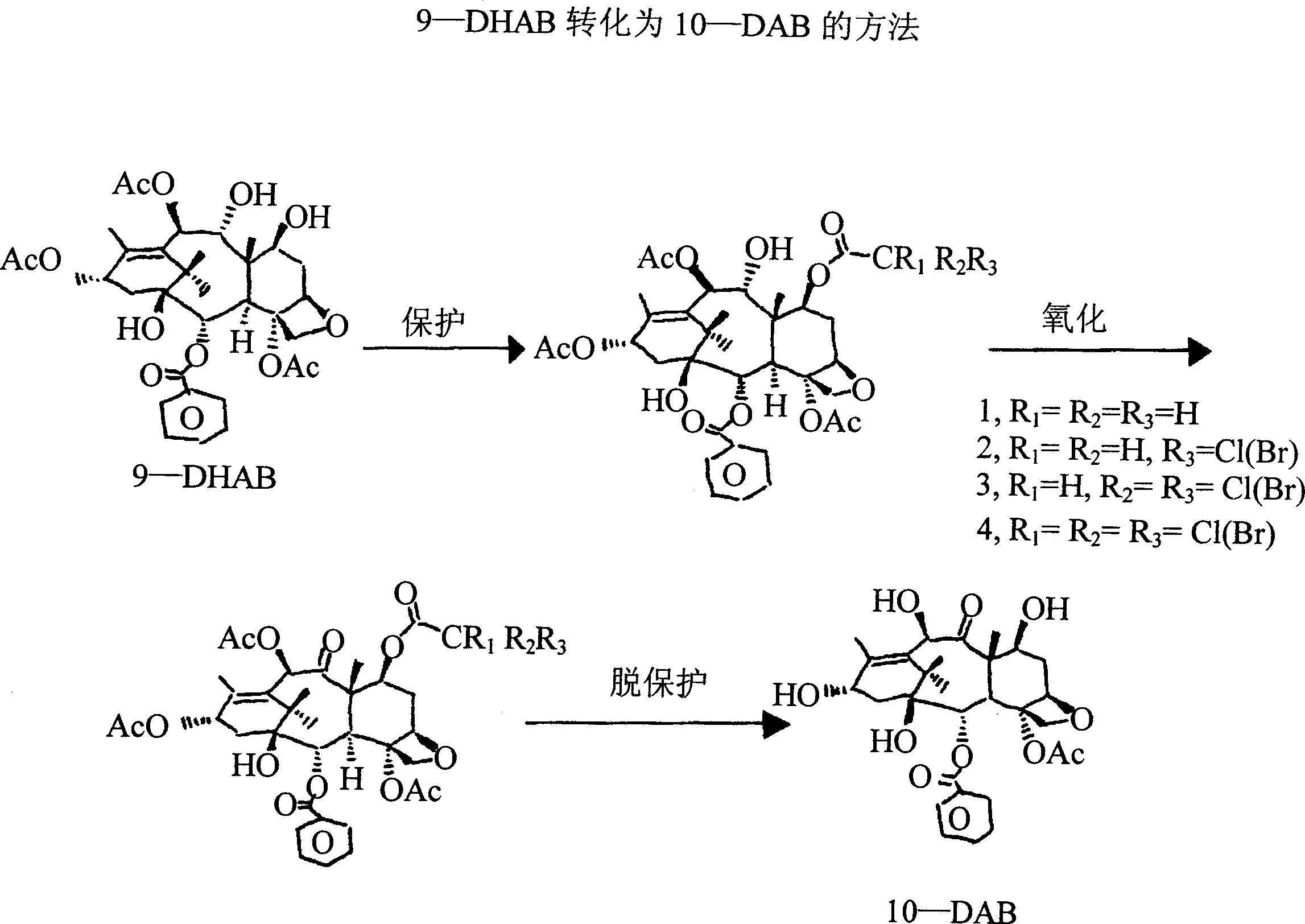
Semi-synthetic route for the preparation of paclitaxel, docetaxel and 10-deacetylbaccatin iii from 9-dihydro-13-acetylbaccatin III
The present invention provides a novel semi-synthetic route in the preparation of docetaxel and paclitaxel. This new process involves the conversion of 9-dihydro-13-acetylbaccatinIII to docetaxel and paclitaxel by the step of converting 9-dihydro-13-acetylbaccatin III into 7-O-triethylsilyl-9,10-diketobaccatin III, and adding docetaxel and paclitaxel side chain precursors, respectively, to form a new class of taxane intermediates, such as 7-O-triethylsilyl-9,10-diketodocetaxel and 7-O-triethylsilyl-9,10-diketopaclitaxeltaxel. These new intermediates then by a series reduction, acetylation of the 10-hydroxyl position for paclitaxel and finally deprotection to yield docetaxel and paclitaxel, the most important anti-cancer drugs.
Owner:6570763 CANADA
Semi-synthesis and isolation of taxane intermediates from a mixture of taxanes
A process is provided for the semi-synthesis and isolation of taxane intermediates useful in the preparation of paclitaxel and docetaxel, in particular, the semi-synthesis and isolation of 10-deacetylbaccatin III, and protected derivatives thereof, from a mixture of taxanes.
