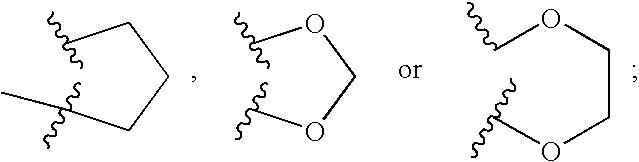
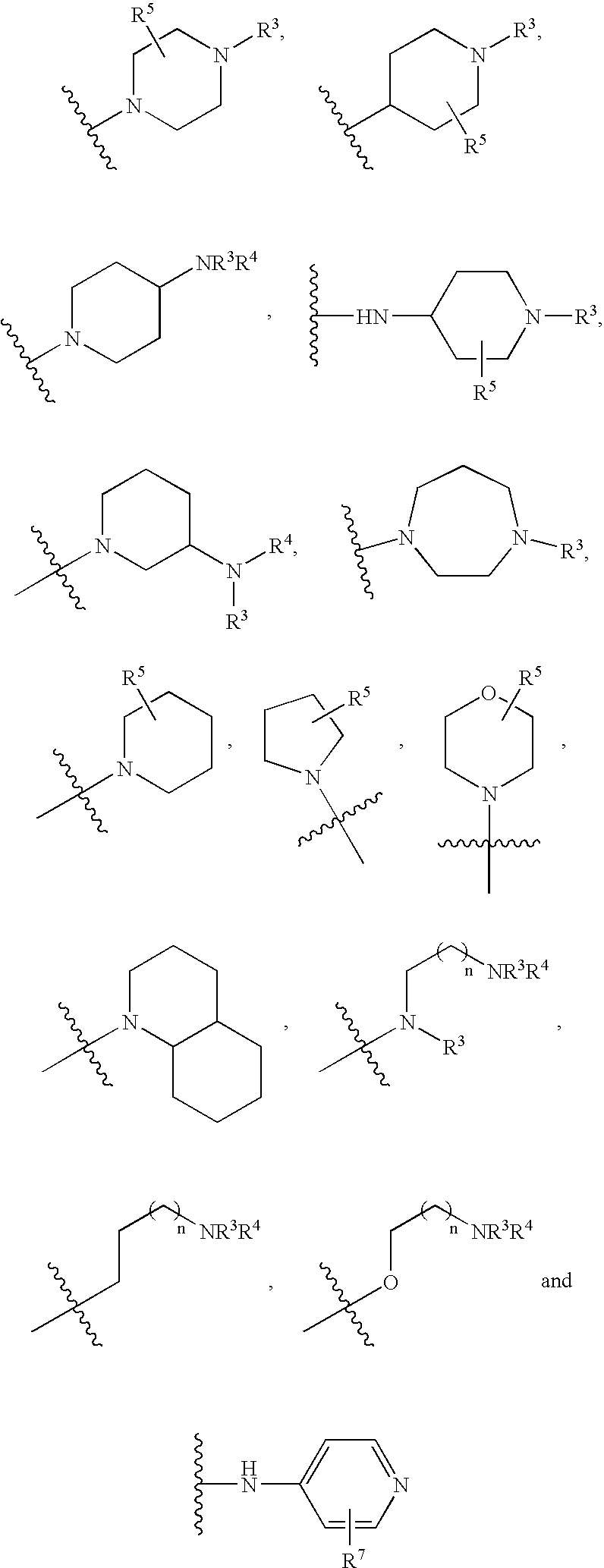
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Dopamine receptor antagonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions of 5-ht3 antagonists and dopamine d2 antagonists for treatment of dopamine-associated chronic conditions
InactiveUS20080004291A1Relieve distressPoor quality of lifeBiocideOrganic active ingredientsDisease5-HT3 antagonist
The present invention provides novel compositions comprising a combination of a 5-HT3 receptor antagonist and a selective dopamine D2 receptor antagonist for the treatment of obsessive, impulsive and compulsive behavioral activities and other dopamine pathway-associated disorders or conditions. Preferably, the pharmaceutical compositions of the present invention comprise amounts of the 5-HT3 receptor antagonist ondansetron and a selective dopamine D2 receptor antagonist, such as risperidone or olanzapine, that are sufficient to control a subjects obsessive, impulsive and compulsive behavioral activities. Kits comprising the combination of antagonists for the treatment of addictive disorders such as alcohol dependence are also provided.
