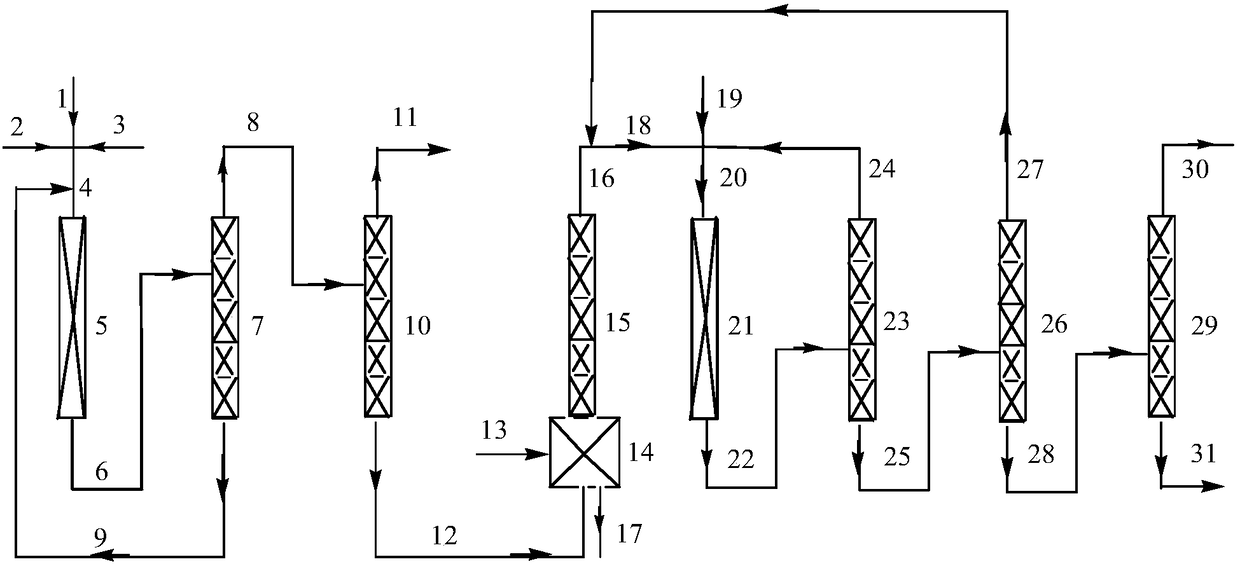
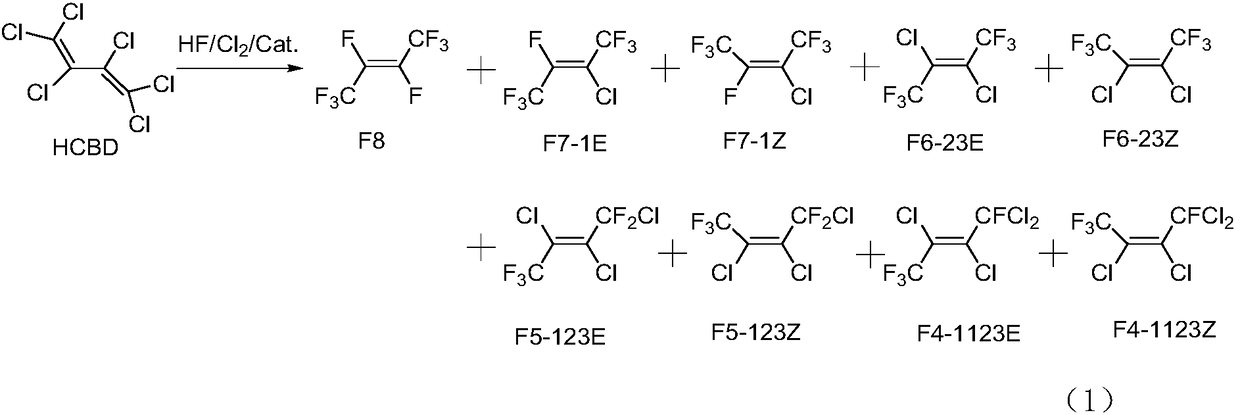
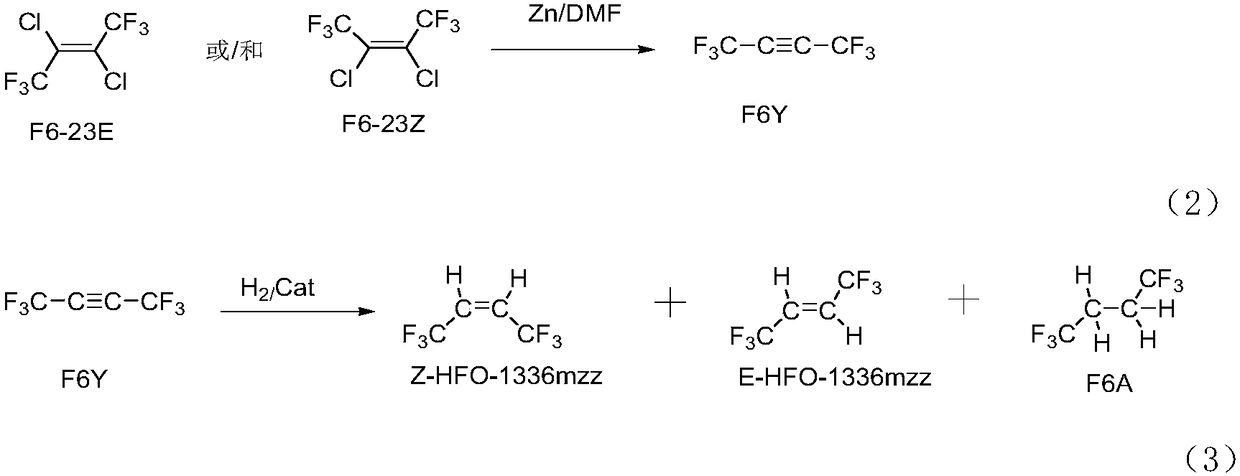
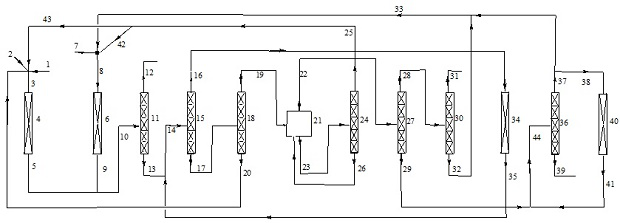
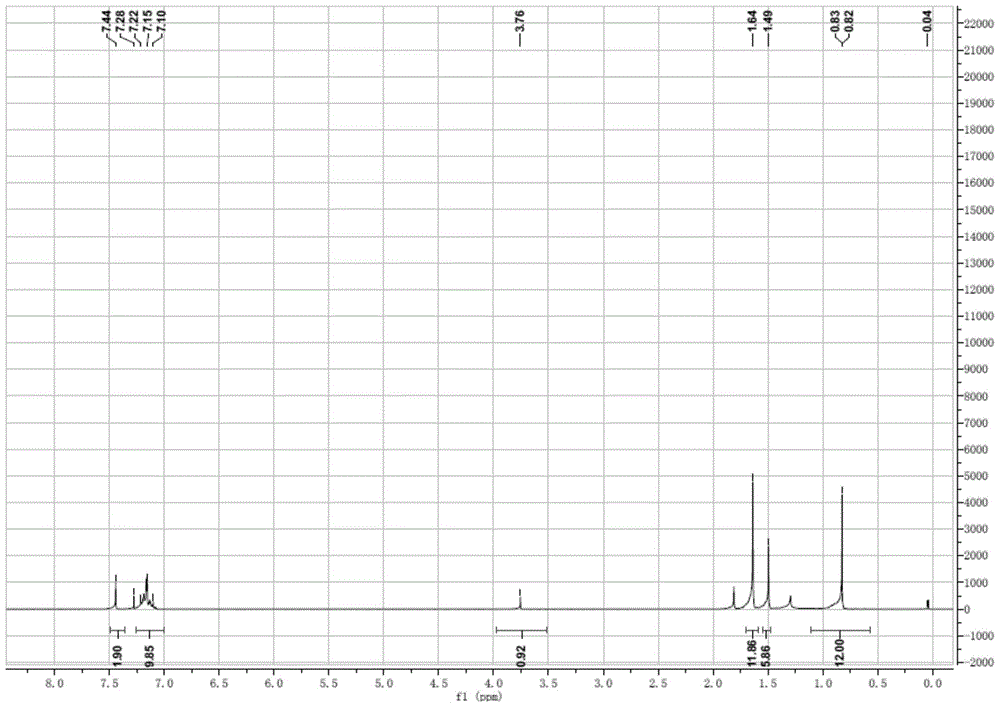
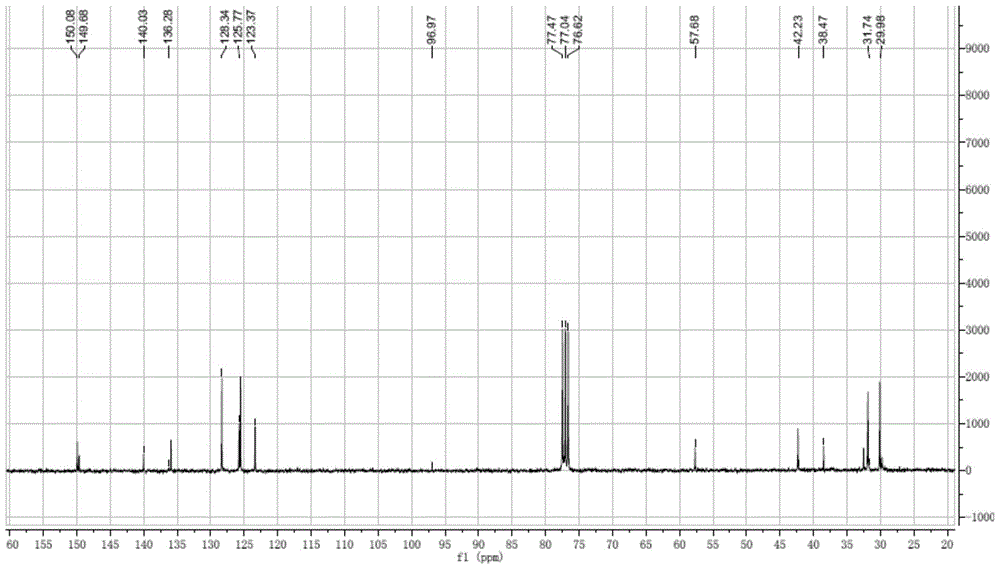
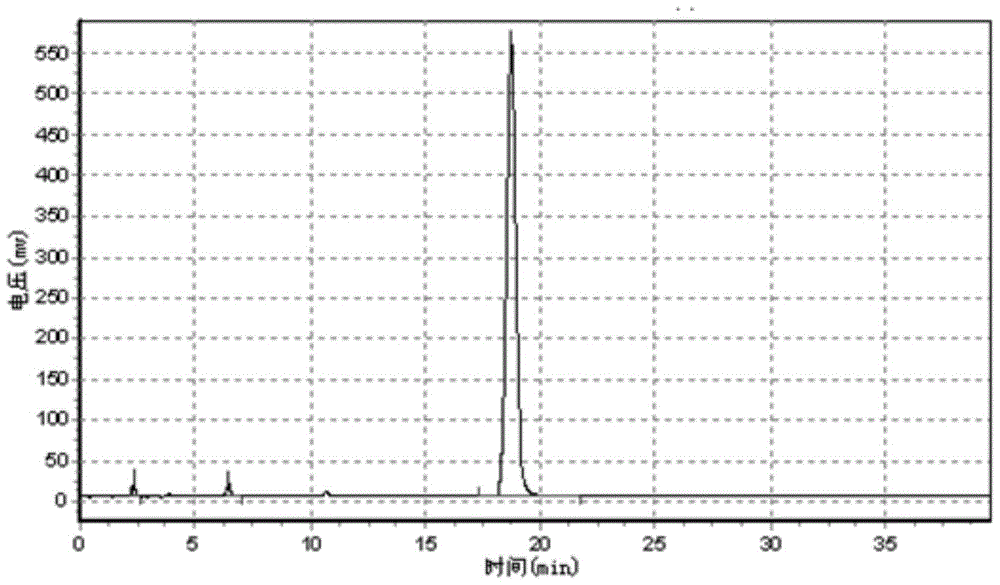
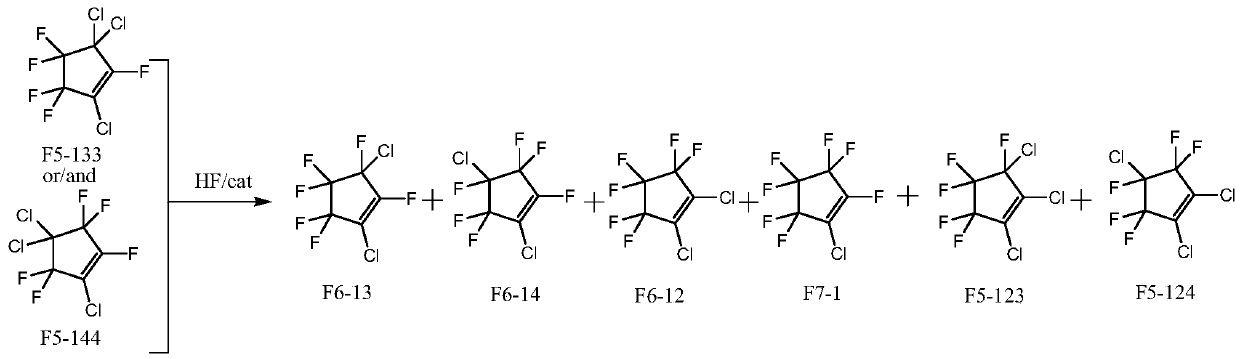
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30results about How to "High productivity per pass" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
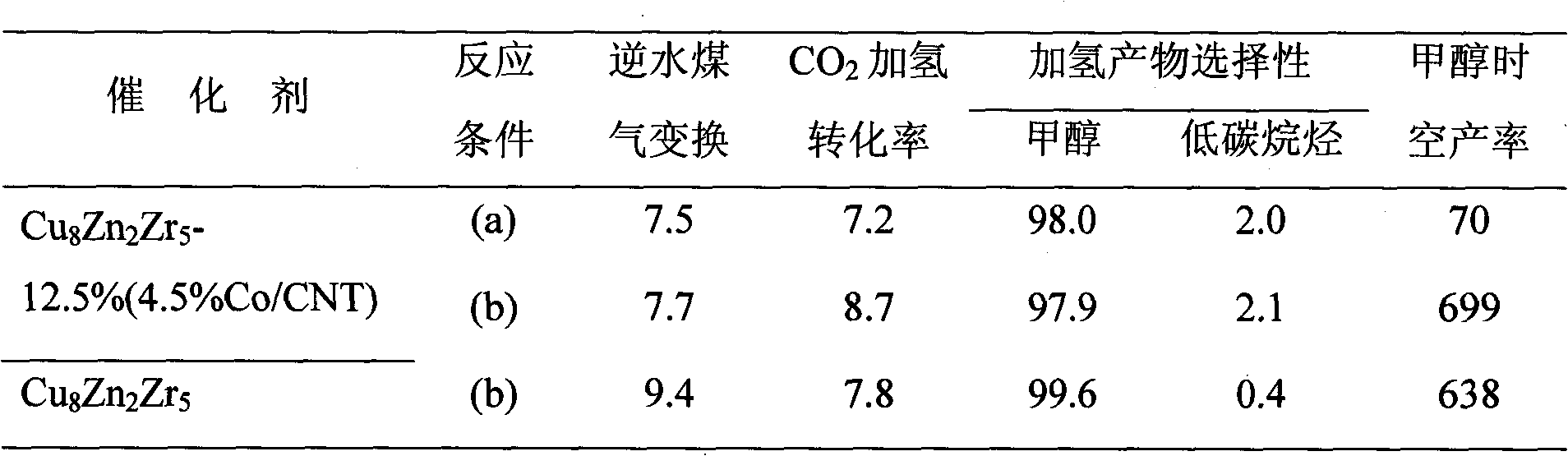
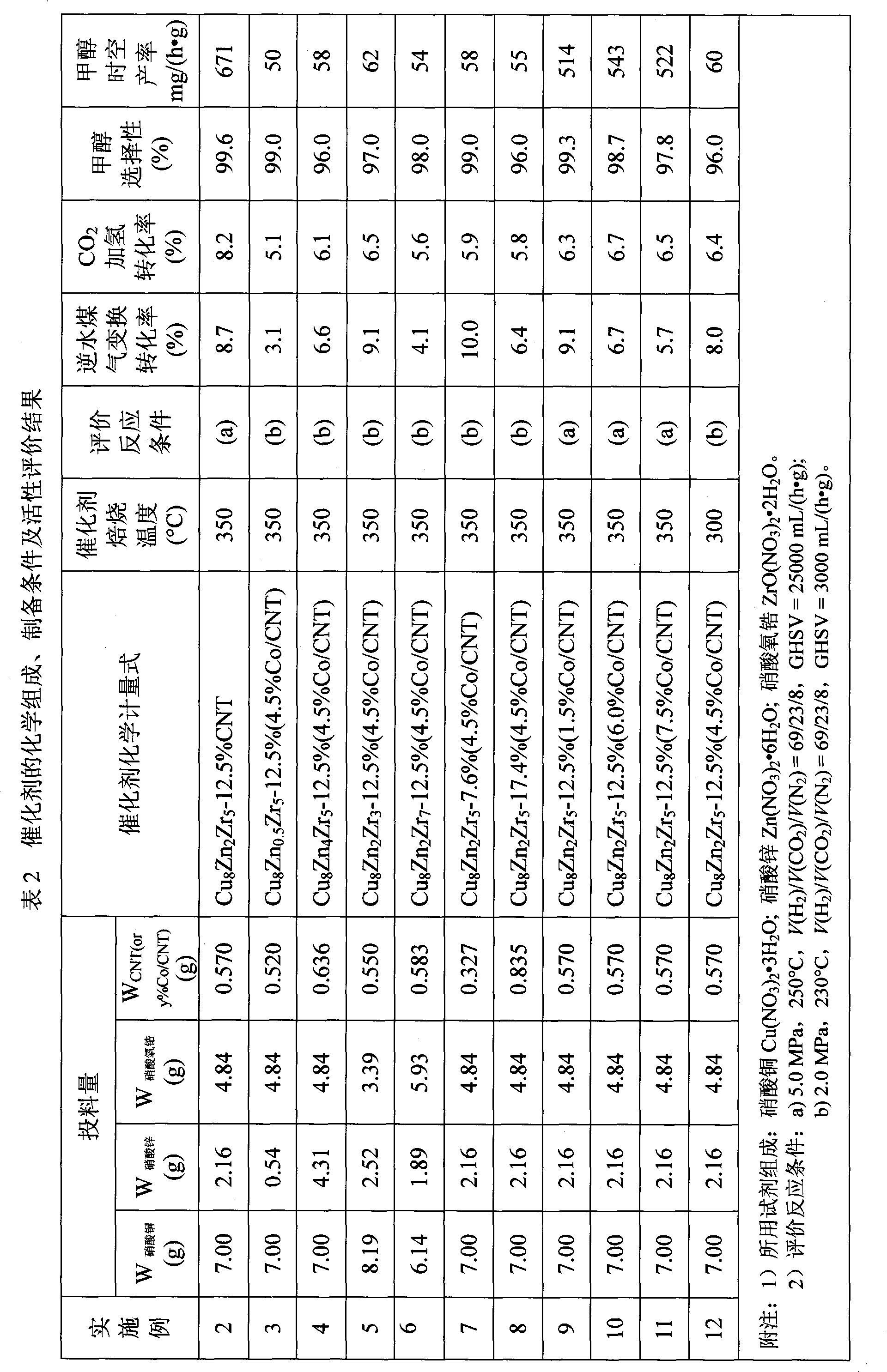
Catalyst for hydrogenation of carbon dioxide to generate methanol and preparation method thereof