Owner:CONOR MEDSYST
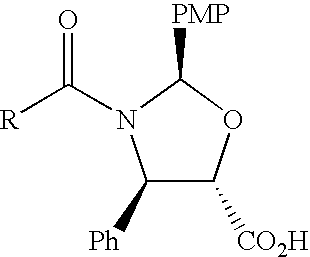
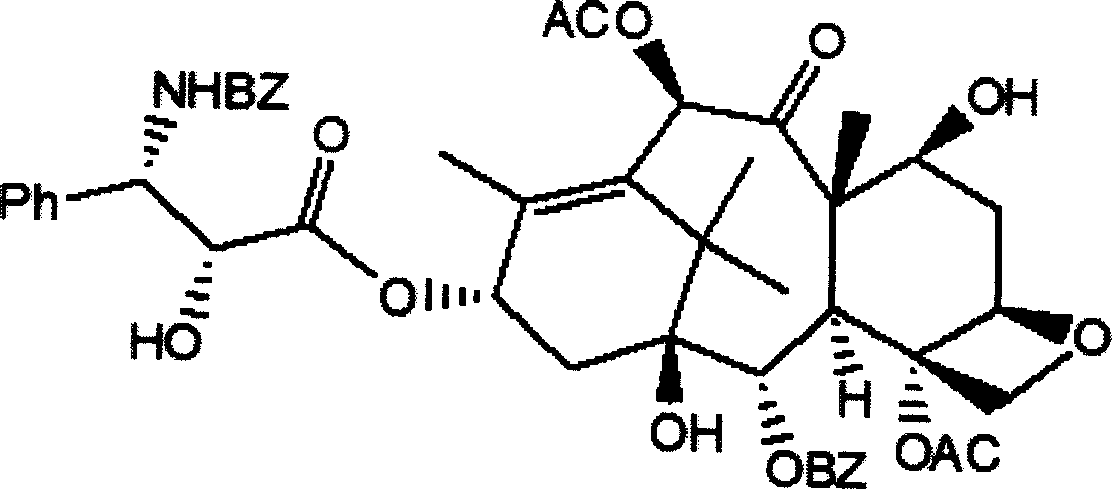
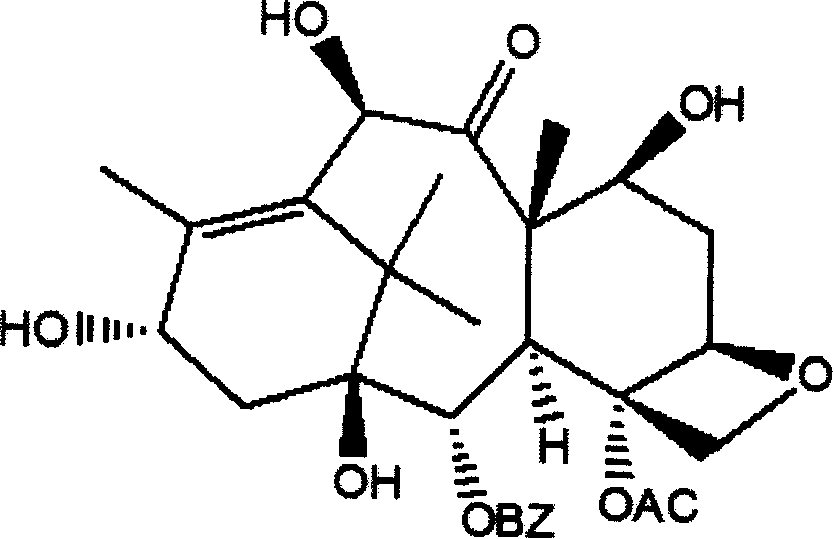
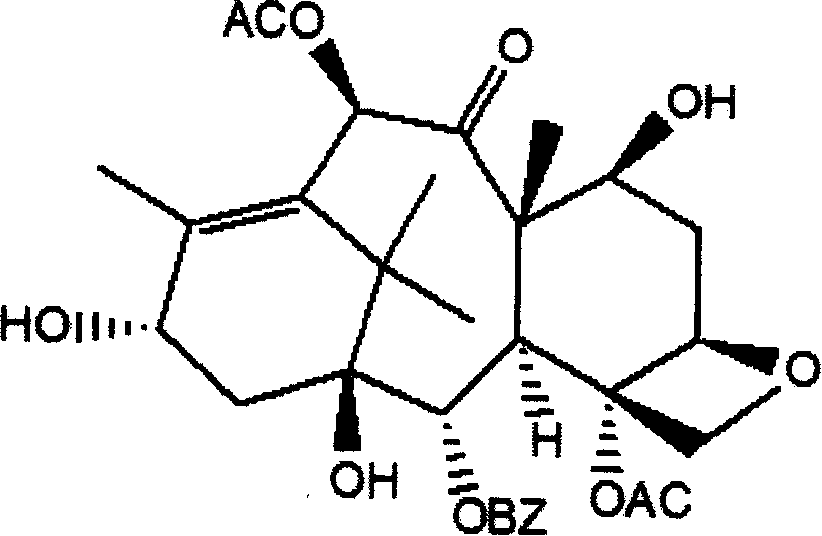
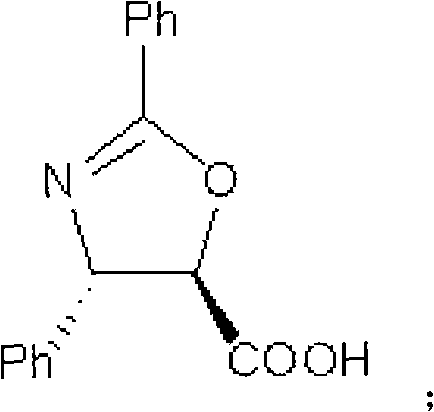
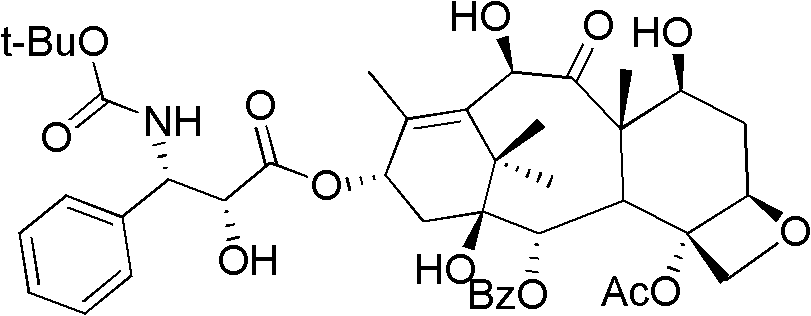
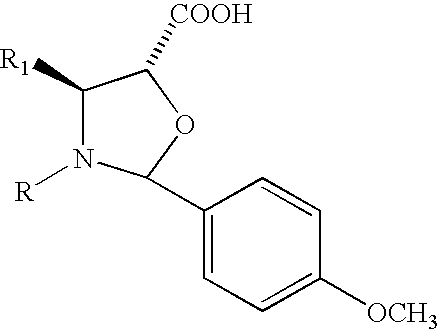
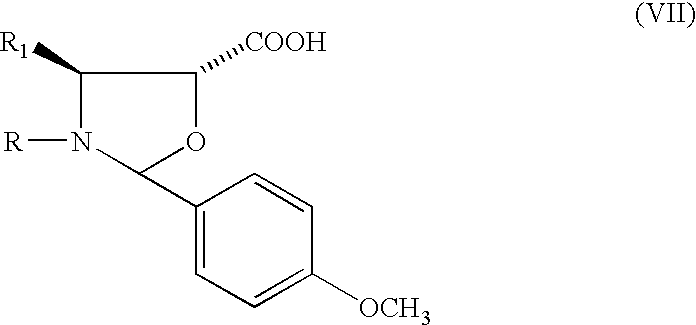
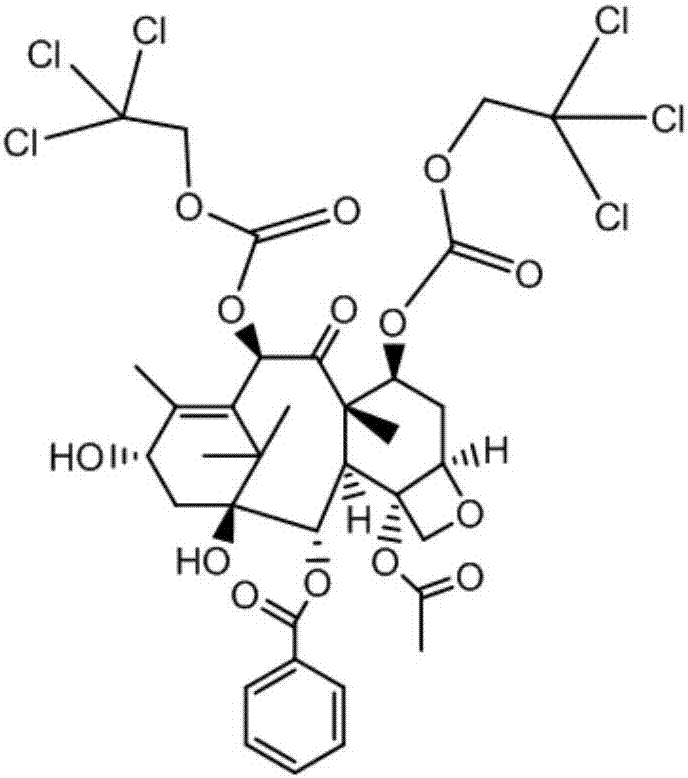
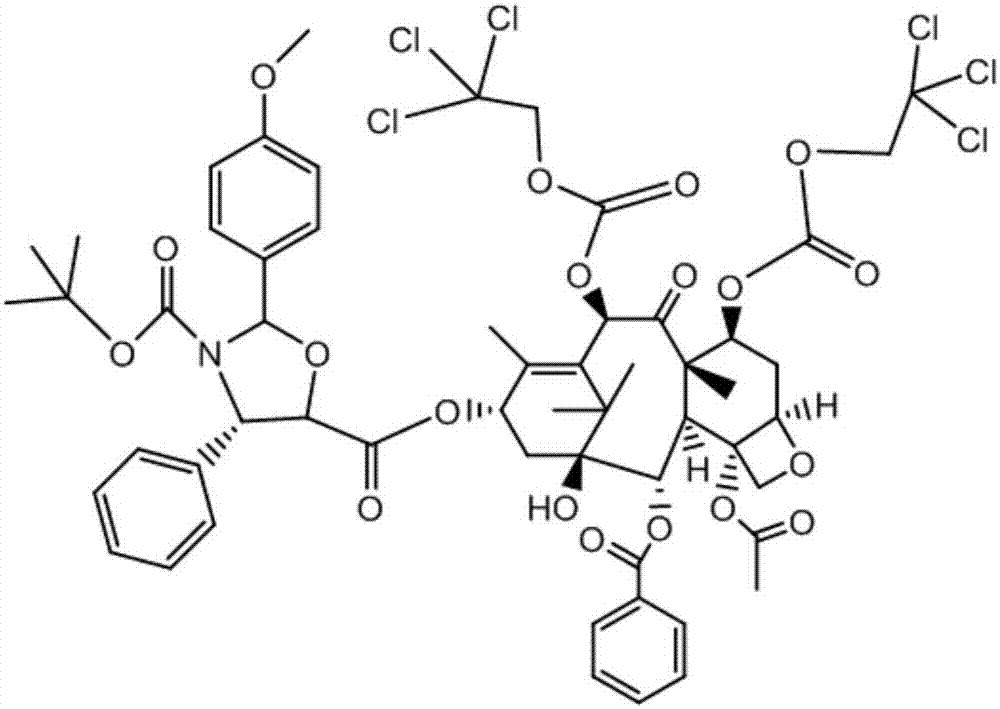
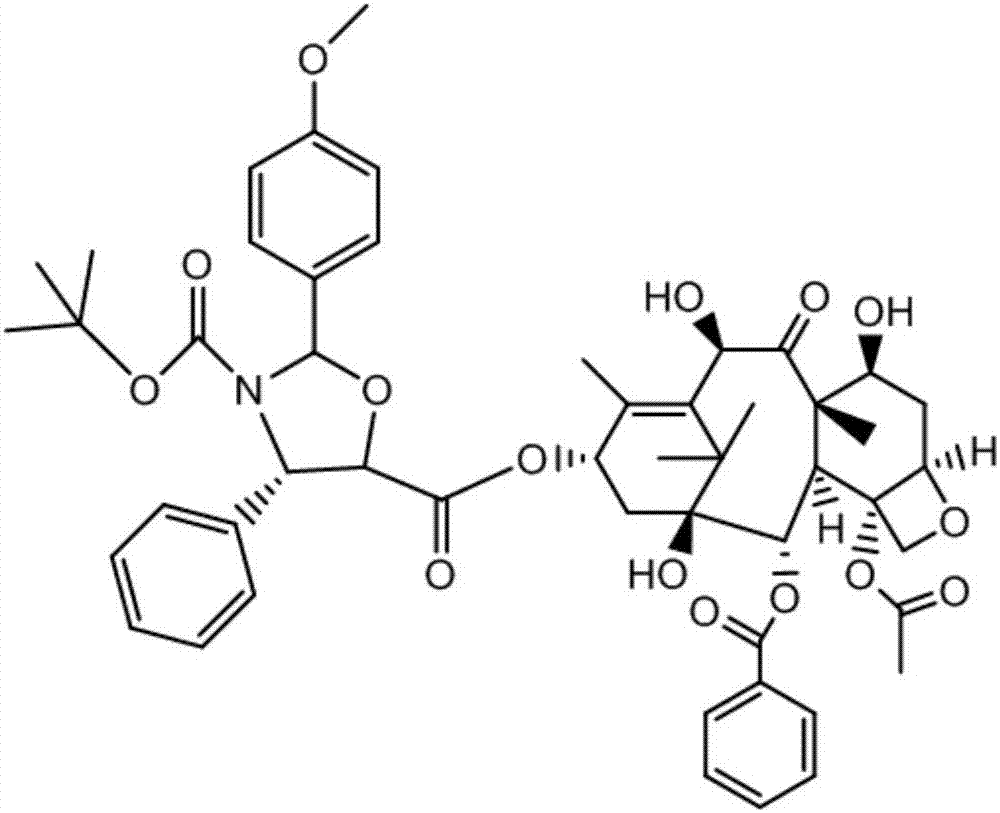
Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl) ethoxycarbonyl]-5-oxazolidine carboxylic acids
InactiveUS7247738B2High purityMinimize degradationOrganic chemistryAntineoplastic agentsDocetaxel-PNPOxazolidine E
This invention relates to a process for preparation of taxanes comprisingsubjecting 7,10-diprotected intermediates 7-O-(2-haloacyl)baccatin III 6c or 7,10-O-di-(2-haloacyl)-10-deacetylbaccatin III 6b to a step of coupling with (4S,5R)-3-[(2-alkyl / aryl-2-trialkylsilyl)ethoxy-carbonyl]-4-aryl-2-substituted-1,3-oxazolidine-5-carboxylic acid 1 in the presence of a condensation agent, an activating agent and an aromatic hydrocarbon to obtain 7-O-[2-(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3(2-unsubstituted / substituted-2-trialkylsilyl)-ethoxycarbonyl-1,3-oxazolidinyl-5-carbonyl]baccatin III 7a or 7,10-di-O[2-(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3-(2-unsubstituted / substituted-2-trialkylsilyl)ethoxy-carbonyl-1,3-oxazolidinyl-5-carbonyl]-10-deacetylbaccatin III 7b;treating the coupled products 7-O-[2-(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3-(2-substituted-2-trialkylsilyl)ethoxy-carbonyl-1,3-oxazolidinyl-5-carbonyl]baccatin III 7a or 7,10-di-O-[2[(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3-(2-substituted-2-trialkylsilyl)ethoxycarbonyl-1,3-oxazolidinyl-5-carbonyl]-10-deacetylbaccatin III 7b with tetraalkylammonium halide in a haloalkane to obtain free amine of structure 8;treating free amine 8 with acid chloride or acid anhydride in the presence of a base in a heterogeneous phase to obtain the intermediates of structure 9;subjecting the intermediates of compound 9 to the deprotection of 2-haloacyl group under mild alkaline condition at −20 to +40° C. for 6–24 h in the presence of ammonia or aliphatic amines or aromatic amines or their combination to obtain paclitaxel or docetaxel.
