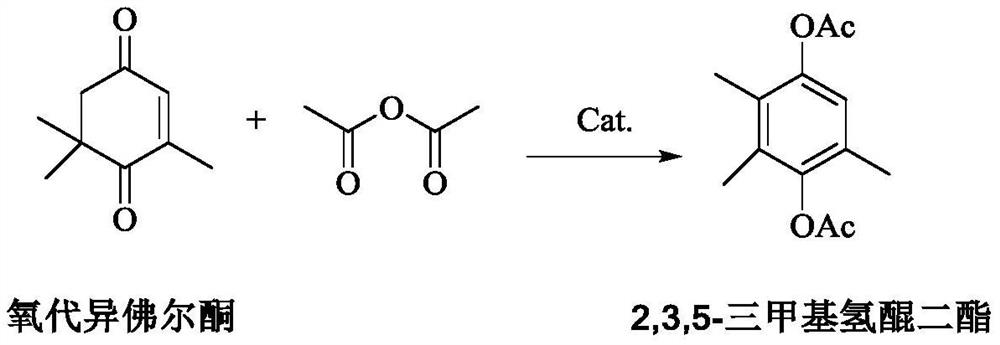
Method for preparing 2,3,5-trimethylhydroquinone diester
A technology of trimethylhydroquinone diester and polyethylene glycol dimethacrylate, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve the cumbersome preparation process of catalysts and product selection. Unsatisfactory performance, strong corrosion of equipment, etc., to achieve the effect of improving the conversion rate of raw materials and product selectivity, improving the utilization rate of raw materials, and stabilizing the loading process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
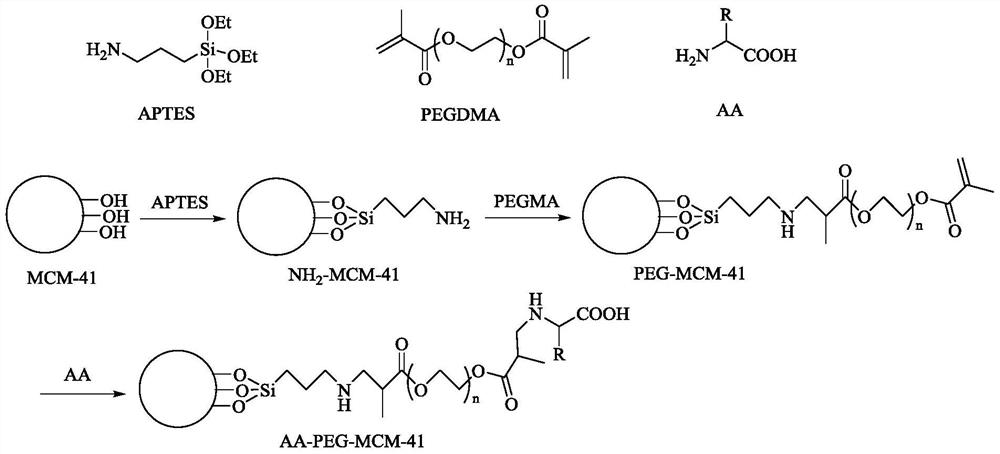
[0055] Add 36g of cetyltrimethylammonium bromide (CTAB) into 1000g of deionized water, and stir until completely dissolved. Add tetraethyl orthosilicate (TEOS) dropwise to the dissolved solution, the molar ratio of CTAB to TEOS is 1:6, and stir vigorously during the dropwise addition. Adjust the pH to 10.5 with ammonia water, then continue stirring for 4 h and transfer to a polytetrafluoroethylene-lined stainless steel kettle for crystallization at 100°C for 36 h. After the crystallization, the solid was separated, washed with water and absolute ethanol, dried and calcined in a muffle furnace at 550°C for 6 hours to obtain MCM-41 molecular sieve.
[0056] Take 50g MCM-41 molecular sieve and 50g APTES and disperse in toluene, reflux for 12h, filter, wash and dry to obtain NH 2 -MCM-41.
[0057] Take 45g NH 2 -MCM-41 was dispersed in an aqueous solution containing 225g PEGDMA (M=1000) and 3000g water, stirred at room temperature for 16 hours, separated, washed to remove the a...
Embodiment 2
[0061] Gly-PEG-MCM-41 was prepared according to the method of Example 1. Take 30g of Gly-PEG-MCM-41 molecular sieve and disperse it in 400mL of aqueous solution containing 15g of silicotungstic acid, stir at room temperature for 12h, separate and wash 3 times with water to obtain amino acid and PEG-modified mesoporous molecular sieve supported solid acid catalyst (denoted as catalyst b).
Embodiment 3
[0063] Add 510.5g (5mol) of acetic anhydride and 7.6g of catalyst a to a 2L reaction flask in sequence, keep stirring at 60°C, add 152.2g (1mol) of oxoisophorone dropwise to the reaction flask, drop it completely in 6 hours, add dropwise Continue the reaction for 3 hours after completion, the conversion rate of oxoisophorone reaches 99.8%, the selectivity of 2,3,5-trimethylhydroquinone diester is 99.9%, and the impurity 3,4,5-trimethylhydroquinone diester The selectivity is 0.01%, and then the reaction solution is filtered, the catalyst is washed with absolute ethanol, dried and used mechanically. The filtrate removes acetic anhydride and the acetic acid obtained by the reaction at an absolute pressure of 2000Pa and 80°C, and then distills at an absolute pressure of 100Pa and a temperature of 130°C to obtain 2,3,5-trimethylhydroquinone diester with a yield of 96.5% , 99.98% purity.
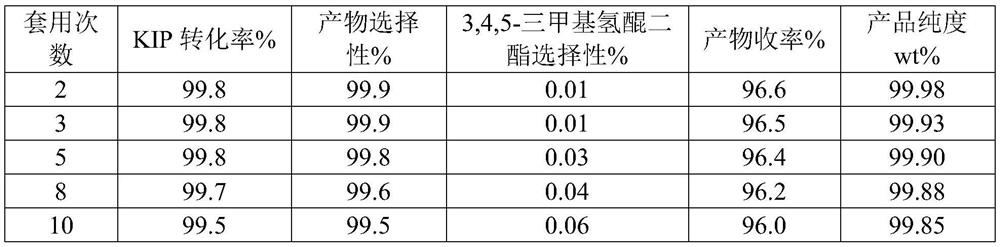
[0064] Catalyst a is applied mechanically, and the experimental data are as follows in Table ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com