InactiveCN101786001AIncreased hydrogenation conversionHigh productivity per passOrganic compound preparationHydroxy compound preparationCarbon nanotubeMetal
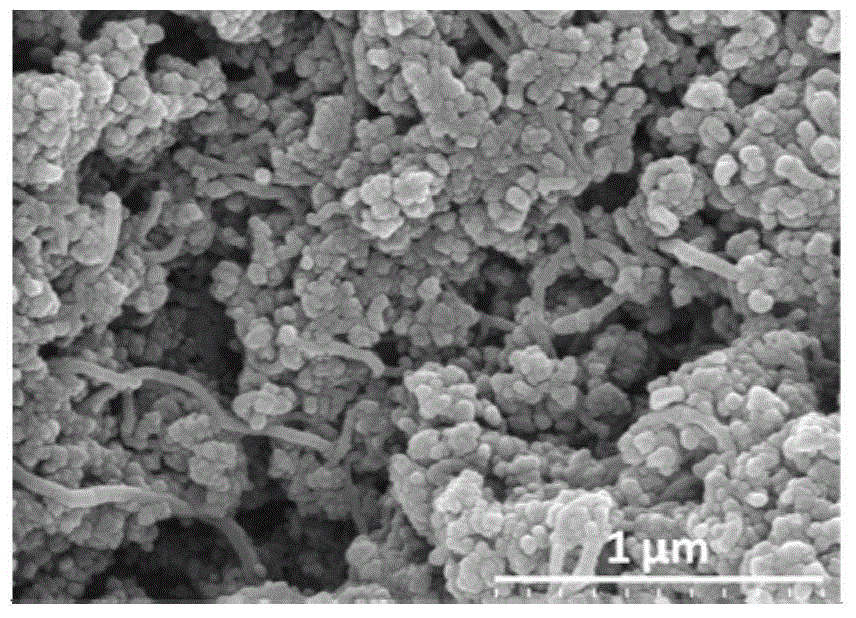
The invention relates to a catalyst, in particular to the catalyst for hydrogenation of carbon dioxide to generate methanol and a preparation method thereof. A carbon nanotube-based material is adopted as an accelerating agent. When being used for the catalytic hydrogenation of the carbon dioxide to generate the methanol, the catalyst can effectively increase the hydrogenation converting rate of the carbon dioxide and the one-path yield rate of the methanol. The catalyst comprises main metal components and the accelerating agent of the carbon nanotube-based material. The main metal components include Cu, Zn and Or. The accelerating agent of the carbon nanotube-based material means CNT or y% Co / CNT, and the chemical formula is CuiZnjZrk-x% (CNT or y% Co / CNT). The mass percent of each component is as follows: Zn: 5 to 20%; Zr: 25 to 50%; the accelerating agent of the carbon nanotube-based material: 1 to 18%; and the rest is Cu. The catalyst is prepared through coprecipitation. A multi-wall carbon nanotube modified by metal cobalt is prepared by utilizing liquid-phase microwaves of polyalcohol to assist in chemical reduction and deposition.