Owner:DABUR PHARM LTD
Semi-synthesis of taxane intermediates from a mixture of taxanes
A process is provided for the semi-synthesis of taxane intermediates useful in the preparation of paclitaxel and docetaxel, in particular, the semi-synthesis of 10-deacetylbaccatin III and baccatin III, and derivatives thereof, from a mixture of taxanes.
Owner:INNOVATIONAL HLDG LLC
Conversion 9-dihydro-13-acetylbaccatin III into 10-deacetylbaccatin III
Owner:UNIVERSITY OF NEW BRUNSWICK
Conversion 9-dihydro-13-acetylbaccatin iii to 10-deacetylbaccatin iii
The present invention relates to a process is provided for the conversion of 9-dihydro-13-acetylbaccatin to 10-deacetylbaccatin III. The process includes four specific interrelated steps. The first step involves protecting the 7-hydroxyl group of 9-dihydro-13-acetylbaccatin and converting that 7-hydroxyl-protected 9-dihydro-13-acetylbaccatin to 7, 13-diacetyl-9-dihydrobaccatin III. The second step involves reacting that 7, 13-diacetyl-9-dihydrobaccatin III with 4-methylmorpholine N-oxide in a suitable solvent and oxidizing that reaction product to yield 7, 13-diacetylbaccatin. The third step involves deacetylating that 7, 13-diacetyl-9-dihydrobaccatin III to yield 7-acetylbaccatin III. The fourth and final step involves converting that 7-acetylbaccatin III to 10-deacetylbaccatin III.
Owner:6570763 CANADA
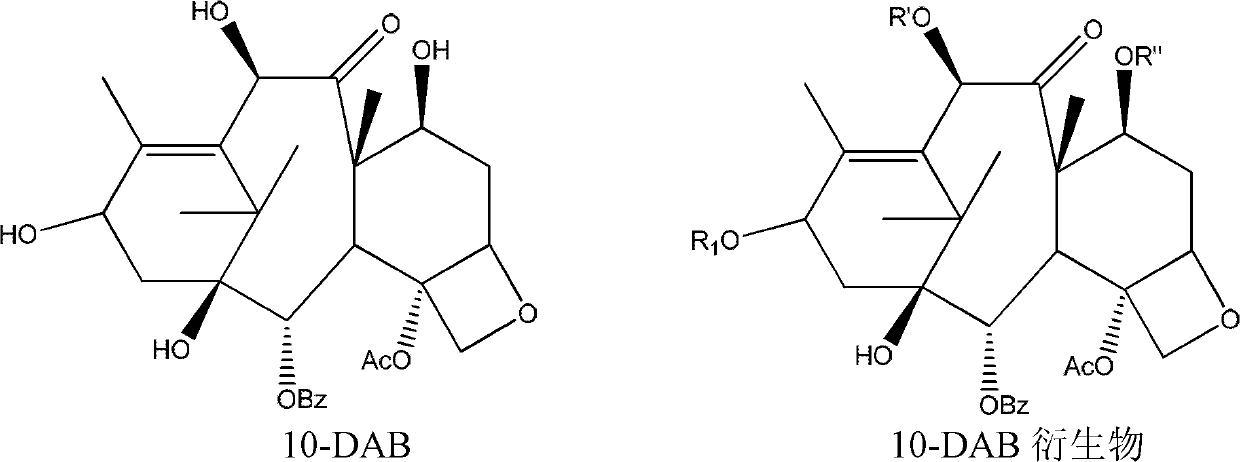
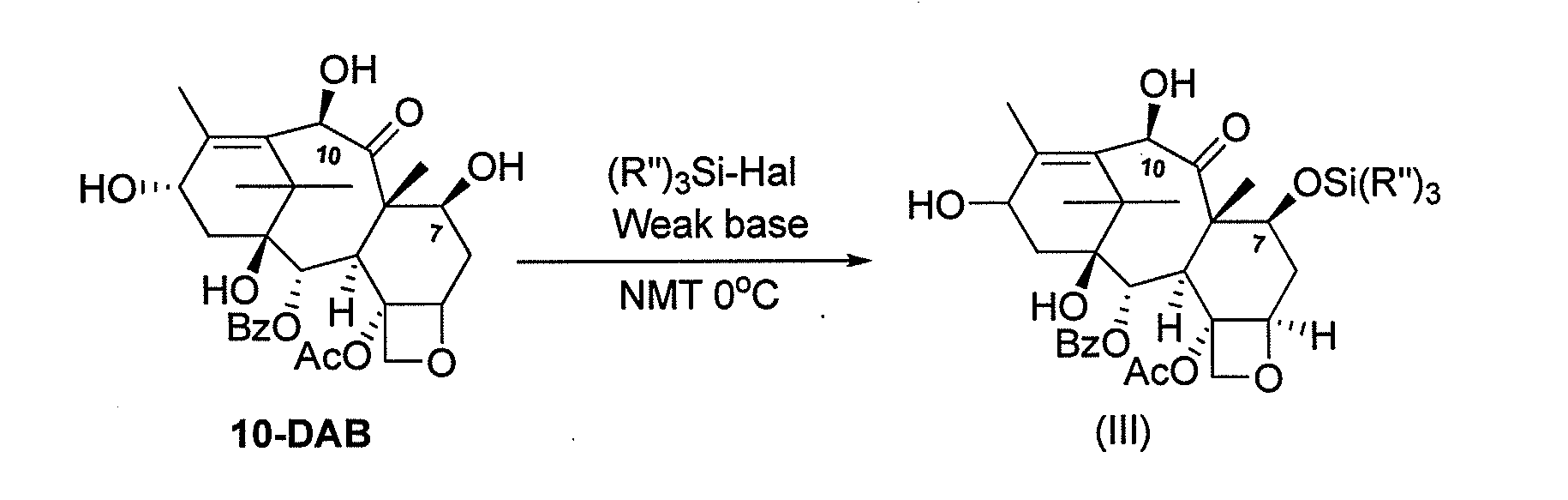
Process for making an intermediate of cabazitaxel
InactiveUS20130090484A1Less efficientOrganic chemistryAntineoplastic agentsCabazitaxel10-Deacetylbaccatin
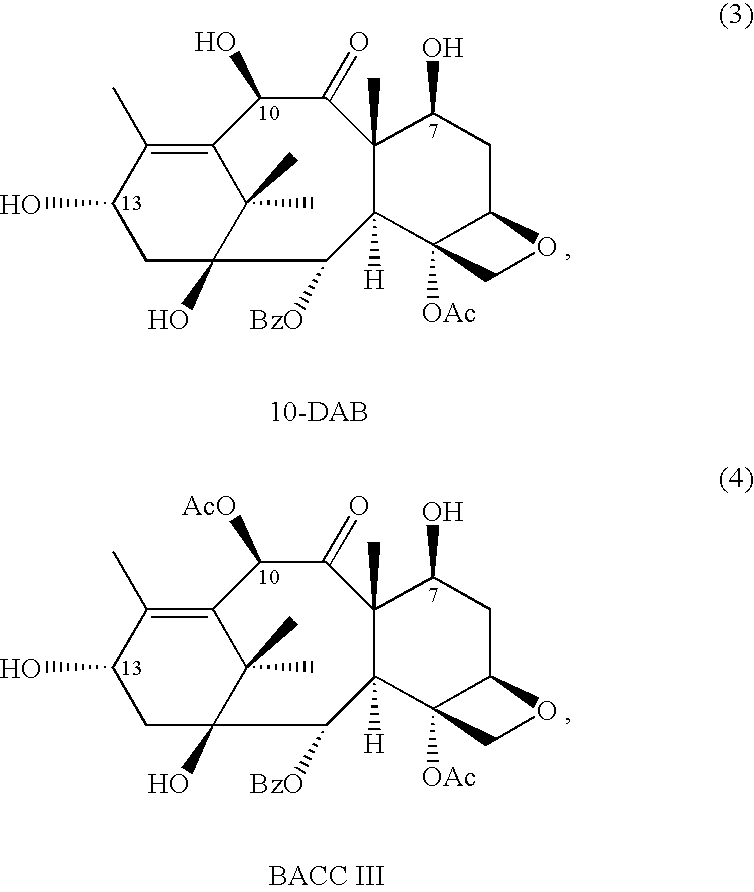
A novel process of making 7,10-dialkyl-10-DAB of formula (I)which is useful as a key intermediate for the preparation of cabazitaxel, comprises selective elaboration of positions 7 and 10 of 10-deacetylbaccatin III.
