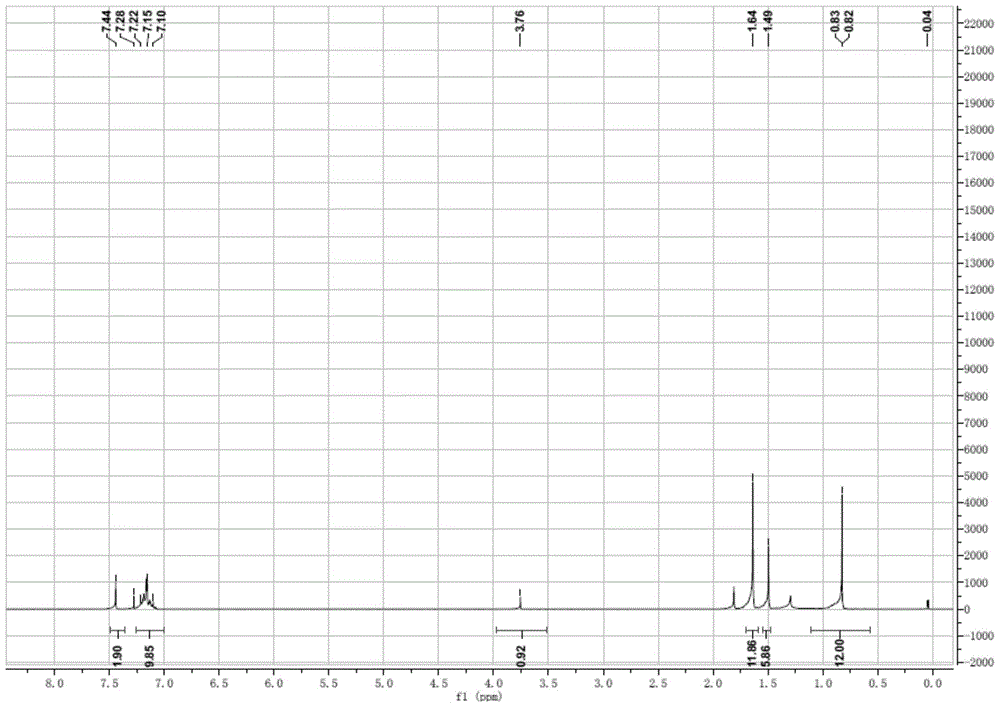
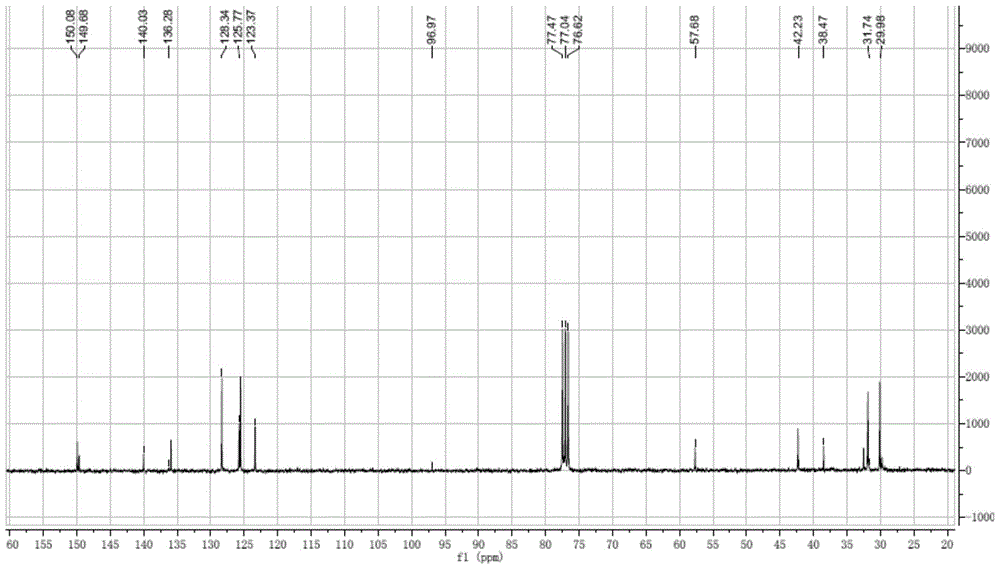
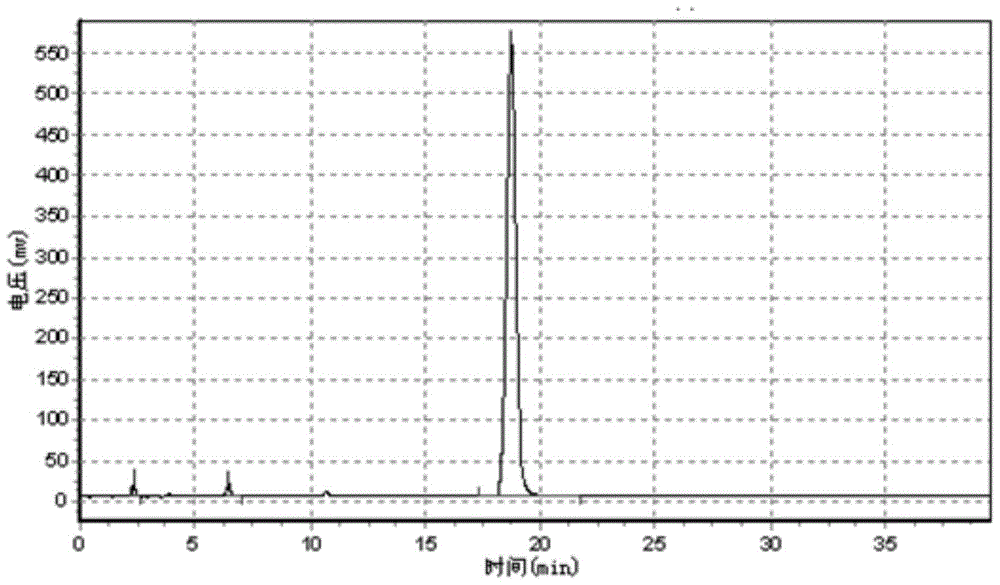
Preparation method of 4-tertoctyl-2,6-dicumylphenol
A technology of tertoctylphenol and cumylphenol, applied in the field of preparation of 4-tertoctyl-2,6-dicumylphenol, which can solve the problems of disproportionation and poor reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0024] Specific embodiment 1: The preparation method of 4-tertoctyl-2,6-dicumylphenol in this embodiment is carried out according to the following steps:
[0025] 1. Preparation of composite catalyst: Add 4-tetraoctylphenol, metal aluminum and rare earth chloride into the reactor, and under the protection of nitrogen, heat the reaction system to 110-140°C for reaction, until no more bubbles are released. Keep warm for 0.4-0.6h, then add ionic liquid, mix evenly to get the mixture of 4-tertoctylphenol and composite catalyst;
[0026] 2. Alkylation reaction: Add α-methylstyrene dropwise to the mixture of 4-tertoctylphenol and composite catalyst, control the rate of addition to keep the system temperature at 100-180°C, wait for α-methylstyrene to drop After the addition was completed, the reaction was continued for 0.8 to 1.2 hours to obtain a reaction mixture;
[0027] 3. Product refining: add dilute sulfuric acid with a mass concentration of 10% to 30% to the reaction mixture ...
specific Embodiment approach 2
[0029] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the composite catalyst in the mixture of 4-tertoctylphenol and composite catalyst obtained in step one is 4-tertoctylphenol aluminum, ionic liquid and chlorinated Rare earth composite catalyst. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0030] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: in step one, the ionic liquid is tetrafluoroborate or p-toluenesulfonate of alkylimidazole, and its general formula is:
[0031]
[0032] R=C 2 h 5 , n-C 4 h 9
[0033]
[0034] Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com