Owner:XIAMEN UNIV
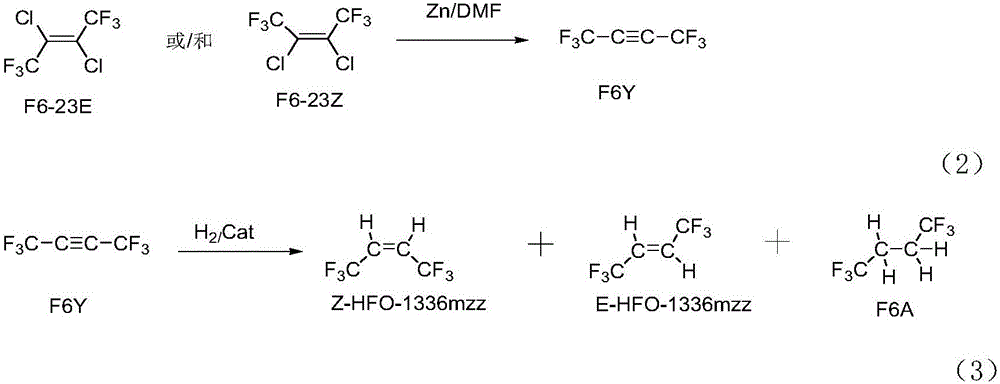
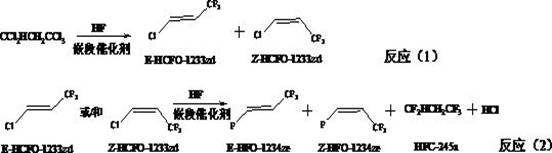
Preparation method of Z-1,1,1,4,4,4-hexafluoro-2-butene
ActiveCN106008147ALow priceHigh activityPreparation by dehalogenationPreparation by halogen replacementChemical synthesisGas phase
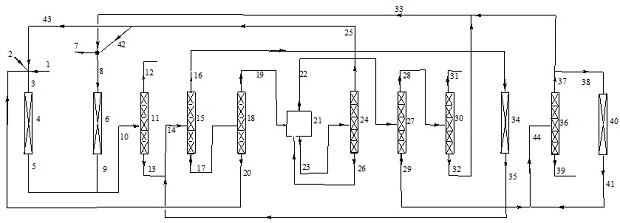
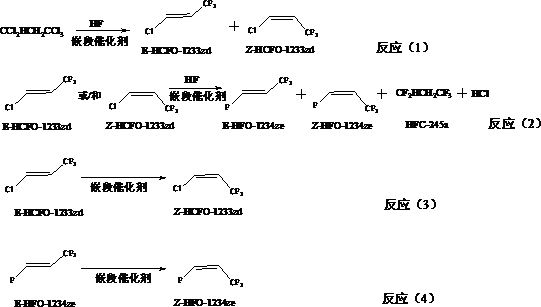
The invention relates to a preparation method of Z-1,1,1,4,4,4-hexafluoro-2-butene, belonging to the field of chemical synthesis. The method comprises the following steps of taking hexachlorobutadiene (HCBD) as a raw material, performing gas-phase catalytic chlorination-fluoridation separation to obtain 2,3-dichlorohexafluoro-2-butene, performing liquid-phase dechlorination to obtain hexafluoro-2-butyne, and preparing the Z-1,1,1,4,4,4-hexafluoro-2-butene (Z-HFO-1336mzz) by means of performing gas-phase catalytic hydrogenation, wherein three reactions are included totally. The Z-1,1,1,4,4,4-hexafluoro-2-butene with high selectivity is obtained by means of performing catalytic hydrogenation by adopting the hexafluoro-2-butyne, and meanwhile the product is easily separated from side products and the raw material. The method disclosed by the invention has the advantages that the original raw material is easily obtained, the activity of a catalyst is high, the service life of the catalyst is long, the raw material can be recycled, and the standard of zero emissions is reached.
Owner:泉州宇极新材料科技有限公司
The preparation method of z-1,1,1,4,4,4-hexafluoro-2-butene
ActiveCN106008147BLow priceHigh activityPreparation by dehalogenationPreparation by halogen replacementChemical synthesisGas phase
The invention relates to a preparation method of Z-1,1,1,4,4,4-hexafluoro-2-butene, belonging to the field of chemical synthesis. The method comprises the following steps of taking hexachlorobutadiene (HCBD) as a raw material, performing gas-phase catalytic chlorination-fluoridation separation to obtain 2,3-dichlorohexafluoro-2-butene, performing liquid-phase dechlorination to obtain hexafluoro-2-butyne, and preparing the Z-1,1,1,4,4,4-hexafluoro-2-butene (Z-HFO-1336mzz) by means of performing gas-phase catalytic hydrogenation, wherein three reactions are included totally. The Z-1,1,1,4,4,4-hexafluoro-2-butene with high selectivity is obtained by means of performing catalytic hydrogenation by adopting the hexafluoro-2-butyne, and meanwhile the product is easily separated from side products and the raw material. The method disclosed by the invention has the advantages that the original raw material is easily obtained, the activity of a catalyst is high, the service life of the catalyst is long, the raw material can be recycled, and the standard of zero emissions is reached.
Owner:泉州宇极新材料科技有限公司
Preparation method of E-1-halogen-3, 3, 3-trifluoropropene
ActiveCN112723985AHigh productivity per passHigh activityPreparation by dehalogenationPhysical/chemical process catalystsHydrogen fluoridePtru catalyst
The invention discloses a preparation method of E-1-halogen-3, 3, 3-trifluoropropene. The preparation method comprises the following steps: in the presence of a block catalyst, carrying out gas-phase fluorination reaction on 1, 1, 1, 3, 3-pentachloropropane and hydrogen fluoride in a tubular reactor to obtain a main product E-1-chlorine-3, 3, 3-trifluoropropene and a small amount of product Z-1-chlorine-3, 3, 3-trifluoropropene; furthermore, in the presence of the block catalyst, E-1-chlorine-3, 3, 3-trifluoropropene or / and Z-1-chlorine-3, 3, 3-trifluoropropene and hydrogen fluoride are subjected to a gas-phase fluorination reaction in the tubular reactor, and a main product E-1, 3, 3, 3-tetrafluoropropene is obtained. The one-way yield of the method is high; especially for the second-step process, only HCl in the product flow in the first step needs to be removed, and the remaining substances can be directly used for fluorination reaction after new HF is added. The block catalyst has the characteristics of high activity and long service life.