Owner:SCINOPHARM TAIWAN LTD +1
Method for separation and purification of 10-deacetylbaccatin III from branches and leaves of taxus chinensis
InactiveCN104892551AAchieve the purpose of separationEasy to separate and extractOrganic chemistryReflux extractionAlcohol
The invention discloses a method for separation and purification of 10-deacetylbaccatin III from branches and leaves of taxus chinensis. The method includes the steps: 1) crushing and drying the branches and the leaves of taxus chinensis, adopting 80% ethyl alcohol for reflux extraction for 5 hours, continuously extracting for 3 times, and subjecting filtrate to vacuum concentration to obtain 10-DAB (deacetylbaccatin) III extracts; 2) dissolving the 10-DAB III extracts, performing dry method sampling to collect eluent containing 10-DAB III, and evaporating to remove solvents so as to obtain a semi-finished 10-DAB III product; 3) dissolving the semi-finished 10-DAB III product, adding imidazole compounds according to a mole ratio of (1:1.5)-4, stirring for 30min, standing, cooling at the temperature of 0 DEG C, filtering and drying to obtain a crystal complex of 10-DAB III and imidazole; 4) dissolving the crystal complex of 10-DAB III and imidazole in water, and extracting, drying and removing solvents to obtain a pure 10-DAB III product with 10-DAB III content being more than 99%.
Owner:JINGGANGSHAN UNIVERSITY
Process for the preparation of taxanes from 10-deacetylbaccatin III
InactiveUSRE40120E1High yieldOrganic active ingredientsOrganic chemistry10-DeacetylbaccatinHydrolysis
A process for the preparation of taxane derivatives by reacting 10-deacetylbaccatin III protected at the 7-and 1-positions with trichloroacetyl groups with a compound of formula and subsequent removal of the protective groups and hydrolysis of the oxazolidine ring.
Owner:INDENA SPA
Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbon1]-5-oxazolidine carboxylic acids
InactiveUS20080064887A1High purityMinimize degradationOrganic chemistryAntineoplastic agentsOxazolidine E10-Deacetylbaccatin
This invention relates to a process for preparation of taxanes comprising subjecting 7,10-diprotected intermediates 7-O-(2-haloacyl)baccatin III 6c or 7,10-O-di-(2-haloacyl)-10-deacetylbaccatin III 6b to a step of coupling with (4S,5R)-3-[(2-alkyl / aryl-2-trialkylsilyl)ethoxy-carbonyl]-4-aryl-2-substituted-1,3-oxazolidine-5-carboxylic acid 1 in the presence of a condensation agent, an activating agent and an aromatic hydrocarbon to obtain 7-O-[2-(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3(2-unsubstituted / substituted-2-trialkylsilyl)-ethoxycarbonyl-1,3-oxazolidinyl-5-carbonyl]baccatin III 7a or 7,10-di-O [2-(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3-(2-unsubstituted / substituted-2-trialkylsilyl)ethoxy-carbonyl-1, 3-oxazolidinyl-5-carbonyl]-10-deacetylbaccatin III 7b; treating the coupled products 7-O-[2-(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3-(2-substituted-2-trialkylsilyl)ethoxy-carbonyl-1, 3-oxazolidinyl-5-carbonyl]baccatin III 7a or 7,10-di-O-[2[(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3-(2-substituted-2-trialkylsilyl)ethoxycarbonyl-1, 3-oxazolidinyl-5-carbonyl-10-deacetylbaccatin III 7b with tetraalkylammonium halide in a haloalkane to obtain free amine of structure 8; treating free amine 8 with acid chloride or acid anhydride in the presence of a base in a heterogeneous phase to obtain the intermediates of structure 9; subjecting the intermediates of compound 9 to the deprotection of 2-haloacyl group under mild alkaline condition at −20 to +40° C. for 6-24 h in the presence of ammonia or aliphatic amines or aromatic amines or their combination to obtain paclitaxel or docetaxel.
Owner:DABUR PHARM LTD
Method for improving yield of 10-deacetylbaccatin III produced by fungi
InactiveCN104975054AHigh yieldReduce pollutionOrganic chemistryMicroorganism based processes10-DeacetylbaccatinMicrobiology
Owner:HENAN INST OF SCI & TECH
Conversion 9-dihydro-13-acetylbaccatin iii into 10-deacetylbaccatin iii
InactiveUS20040063976A1Efficient methodOrganic chemistryBulk chemical production10-DeacetylbaccatinProtecting group
9-dihydro-13-acetylbaccatin III, one of the chemicals obtained from Taxus canadensis is used to produce, inter alia, 10-decetylbaccatin III, a useful intermediate for the preparation of paclitaxel and analogues thereof The 9-dihydro-13-acetylbaccatin III is converted into the 10-deacetylbaccatin III by a simple three step process involving (a) replacement of the C-7 hydroxyl group of the 9-dihydro compound with a protecting group, (b) the oxidizing of the C-7 protected compound to produce a C-9 keytone, and (c) the deprotecting of the C-9 keytone to produce 10-deacetylbaccatin III
Owner:UNIVERSITY OF NEW BRUNSWICK
Method for semisynthesis of Docetaxel and intermediate of Docetaxel
InactiveCN107141272AHigh molar yieldEasy to prepareOrganic chemistrySide chainHydrogenation reaction
The invention relates to a method for semisynthesis of Docetaxel and an intermediate of the Docetaxel. The method provided by the invention comprises the steps of firstly, carrying out hydroxyl protection on C7 and C10 of 10-DAB III (10-deacetylbaccatin III) by using 2,2,2-trichloroethylchloroformate so as to obtain an intermediate I, subjecting the intermediate I to a reaction with a side chain radical compound so as to prepare an intermediate II, subjecting the intermediate II to a hydrogenation reaction under the catalysis of palladium-charcoal so as to prepare an intermediate III, subjecting the intermediate III to a reaction under acidic conditions so as to obtain a Docetaxel crude product, and subjecting the Docetaxel crude product to purification, thereby obtaining a purified product. According to the method provided by the invention, the 10-DAB III raw material can be sufficiently utilized, finally-produced byproducts are few, the final product Docetaxel has the purity of 99.6% to 99.9%, the mole yield reaches up to 73% to 82%, and the utilization ratio of the 10-DAB III can be greatly increased.