Owner:TRANSCEPT PHARMA
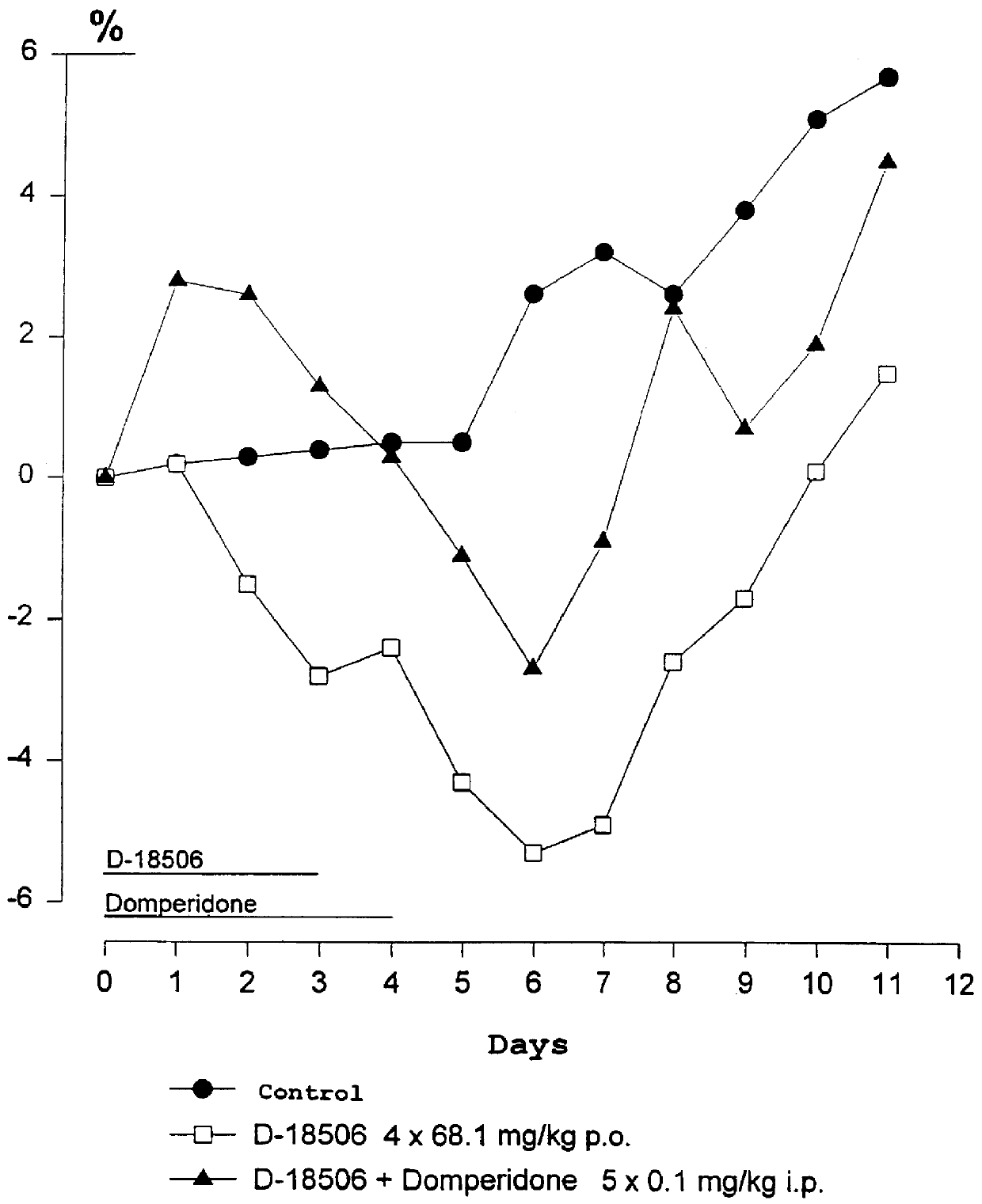
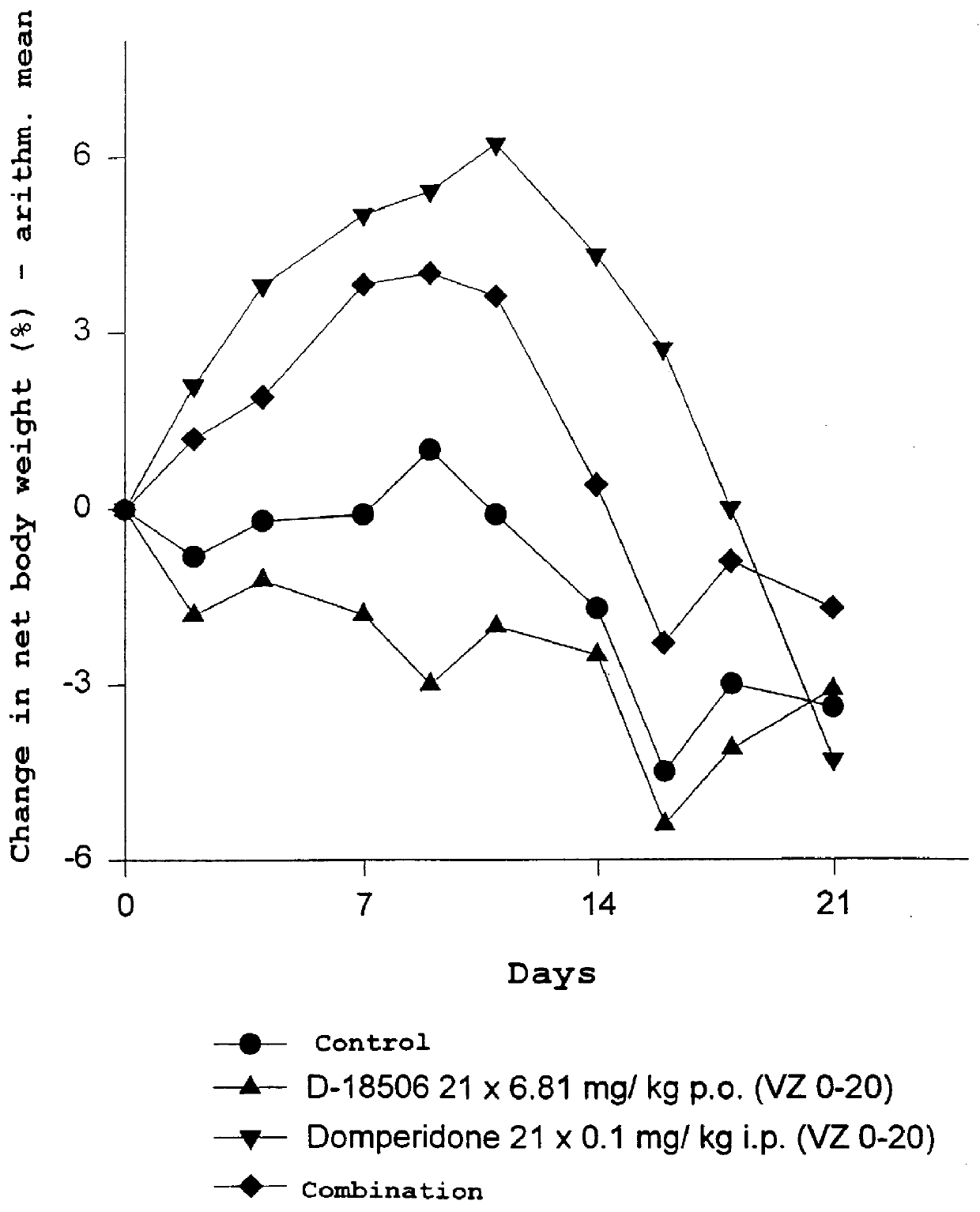
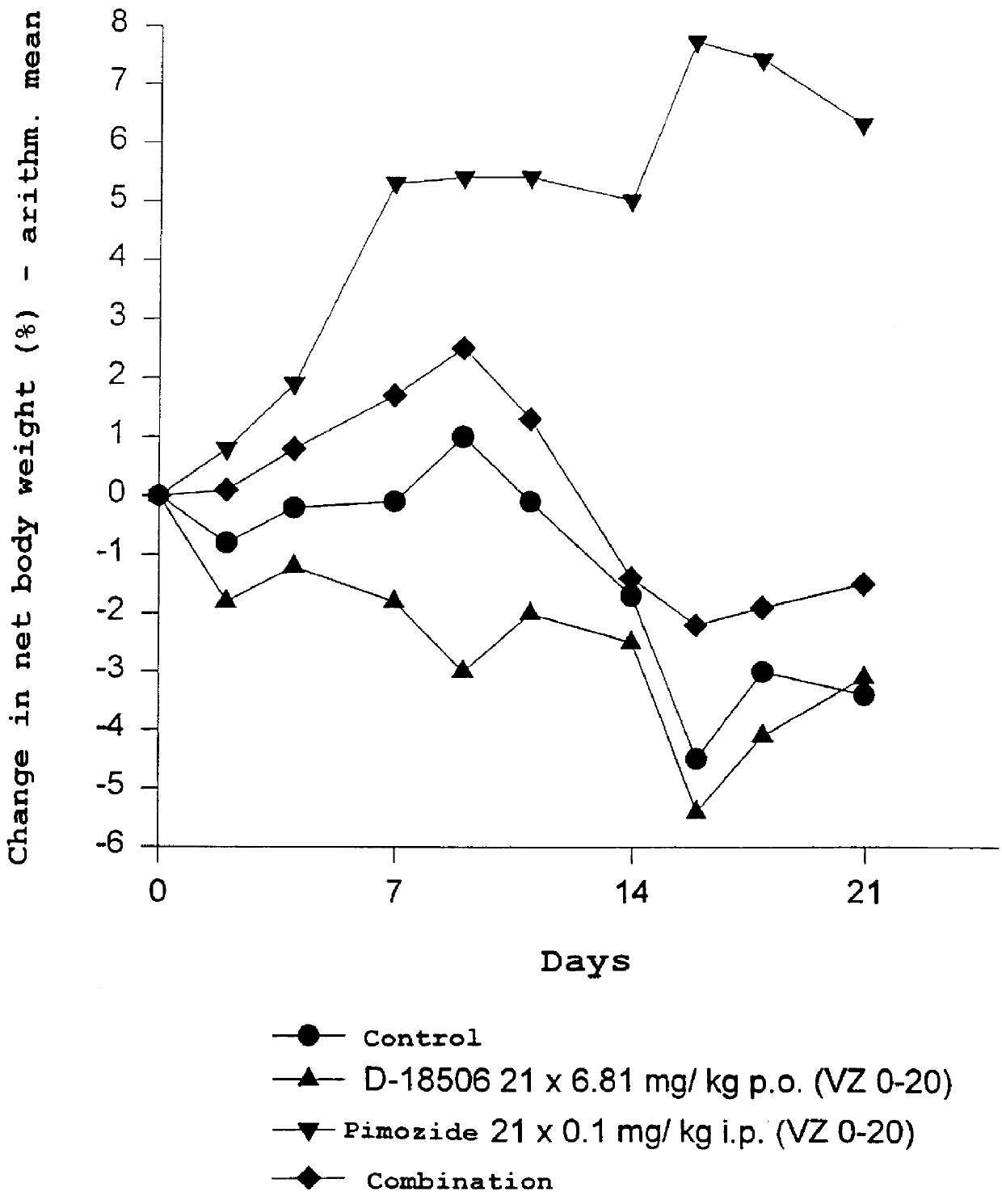
Use of dopamine receptor antagonists in palliative tumor therapy
The side effect of decrease in body weight caused by the alkylphosphocholines such as miltefosine can be antagonized by certain acetylcholine receptor antagonists such as domperidone and pimozide. The combination of alkylphosphocholine plus the antagonist does not have any effect on the anti-tumor action of the alkylphosphocholine. The combination also caused no new side effects in the animals.