Owner:泉州宇极新材料科技有限公司
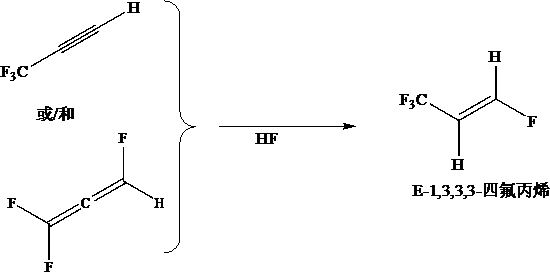
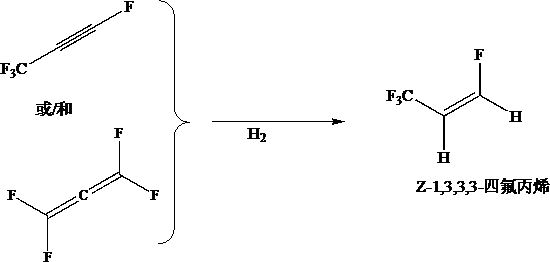
Preparation method of Z-1, 3, 3, 3-tetrafluoropropene
ActiveCN112811978AHigh productivity per passHigh activityHalogenated hydrocarbon preparationPtru catalystCombinatorial chemistry
The invention discloses a preparation method of Z-1, 3, 3, 3-tetrafluoropropene, which comprises the following steps: by taking 1, 3, 3, 3-tetrafluoropropyne or / and an isomer 1, 1, 3, 3-tetrafluoropropadiene thereof as a raw material, carrying out gas-phase selective hydrogenation in the presence of a hydrogenation catalyst to obtain the Z-1, 3, 3, 3-tetrafluoropropene. The method provided by the invention is mainly used for producing Z-1, 3, 3, 3-tetrafluoropropene in a high-efficiency and gas-phase continuous circulation manner.
Owner:泉州宇极新材料科技有限公司
The preparation method of e-1-halo-3,3,3-trifluoropropene
ActiveCN112723985BHigh productivity per passHigh activityPreparation by dehalogenationPhysical/chemical process catalystsHydrogen fluoridePtru catalyst
Owner:泉州宇极新材料科技有限公司
Method for producing propylene from n-butene
ActiveCN104557397AHigh yieldHigh productivity per passMolecular sieve catalystsBulk chemical productionMolecular sieveAdhesive
The invention discloses a method for producing propylene from n-butene. Under the action of a catalyst, a raw material containing n-butene is subjected to cracking reaction under the reaction temperature of 500-600 DEG C, preferably 500-550 DEG C and the total reaction pressure (the absolute pressure) is 0-0.25MPa; the weight space velocity is 1-10h<-1>, preferably 1-5h<-1>; the catalyst comprises the following components in percentage by weight: 30-60 percent of a phosphorus-modified SAPO-11 molecular sieve, 20-30 percent of a hydrogen type ZSM-5 or ZSM-22 molecular sieve and the balance of an adhesive; the load of the phosphorus-modified SAPO-11 molecular sieve is 0.2-1 percent by weight (not including phosphorus in the SAPO-11 molecular sieve). The method used in the industrial process of producing propylene from the mixed carbon-4 raw material containing n-butene has the advantage of high once-through yield of propylene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of Z-1-halogen-3, 3, 3-trifluoropropene
ActiveCN112723983AHigh productivity per passGood choiceOrganic chemistry methodsPreparation by halogen replacementLiquid wasteIsomerization
The invention discloses a preparation method of Z-1-halogen-3, 3, 3-trifluoropropene. The preparation method comprises the following steps: in the presence of a block catalyst, E-1-halogen-3, 3, 3-trifluoropropene is subjected to a gas phase isomerization reaction in a tubular reactor to obtain Z-1-halogen-3, 3, 3-trifluoropropene, and halogen is fluorine or chlorine. According to the preparation method, 1, 1, 1, 3, 3-pentachloropropane is used as an initial raw material, Z-1-chloro-3, 3, 3-trifluoropropene or Z-1, 3, 3, 3-tetrafluoropropene secondary product is prepared through a gas-phase fluorination reaction and an isomerization reaction, the materials which are not completely reacted are independently circulated through a gas-phase independent circulation process so that the initial raw materials can be almost completely converted into the target product, and finally, the target product is extracted from a process system, and thus liquid waste and waste gas are not generated, and green production is realized.
Owner:泉州宇极新材料科技有限公司
Method for preparing propylene from n-butene
ActiveCN106673945ALow reaction temperatureReduced activityMolecular sieve catalystsBulk chemical productionButeneReaction temperature
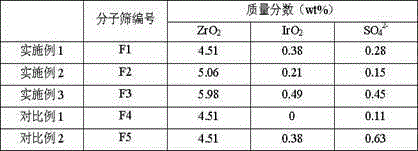
The invention discloses a method for preparing propylene from n-butene. A raw material containing the n-butene is in contact with a cracking catalyst under a catalytic cracking reaction condition, wherein the reaction temperature is 450-550 DEG C, preferably 450-500 DEG C, the reaction total pressure (absolute pressure) is 0-0.5MPa, preferably 0-0.15MPa, the weight space velocity is 1-10h<-1>, preferably 2-4h<-1>; according to the cracking catalyst, the content of FER molecular sieve and modified ZSM-5 molecular sieve is no less than 50%, and the mass ratio of the FER molecular sieve to the modified ZSM-5 molecular sieve is (1 to 1) to (3 to 1); the modified ZSM-5 molecular sieve comprises the following components in percentage by weight: 4.5-6.0% of zirconium dioxide, 0.2-0.5% of iridium oxide, 0.15-0.45% of SO4<2->, and the remaining of ZSM-5 molecular sieve, and molar ratio of silicon to aluminum of the FER molecular sieve is 50-100. The method has the advantages of low reaction temperature and high single-pass propylene yield for use in the industrial process of producing the propylene from the raw material containing n-butene mixed C4.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing 3, 3, 3-trifluoropropyne through gas-phase dehydrohalogenation
ActiveCN113004117AHigh productivity per passHigh selectivityPreparation by hydrogen halide split-offCatalyst activation/preparationHydrogen halidePtru catalyst
The invention discloses a method for preparing 3, 3, 3-trifluoropropyne through gas-phase dehydrohalogenation. The method comprises the following steps: taking 1-halogen-3, 3, 3-trifluoropropene or / and 2-halogen-3, 3, 3-trifluoropropene (halogen = F or Cl or Br or I) as a raw material, and carrying out gas-phase dehydrohalogenation reaction in the presence of a catalyst to obtain the 3, 3, 3-trifluoropropyne. The method disclosed by the invention is mainly used for producing the 3, 3, 3-trifluoropropyne in a gas-phase continuous circulation manner at a high conversion rate and high selectivity.
Owner:泉州宇极新材料科技有限公司
Preparation method of E-1, 3, 3, 3-tetrafluoropropene
ActiveCN112811973AHigh productivity per passHigh activityCatalyst activation/preparationHalogenated hydrocarbon preparationPtru catalystCombinatorial chemistry
The invention discloses a preparation method of E-1, 3, 3, 3-tetrafluoropropene, which comprises the following steps: by taking 3, 3, 3-trifluoropropyne or / and an isomer 1, 3, 3-trifluoropropadiene thereof as a raw material, carrying out gas-phase selective fluorination reaction in the presence of a fluorination catalyst to obtain E-1, 3, 3, 3-tetrafluoropropene. The method provided by the invention is mainly used for producing E-1, 3, 3, 3-tetrafluoropropene in a high-efficiency and gas-phase continuous circulation manner.