Owner:CHONGQING BEISHENG PHARMA TECH CO LTD
Semi-synthetic method of antineoplastic drug paclitaxel
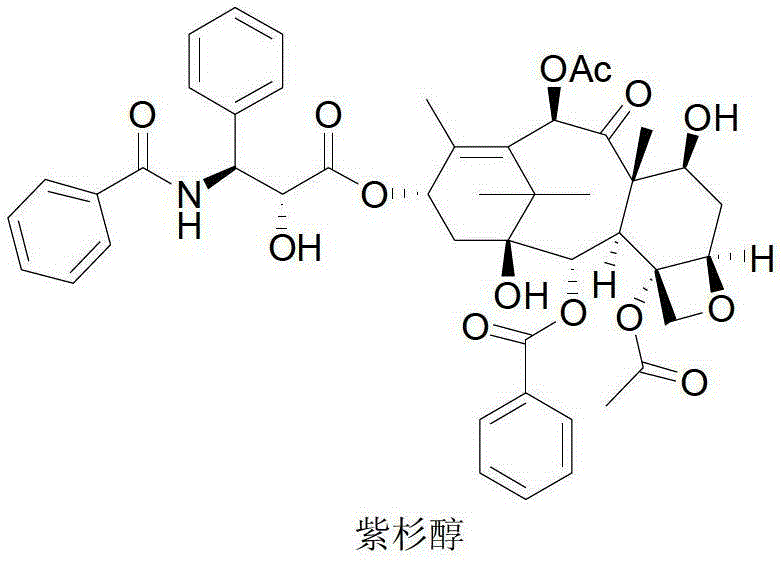
ActiveCN103130753BHigh purityHigh yieldOrganic chemistryBulk chemical productionSynthesis methods10-Deacetylbaccatin
The invention relates to a semi-synthesis method of antitumor drug taxol, and more particularly relates to a method for preparing taxol by the processes of taking 10-deacetylbaccatin III (10-DAB) as an initial material, selective acetylation, protection, condensation, open loop, and deprotection.
Owner:重庆赛诺生物药业股份有限公司
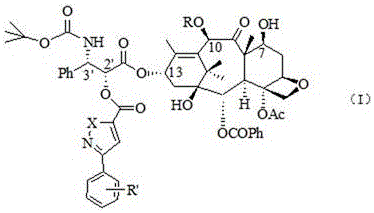
Docetaxel side chain 2'-derived novel taxanes antitumor compound as well as synthesis method and application thereof
The invention discloses a docetaxel side chain 2'-derived novel taxanes antitumor compound shown as the general structure formula (I) as well as a synthesis method and application thereof. In the formula, X is N or O, R is H or acetyl, and R' is H, nitryl, cyano, methoxyl or a halogen group. The synthesis method takes 10-deacetylbaccatin is used as a raw material; after 7-OH and 10-OH are protected, condensation with phenylisoserine (side chain) protecting 3'-NHBoc and 2'-OH in the presence of condensation agents DCC (Dicyclohexylcarbodiimide) and DMAP (Dimethylaminopyridine) is performed; esterification with substituted phenyl isoxazole carboxylic acid or substituted phenyl oxadiazole methyl carboxylic acid in the presence of the DCC and the DMAP is performed; finally, a protecting group is removed to obtain the compound. The compound disclosed by the invention has relatively high activity on tumor cells.
Owner:JINLIN MEDICAL COLLEGE +1
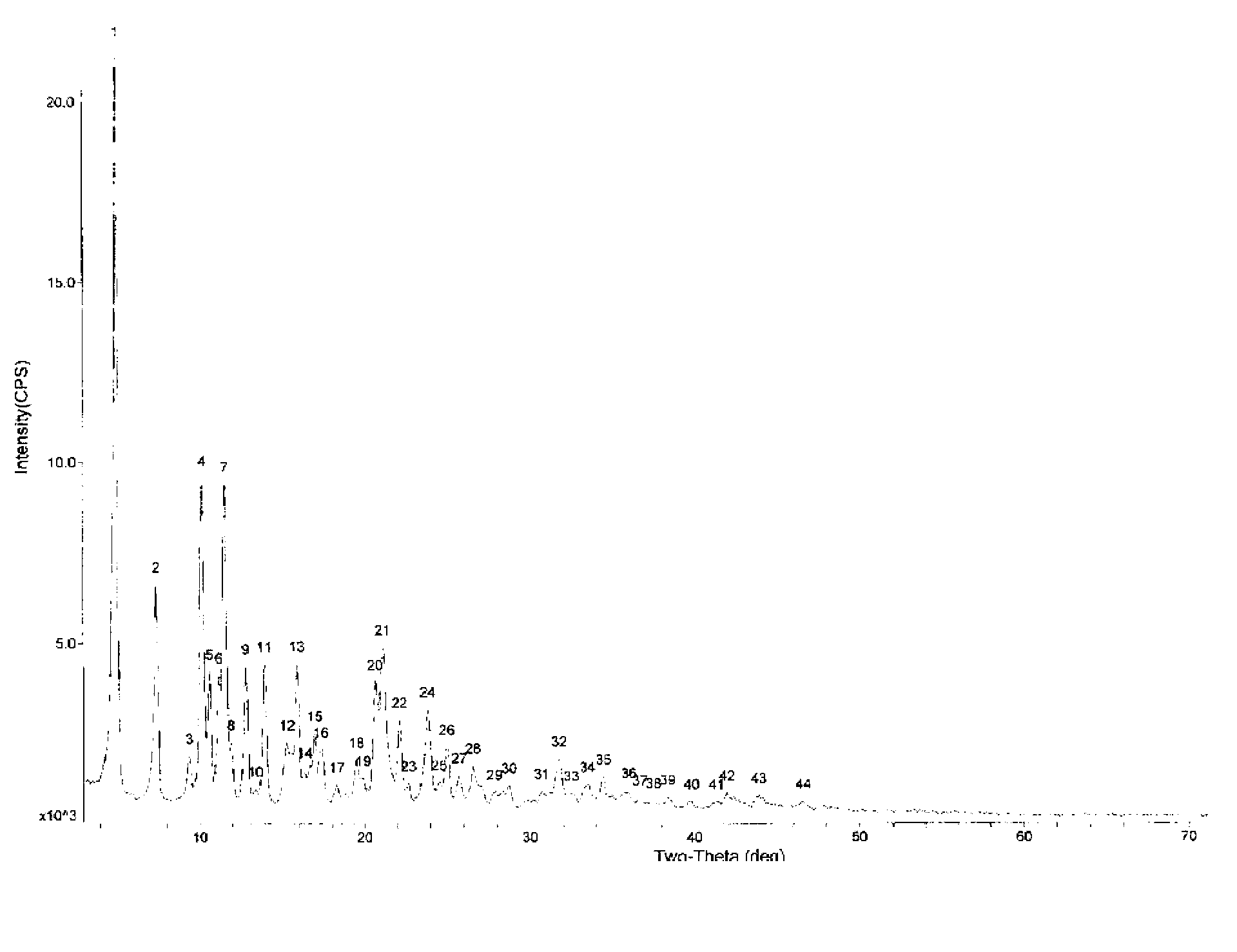
Crystallization purification method for 10-deacetylbaccatin III
A disclosed crystallization purification method for 10-deacetylbaccatin III (10-DAB III) is performed according to the following steps: a, adding acetone into a 10-DAB III crude product, and performing ultrasonic stirring so as to completely dissolve 10-DAB III; b, adding acetonitrile when 10-DAB III is in the state of ultrasonic stirring, and filtering after a crystal is precipitated, so as to obtain a wet crystal; and c, drying the wet crystal, so as to obtain a high-purity 10-DAB III crystal. According to the crystallization purification method for 10-DAB III, by employing the acetone-acetonitrile mixed solvent for crystallization, the purity of the 10-DAB III crude product possessing the purity of 35% and being subjected to once crystallization is improved to 90% or more, the yield is 65% or more, the crystal purity is high, the yield is high, and the method is simple and convenient to operate, and helps to substantially improved the purification yield of 10-DAB III.
