Preparation method of trans-stilbene compound
A technology of stilbene and compound, which is applied in the field of preparation of trans-stilbene compound, can solve the problems of complicated operation, poor functional group tolerance, etc., and achieves the effects of simple post-processing, simple and convenient operation, and simple post-processing operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
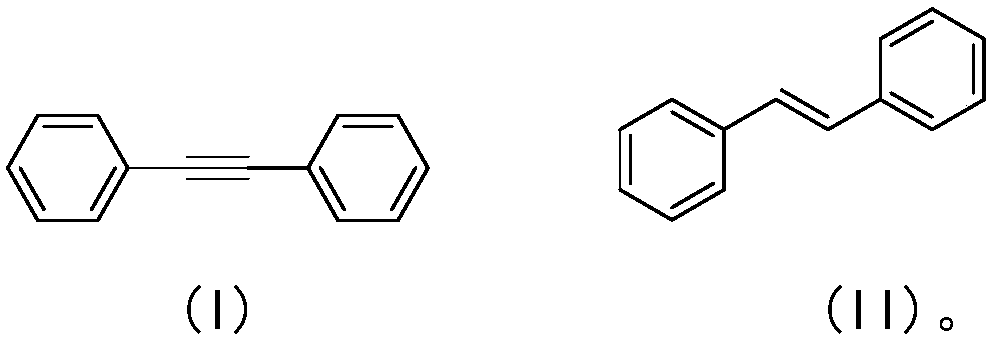
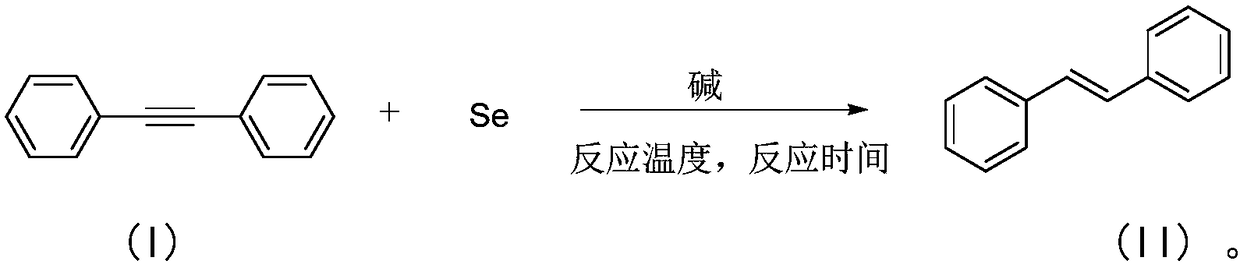
[0044] A kind of preparation method of trans-stilbene compound, in organic solvent, under nitrogen or inert gas condition, with toluene acetylene having the structure shown in formula (I) as reaction substrate, in the common of alkali and elemental selenium Under the action of promotion, the carbon-carbon triple bond in the tolanne structure is chemoselectively reduced to obtain a reaction solution, and the reaction solution is post-treated to obtain trans-stilbene with the structure shown in formula (II).
[0045] Above-mentioned reaction process, available following reaction equation expression:
[0046]
[0047] (1) alkali
[0048] The alkali selected in the present invention is at least one of potassium carbonate, potassium acetate, sodium acetate, lithium acetate, sodium bicarbonate, ammonium acetate, calcium acetate, zinc acetate, barium acetate, silver acetate, preferably potassium acetate;
[0049] In terms of molar weight, the ratio of the amount of the base to th...
Embodiment 1
[0068] Synthesis of (E)-1,2-stilbene
[0069]
[0070] At room temperature, (E)-1,2 tolanylacetylene (0.4mmol, 1equiv), selenium powder (1.2mmol, 3equiv) and potassium acetate (KOAc) (0.8mmol, 2equiv) were added to the reaction tube, and then Gas-nitrogen replacement three times, add 12.4mmol N, N-dimethylformamide (DMF), stir at 150 ° C reaction temperature for 24h, after the reaction is monitored by thin layer chromatography, the reaction liquid is cooled, and then the reaction liquid Post-processing is to obtain (E)-1,2-stilbene.
[0071] Postprocessing includes the following steps:
[0072] Add ethyl acetate to the reaction solution for dilution, and concentrate under reduced pressure to obtain a concentrate, wherein: the volume ratio of the reaction solution to ethyl acetate is 1:5;
[0073] The concentrate was separated by column chromatography to obtain an eluate in which:
[0074] The silica gel separated by column chromatography is 300-400 mesh silica gel;
[0...
Embodiment 2
[0082] Synthesis of (E)-4-Styrylbenzaldehyde
[0083]
[0084] At room temperature, (E)-4-phenylethynyl benzaldehyde (0.4mmol, 1equiv), selenium powder (0.4mmol, 1equiv) and KOAc (1.6mmol, 4equiv) were added to the reaction tube, then pumped-filled Nitrogen was replaced three times, 26 mmol dimethyl sulfoxide was added, and stirred at a reaction temperature of 160° C. for 12 h. After the end of the reaction is monitored by thin-layer chromatography, the reaction solution is cooled, and then the reaction solution is post-treated to obtain (E)-4-styryl benzaldehyde.
[0085] Postprocessing includes the following steps:
[0086] Add ethyl acetate to the reaction solution for dilution, and concentrate under reduced pressure to obtain a concentrate, wherein: the volume ratio of the reaction solution to ethyl acetate is 1:10;
[0087] The concentrate was separated by column chromatography to obtain an eluate in which:
[0088] The silica gel separated by column chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com