Owner:泉州宇极新材料科技有限公司
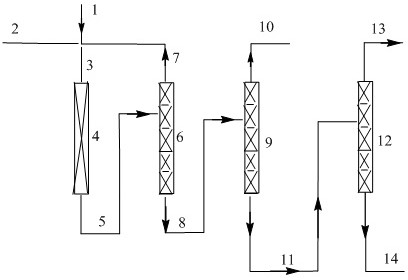
Method for simultaneously preparing dichlorohexafluorocyclopentene isomers
ActiveCN107082737ARaw materials are easy to getStable activityPreparation by halogen replacementHydrogen fluorideSingle pass
The invention relates to a method for simultaneously preparing dichlorohexafluorocyclopentene isomers. The method comprises the following steps: taking hydrogen fluoride and trichloropentafluorocyclopentene as reaction raw materials; carrying out gas-phase catalysis fluorine-chlorine exchange reaction in the presence of a fluorination catalyst to obtain the dichlorohexafluorocyclopentene isomers. By adopting the method provided by the invention, 1,2-dichlorohexafluorocyclopentene, 1,3-dichlorohexafluorocyclopentene and 1,4-dichlorohexafluorocyclopentene can be coproduced, wherein the single-pass yield of the 1,3-dichlorohexafluorocyclopentene and the 1,4-dichlorohexafluorocyclopentene is relatively high and the sum of the yield of the 1,3-dichlorohexafluorocyclopentene and the 1,4-dichlorohexafluorocyclopentene is greater than 57 percent; the raw materials are easy to obtain and the fluorination catalyst has stable activity, so that the method is suitable for simultaneously and continuously preparing the 1,2-dichlorohexafluorocyclopentene, the 1,3-dichlorohexafluorocyclopentene and the 1,4-dichlorohexafluorocyclopentene in a large scale in a gas phase.
Owner:BEIJING YUJI SCI & TECH +1
Method for synthesizing fluoroisobutylene by taking hexafluoropropylene as initial raw material
ActiveCN112794788AHigh productivity per passHigh activityPhysical/chemical process catalystsPreparation by hydrogen halide split-offHydrogen fluoridePtru catalyst
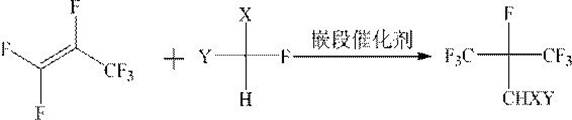
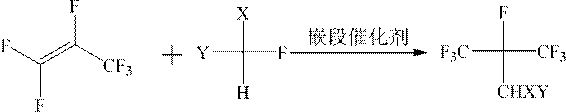
The invention discloses a method for synthesizing fluoroisobutylene by taking hexafluoropropylene as an initial raw material. The method comprises the following steps: (1) gas phase addition reaction: in the presence of a block catalyst, hexafluoropropylene and fluoromethane CHFXY (X, Y = F or H) are subjected to gas phase addition to obtain (CF3)2CFCHXY, and (2) dehydrofluorination reaction: in the presence of a dehydrofluorination catalyst, the (CF3)2CFCHXY as a raw material is subjected to dehydrofluorination reaction to obtain (CF3)2C = CXY. The block catalyst and the dehydrofluorination catalyst in the invention have the characteristics of high activity and long service life, and can be used for high-efficiency and gas-phase continuous cycle production of fluoroisobutylene.
Owner:泉州宇极新材料科技有限公司
Method for preparing heptafluoroisobutyronitrile through gas-phase fluorocyaniding
PendingCN113683530AThorough responseTake advantage ofPhysical/chemical process catalystsPreparation by cyanogen halide reactionChemical synthesisHydrogen fluoride
The invention relates to a method for preparing heptafluoroisobutyronitrile through gas-phase fluorocyaniding, and belongs to the field of chemical synthesis. Hexafluoropropylene is used as a raw material, in the presence of a fluorination catalyst, hexafluoropropylene, hydrogen fluoride and X-CN are subjected to a gas phase catalytic reaction to obtain heptafluoroisobutyronitrile, unreacted hexafluoropropylene, hydrogen fluoride and X-CN in a product flow are circulated to a reactor filled with the fluorination catalyst for continuous reaction, and when X-CN is F-CN, the raw material HF can be zero. The method has the characteristics of easily available initial raw materials, high activity of the fluorination catalyst and long service life, and only the main product heptafluoroisobutyronitrile and the possible byproduct HX are extracted from the whole system by adopting a continuous cycle process technology, so that the zero emission standard is achieved.
Owner:CHEM & CHEM ENG GUANGDONG LAB +1
Nickel-based methanation catalyst promoted by in-situ growth of carbon nanotubes and preparation method thereof
InactiveCN103769106BHigh productivity per passHigh reactivityGaseous fuelsMetal/metal-oxides/metal-hydroxide catalystsMethanationCarbon impurities
The invention discloses a nickel-based methanation catalyst promoted by an in-situ grew carbon nano tube and a preparation method for the nickel-based methanation catalyst and relates to a methanation catalyst. The catalyst comprises a main component and a promoter, wherein the main component comprises Ni and Mg; the promoter is the carbon nano tube and is represented by Ni / MgO-CNTs. The preparation method comprises the steps of mixing Ni(NO3)2*6H2O and Mg(NO3)2*6H2O, and dissolving the mixture in water to prepare a solution A, dissolving NaOH and NaHCO3 in water to prepare a solution B; adding the solution A and the solution B into a reaction container for reaction, aging and filtering precipitation liquid, washing until the filtrate is neutralized, drying and roasting to obtain an Ni / MgO catalyst precursor, then performing tabletting and screening, performing mixed dilution through quartz sand, putting the precursor in a rector, heating to 873K, reducing the precursor to obtain an Ni / MgO catalyst in a reduced state, converting gas flow into CO gas reaction, growing the carbon nano tube in situ, cooling to 823K to remove carbon impurities, and cooling to 473K to obtain a product.
Owner:XIAMEN UNIV
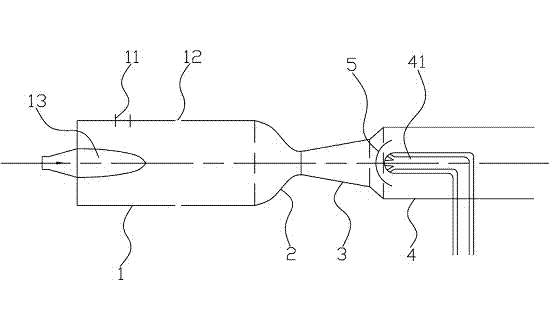
Pneumatic heating and quick warming process and equipment for same
InactiveCN103087760AIncrease temperatureHigh productivity per passCatalytic crackingHydrocarbon by hydrocarbon crackingShock waveWarming process
The invention provides a pneumatic heating and quick warming process and equipment for the process. According to the pneumatic heating and quick warming process, the high-speed liquid and the liquid to be heated are contacted to generate a shock wave, the static temperature of the high-speed liquid is rapidly raised after the shock wave is generated, then the high-speed liquid is quickly mixed with the liquid to be heated, and thus the temperature of the liquid to be heated can be quickly raised. When the liquid to be heated is the petroleum hydrocarbon liquid, the temperature of the petroleum hydrocarbon liquid is instantaneously raised, the petroleum hydrocarbon liquid is cracked in a cracking area and the ethylene is separated out, so that the standing time in the reaction is short, and the per-pass yield of the ethylene is increased; in addition, the phenomenon of coking in the furnace can be avoided by utilizing the equipment.
Owner:SHENZHEN LK SCI&TECH
The preparation method of z-1,3,3,3-tetrafluoropropene
ActiveCN112811978BHigh productivity per passHigh activityHalogenated hydrocarbon preparationPtru catalystPropadiene
The invention discloses a preparation method of Z-1,3,3,3-tetrafluoropropene, which uses 1,3,3,3-tetrafluoropropyne or / and its isomer 1,1,3 ,3-tetrafluoropropadiene is used as a raw material, and Z-1,3,3,3-tetrafluoropropene is obtained through selective hydrogenation in the gas phase in the presence of a hydrogenation catalyst. The invention is mainly used for producing Z-1,3,3,3-tetrafluoropropene with high efficiency and continuous gas phase circulation.