Owner:CHONGQING ZHENYUAN HONGDOUSHAN DEV CO LTD
Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbonyl]-5-oxazolidine carboxylic acids
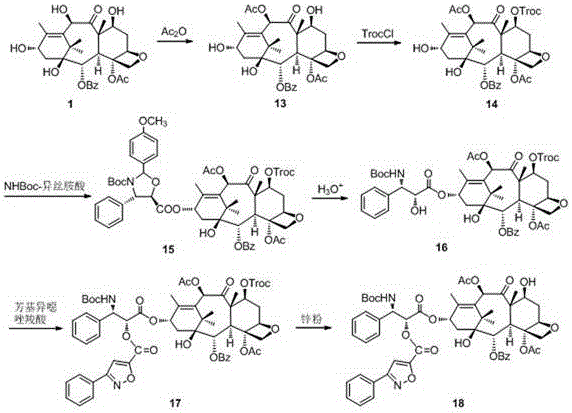
InactiveUS20030229135A1High purityMinimize degradationOrganic active ingredientsBiocideOxazolidine EAlfaxalone
This invention relates to a process for preparation of taxanes comprising subjecting 7,10-diprotected intermediates 7-O-(2-haloacyl) baccatin III 6c or 7,10-O-di-(2-haloacyl)-10-deacetylbaccatin III 6b to a step of coupling with (4S,5R)-3-[(2-alkyl / aryl-2-trialkylsilyl) ethoxy-carbonyl]-4-aryl-2-substituted-1,3-oxazolidine-5-carboxylic acid 1 in the presence of a condensation agent, an activating agent and an aromatic hydrocarbon to obtain 7-O-[2-(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3(2-unsubstituted / substituted-2-trialkylsilyl)-ethoxycarbonyl-1,3-oxazolidinyl-5-carbonyl]baccatin III 7a or 7,10-di-O[2-(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3-(2-unsubstituted / substituted-2-trialkylsilyl)ethoxy-carbonyl-1,3-oxazolidinyl-5-carbonyl]-10-deacetylbaccatin III 7b; treating the coupled products 7-O-[2-(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3-(2-substituted-2-trialkylsilyl)ethoxy-carbonyl-1,3-oxazolidinyl-S-carbonyl]baccatin III 7a or 7,10-di-0-[2[(haloacyl)]-13-[(4S,5R)-4-aryl-2-substituted-3-(2-substituted-2-trialkylsilyl)ethoxycarbonyl-1,3-oxazolidinyl-5-carbonyl]-10-deacetylbaccatin III 7b with tetraalkylammonium halide in a haloalkane to obtain free amine of structure 8; treating free amine 8 with acid chloride or acid anhydride in the presence of a base in a heterogeneous phase to obtain the intermediates of structure 9; subjecting the intermediates of compound 9 to the deprotection of 2-haloacyl group under mild alkaline condition at -20 to +40° C. for 6-24 h in the presence of ammonia or aliphatic amines or aromatic amines or their combination to obtain paclitaxel or docetaxel.
Owner:DABUR PHARM LTD
Semi-synthesis and isolation of taxane intermediates from a mixture of taxanes
A process is provided for the semi-synthesis and isolation of taxane intermediates useful in the preparation of paclitaxel and docetaxel, in particular, the semi-synthesis and isolation of 10-deacetylbaccatin III, and protected derivatives thereof, from a mixture of taxanes.
Owner:INNOVATIONAL HLDG LLC
Method for extracting and purifying two kinds of taxane compound from yew branches and leaves
The invention relates to a method for using specific resin and silicon gel column chromatography purification via a negative pressure cavitation water extraction method to produce 10-deacetylbaccatin III and 7-xylose-10-deacetylpaclitaxel at high efficiency. The method comprises using taxus branch leaves as raw material, drying and breaking the raw material to process negative pressure cavitationwater extraction, filtering, using AB-8 resin to process dynamic absorption on filter liquor to concentrate 10-deacetylbaccatin III and 7-xylose-10-deacetylpaclitaxel and carrying out a further purification by middle pressure silicon gel column chromatography, to obtain the 10-deacetylbaccatin III of 60% purity or more and 7-xylose-10-deacetylpaclitaxel of 67% purity or more. The invention can use renewable taxus branch leaves to protect ecological resource and effectively extract effective component and biological semi synthesis precursor materials. The invention has simple operation, thus is suitable for industrial application and is important for industrial production.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