Owner:泉州宇极新材料科技有限公司
Method for continuously preparing 3,3,3-trifluoro-2-(trifluoromethyl)-1-propene in gas phase
ActiveCN112794787BHigh productivity per passHigh activityPreparation by hydrogen halide split-offLiquid wastePtru catalyst
The invention discloses a method for continuously preparing 3,3,3-trifluoro-2-(trifluoromethyl)-1-propene in gas phase, which uses octafluoroisobutene as a raw material through hydrogenation-dehydrofluorination-hydrogenation-dehydrogenation Four-step gas-phase continuous reaction of hydrogen fluoride to obtain 3,3,3-trifluoro-2-(trifluoromethyl)-1-propene. Both the hydrogenation catalyst and the dehydrofluorination catalyst of the present invention have the characteristics of high activity and long service life; and through the gas phase independent circulation process, the incompletely reacted materials can be independently circulated, so that the initial raw materials can be almost completely converted3,3, 3‑trifluoro‑2‑(trifluoromethyl)‑1‑propene, and finally the product 3,3,3‑trifluoro‑2‑(trifluoromethyl) is extracted from the process system with high efficiency and continuous gas phase circulation )‑1‑propylene, so that no liquid waste and waste gas are produced, and green production is realized.
Owner:泉州宇极新材料科技有限公司
Method for continuously preparing 3, 3, 3-trifluoro-2-(trifluoromethyl)-1-propylene in gas phase
ActiveCN112794787AHigh productivity per passHigh activityPreparation by hydrogen halide split-offLiquid wastePtru catalyst
The invention discloses a method for continuously preparing 3, 3, 3-trifluoro-2-(trifluoromethyl)-1-propylene in a gas phase, and the method comprises the following steps: by taking octafluoroisobutylene as a raw material, carrying out hydrogenation-dehydrofluorination-hydrogenation-dehydrofluorination four-step gas phase continuous reaction to obtain the 3, 3, 3-trifluoro-2-(trifluoromethyl)-1-propylene. The hydrogenation catalyst and the dehydrofluorination catalyst have the characteristics of high activity and long service life; according to the present invention, by using the gas phase independent circulation process, the incomplete reaction material is subjected to independent circulation so as to completely convert the initial raw material into the 3, 3, 3-trifluoro-2-(trifluoromethyl)-1-propylene, and finally the product 3, 3, 3-trifluoro-2-(trifluoromethyl)-1-propylene is efficiently and continuously and circularly extracted from the process system in the gas phase; therefore, liquid waste and waste gas are not generated, and green production is realized.
Owner:泉州宇极新材料科技有限公司
Method for synthesizing fluorinated isobutene with hexafluoropropylene as starting material
ActiveCN112794788BHigh productivity per passHigh activityPreparation by hydrogen halide split-offPhysical/chemical process catalystsHydrogen fluoridePtru catalyst
The invention discloses a method for synthesizing fluorinated isobutene with hexafluoropropylene as a starting material, which comprises the following steps: (1) gas phase addition reaction: in the presence of a block catalyst, hexafluoropropylene and fluoromethane CHFXY(X , Y=F or H) undergoes gas phase addition to obtain (CF 3 ) 2 CFCHXY, (2) dehydrofluorination reaction: in the presence of dehydrofluorination catalyst, (CF 3 ) 2 CFCHXY is a raw material for dehydrofluorination reaction to obtain (CF 3 ) 2 C=CXY. Both the block catalyst and the dehydrofluorination catalyst in the invention have the characteristics of high activity and long service life, and can be used to produce fluorinated isobutene with high efficiency and continuous gas phase circulation.
Owner:泉州宇极新材料科技有限公司
A method for producing propylene from n-butene
ActiveCN104557397BHigh productivity per passMolecular sieve catalystsBulk chemical productionButeneHydrogen
The invention discloses a method for producing propylene from n-butene. Under the action of a catalyst, a raw material containing n-butene is subjected to cracking reaction under the reaction temperature of 500-600 DEG C, preferably 500-550 DEG C and the total reaction pressure (the absolute pressure) is 0-0.25MPa; the weight space velocity is 1-10h<-1>, preferably 1-5h<-1>; the catalyst comprises the following components in percentage by weight: 30-60 percent of a phosphorus-modified SAPO-11 molecular sieve, 20-30 percent of a hydrogen type ZSM-5 or ZSM-22 molecular sieve and the balance of an adhesive; the load of the phosphorus-modified SAPO-11 molecular sieve is 0.2-1 percent by weight (not including phosphorus in the SAPO-11 molecular sieve). The method used in the industrial process of producing propylene from the mixed carbon-4 raw material containing n-butene has the advantage of high once-through yield of propylene.
Owner:CHINA PETROLEUM & CHEM CORP +1
A kind of method that n-butene prepares propylene
ActiveCN106673945BLow reaction temperatureReduced activityMolecular sieve catalystsBulk chemical productionButeneMolecular sieve
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of 4-tertoctyl-2,6-dicumylphenol
InactiveCN105061157BSynthesis temperature is lowAvoid generatingOrganic chemistryOrganic compound preparationMetallic aluminumRare earth
The preparation method of 4-teroctyl-2,6-dicumylphenol relates to a preparation method of 4-teroctyl-2,6-dicumylphenol. The invention is to solve the problems of poor reaction selectivity and disproportionation at high temperatures in the existing 4-teroctyl-2,6-dicumylphenol production process. Method: 1. Add 4-teroctylphenol, metal aluminum and rare earth chloride into the reactor, heat the reaction until no more bubbles are released, then keep it warm, add ionic liquid, mix evenly to obtain the mixture; 2. Add the mixture to the mixture. Add α-methylstyrene dropwise, and after the addition of α-methylstyrene is completed, continue the reaction to obtain a reaction mixture; 3. Add dilute sulfuric acid to the reaction mixture, add organic solvent, separate the water layer, and directly cool to precipitate crystals , filter to obtain the crude product, and recrystallize it with ethanol-water mixed solvent to obtain 4-teroctyl-2,6-dicumylphenol. This method greatly improves the selectivity of 4-teroctyl-2,6-dicumylphenol. It effectively avoids the disproportionation of 4-terxinylphenol into phenol. Used in the preparation of 4-teroctyl-2,6-dicumylphenol.
Owner:HARBIN UNIV OF SCI & TECH
Method for simultaneously preparing dichlorohexafluorocyclopentene isomers
ActiveCN107082737BHigh productivity per passHigh activityPreparation by halogen replacementHydrogen fluorideCyclopentene
The invention relates to a method for simultaneously preparing dichlorohexafluorocyclopentene isomers. The method comprises the following steps: taking hydrogen fluoride and trichloropentafluorocyclopentene as reaction raw materials; carrying out gas-phase catalysis fluorine-chlorine exchange reaction in the presence of a fluorination catalyst to obtain the dichlorohexafluorocyclopentene isomers. By adopting the method provided by the invention, 1,2-dichlorohexafluorocyclopentene, 1,3-dichlorohexafluorocyclopentene and 1,4-dichlorohexafluorocyclopentene can be coproduced, wherein the single-pass yield of the 1,3-dichlorohexafluorocyclopentene and the 1,4-dichlorohexafluorocyclopentene is relatively high and the sum of the yield of the 1,3-dichlorohexafluorocyclopentene and the 1,4-dichlorohexafluorocyclopentene is greater than 57 percent; the raw materials are easy to obtain and the fluorination catalyst has stable activity, so that the method is suitable for simultaneously and continuously preparing the 1,2-dichlorohexafluorocyclopentene, the 1,3-dichlorohexafluorocyclopentene and the 1,4-dichlorohexafluorocyclopentene in a large scale in a gas phase.