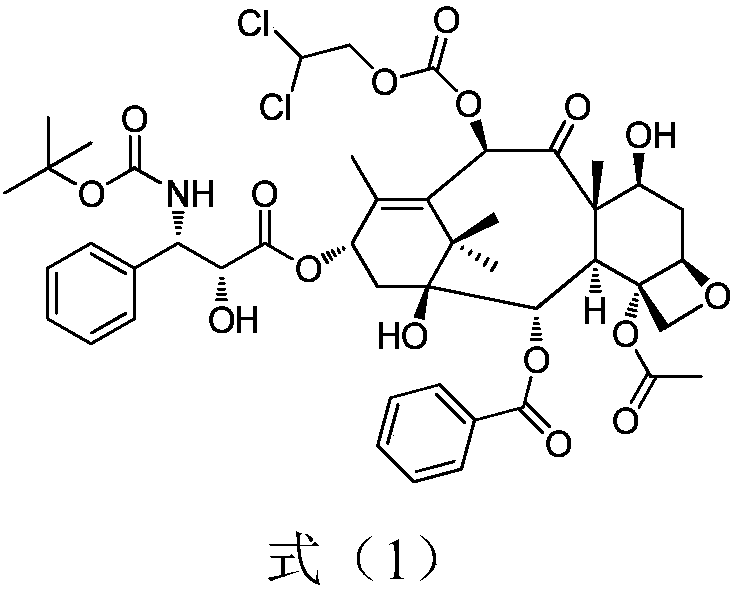
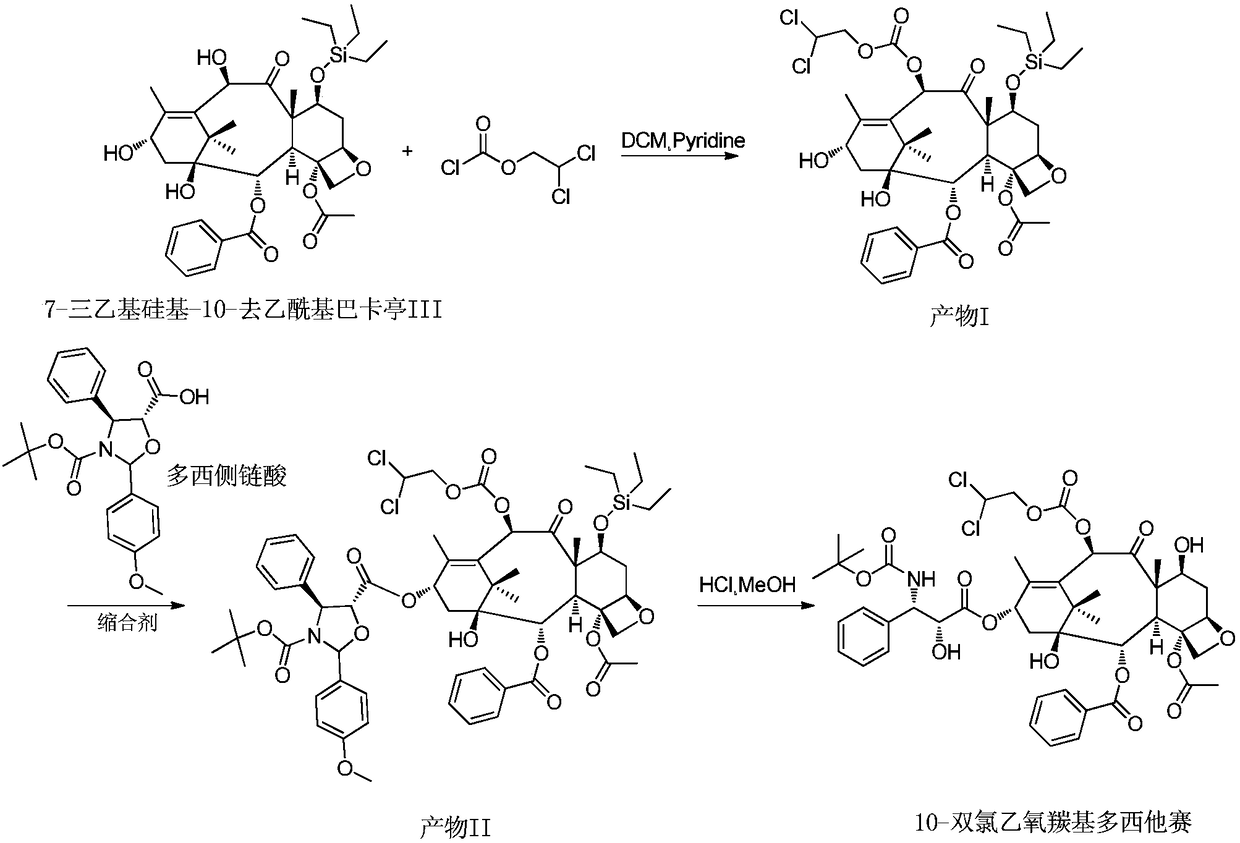
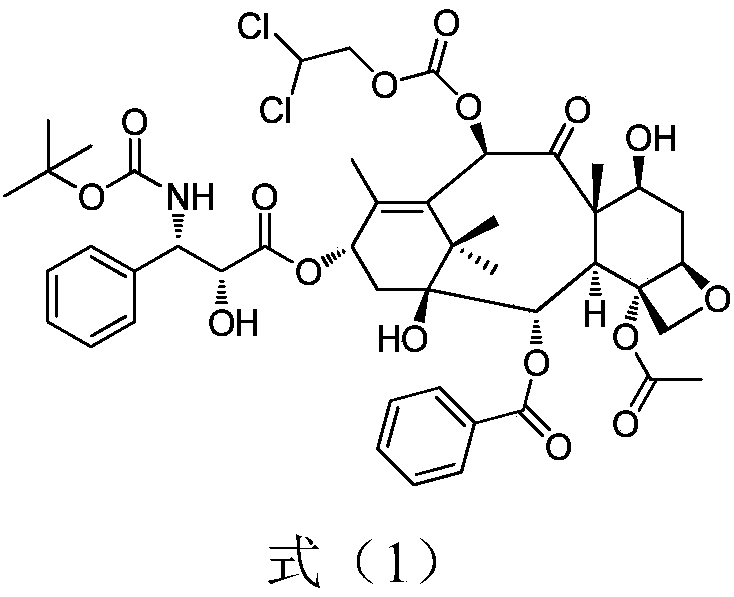
Preparation method for docetaxel impurity
InactiveCN108947942AFew reaction stepsHigh reaction yieldOrganic chemistryDocetaxel10-Deacetylbaccatin
The invention provides a preparation method for a docetaxel impurity, namely, 10-dichloroethoxycarbonyl docetaxel. The preparation method comprises the following steps: (1) taking 7-triethyl silicon-10-deacetylbaccatin III as a raw material and reacting with chloroformic-2,2-dichloro ethyl ester, thereby acquiring a product I; (2) triggering the product I to react with polysillic acid under the effect of a condensing agent, thereby acquiring a product II; (3) triggering the product III to react under the effect of hydrochloric acid, thereby acquiring the 10-dichloroethoxycarbonyl docetaxel. The method is simple in operation, high in yield and high in product purity. The acquired product has a great significance in researching docetaxel quality.
Owner:无锡紫杉药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl) ethoxycarbonyl]-5-oxazolidine carboxylic acids Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl) ethoxycarbonyl]-5-oxazolidine carboxylic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/26d57529-318d-4346-811c-dce4cfedf808/US07247738-20070724-C00001.png)
![Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl) ethoxycarbonyl]-5-oxazolidine carboxylic acids Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl) ethoxycarbonyl]-5-oxazolidine carboxylic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/26d57529-318d-4346-811c-dce4cfedf808/US07247738-20070724-C00002.png)
![Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl) ethoxycarbonyl]-5-oxazolidine carboxylic acids Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl) ethoxycarbonyl]-5-oxazolidine carboxylic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/26d57529-318d-4346-811c-dce4cfedf808/US07247738-20070724-C00003.png)
![Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbon1]-5-oxazolidine carboxylic acids Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbon1]-5-oxazolidine carboxylic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0b801fd5-ff34-4113-a1e1-f7d7d2658881/US20080064887A1-20080313-C00001.png)
![Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbon1]-5-oxazolidine carboxylic acids Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbon1]-5-oxazolidine carboxylic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0b801fd5-ff34-4113-a1e1-f7d7d2658881/US20080064887A1-20080313-C00002.png)

![Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbonyl]-5-oxazolidine carboxylic acids Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbonyl]-5-oxazolidine carboxylic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b774c693-8161-42e5-8b07-4a5f955b60a7/US20030229135A1-20031211-C00001.png)
![Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbonyl]-5-oxazolidine carboxylic acids Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbonyl]-5-oxazolidine carboxylic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b774c693-8161-42e5-8b07-4a5f955b60a7/US20030229135A1-20031211-C00002.png)
![Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbonyl]-5-oxazolidine carboxylic acids Method of preparation of anticancer taxanes using 3-[(substituted-2-trialkylsilyl)ethoxycarbonyl]-5-oxazolidine carboxylic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b774c693-8161-42e5-8b07-4a5f955b60a7/US20030229135A1-20031211-P00999.png)