Owner:BEIJING YUJI SCI & TECH +1
The preparation method of e-1,3,3,3-tetrafluoropropene
ActiveCN112811973BHigh productivity per passHigh activityCatalyst activation/preparationHalogenated hydrocarbon preparationPtru catalystCombinatorial chemistry
The invention discloses a preparation method of E-1,3,3,3-tetrafluoropropene, which uses 3,3,3-trifluoropropyne or / and its isomer 1,3,3-trifluoropropene Fluoropropadiene is used as a raw material, and E-1,3,3,3-tetrafluoropropene is obtained through gas-phase selective fluorination in the presence of a fluorination catalyst. The invention is mainly used for producing E-1,3,3,3-tetrafluoropropene with high efficiency and continuous gas phase circulation.
Owner:泉州宇极新材料科技有限公司
Method for preparing 3,3,3-trifluoropropyne by gas-phase dehydrohalogenation
ActiveCN113004117BHigh productivity per passHigh selectivityPreparation by hydrogen halide split-offCatalyst activation/preparationHydrogen halidePtru catalyst
The invention discloses a method for preparing 3,3,3-trifluoropropyne by gas-phase dehydrohalogenation. ‑Trifluoropropene (halogen = F or Cl or Br or I) as a raw material, in the presence of a catalyst, undergoes a gas phase dehydrohalogenation reaction to obtain 3,3,3‑trifluoropropyne. The invention is mainly used for producing 3,3,3-trifluoropropyne with conversion rate and high selectivity in gas phase continuous circulation.
Owner:泉州宇极新材料科技有限公司
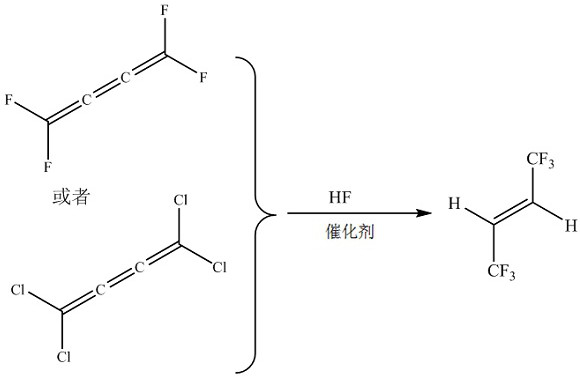
Method for preparing E-1, 1, 1, 4, 4, 4-hexafluoro-2-butene by gas phase fluorination
ActiveCN112723981AHigh productivity per passHigh activityOrganic chemistry methodsPreparation by halogen replacementHydrogen fluorideButene
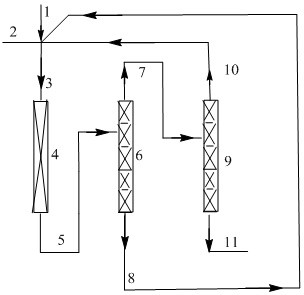
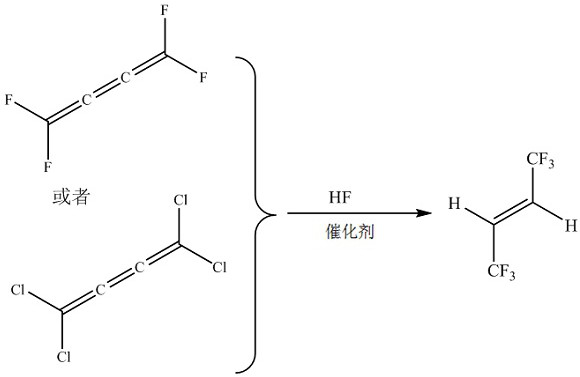
The invention discloses a method for preparing E-1, 1, 1, 4, 4, 4-hexafluoro-2-butene by gas-phase fluorination. The method comprises the following steps: in the presence of a fluorination catalyst, carrying out fluorination reaction on tetrafluorobutane or tetrachlorobutane and hydrogen fluoride to obtain the E-1, 1, 1, 4, 4, 4-hexafluoro-2-butene. The method is mainly used for efficiently and continuously producing E-1, 1, 1, 4, 4, 4-hexafluoro-2-butene in a circulating manner.
Owner:泉州宇极新材料科技有限公司
The preparation method of z-1-halo-3,3,3-trifluoropropene
ActiveCN112723983BHigh productivity per passGood choiceOrganic chemistry methodsPreparation by halogen replacementIsomerizationPtru catalyst
The invention discloses a preparation method of Z‑1‑halo‑3,3,3‑trifluoropropene. In the presence of a block catalyst, in a tubular reactor, E‑1‑halo‑3,3,3 -Trifluoropropene undergoes gas phase isomerization reaction to obtain Z-1-halo-3,3,3-trifluoropropene, wherein halogen=fluorine or chlorine, the present invention uses 1,1,1,3,3-pentachloro Propane is used as the starting material, and Z-1-chloro-3,3,3-trifluoropropene or Z-1,3,3,3-tetrafluoropropene are prepared by gas-phase fluorination reaction and isomerization reaction Products, the present invention independently circulates the incompletely reacted materials through the gas phase independent circulation process, so that the initial raw materials can be almost completely converted into the target product, and finally the target product is extracted from the process system, so that no liquid waste and waste gas are generated, and the realization of Green production.
Owner:泉州宇极新材料科技有限公司
Method for synthesizing 2, 3, 3, 3-tetrafluoro-2-(trifluoromethyl)propionitrile through gas-phase catalytic fluorination
PendingCN114085163AHigh productivity per passWon't happenPreparation by hydrocarbon ammoxidationMetal/metal-oxides/metal-hydroxide catalystsHydrogen fluoridePtru catalyst
The invention discloses a method for preparing 2, 3, 3, 3-tetrafluoro-2-(trifluoromethyl)propionitrile through gas-phase catalytic fluorination. In the presence of a fluorine-halogen exchange catalyst, 2-R1-3, 3, 3-trifluoro-2-(trifluoromethyl) propionitrile (R1 is H, Cl, Br or I) is used as a raw material and is subjected to gas-phase catalytic fluorination reaction with hydrogen fluoride and halogen simple substances to obtain 2, 3, 3, 3-tetrafluoro-2-(trifluoromethyl)propionitrile, and the raw material 2-R1-3, 3, 3- trifluoro-2-(trifluoromethyl)propionitrile can be synthesized through a series of gas phase reactions by taking octafluoroisobutylene or saturated or unsaturated nitrile as an initial raw material. The method is mainly used for preparing the 2, 3, 3, 3-tetrafluoro-2-(trifluoromethyl)propionitrile in a gas phase manner. The preparation method has the advantages of short route, high single-pass yield, high catalyst activity and no generation of liquid waste.
Owner:CHEM & CHEM ENG GUANGDONG LAB +1
Method for preparing e-1,1,1,4,4,4-hexafluoro-2-butene by gas phase fluorination
ActiveCN112723981BHigh productivity per passHigh activityOrganic chemistry methodsPreparation by halogen replacementHydrogen fluorideButene
The invention discloses a method for preparing E-1,1,1,4,4,4-hexafluoro-2-butene by gas-phase fluorination, namely: in the presence of a fluorination catalyst, tetrafluorobutatriene or tetrafluorobutatriene Chloroprene, fluorinated with hydrogen fluoride to give E-1,1,1,4,4,4-hexafluoro-2-butene. The invention is mainly used for producing E-1,1,1,4,4,4-hexafluoro-2-butene with high efficiency and continuous circulation.
Owner:泉州宇极新材料科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com