Owner:AETERNA ZENTARIS GMBH
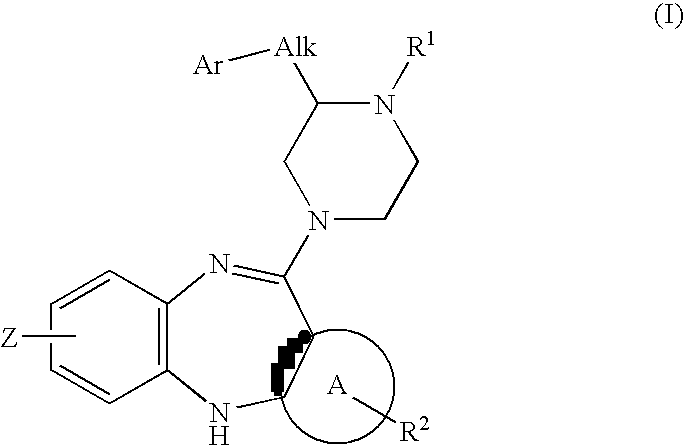
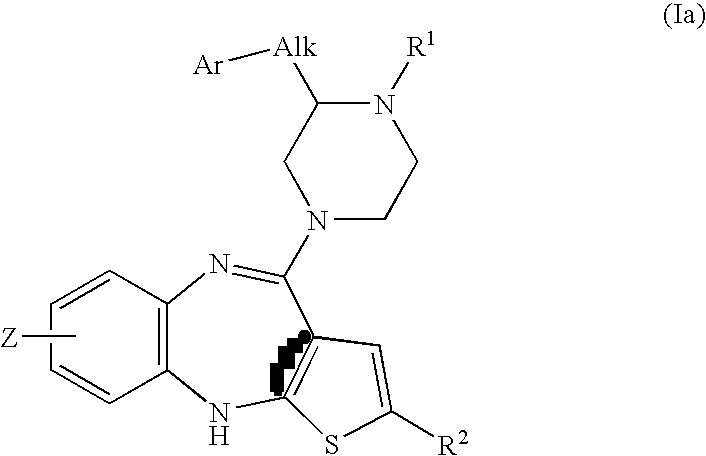
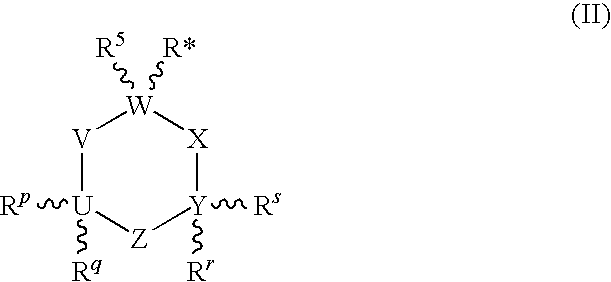
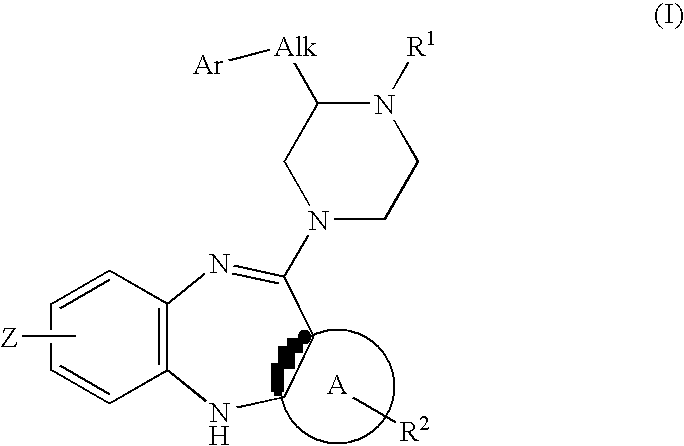
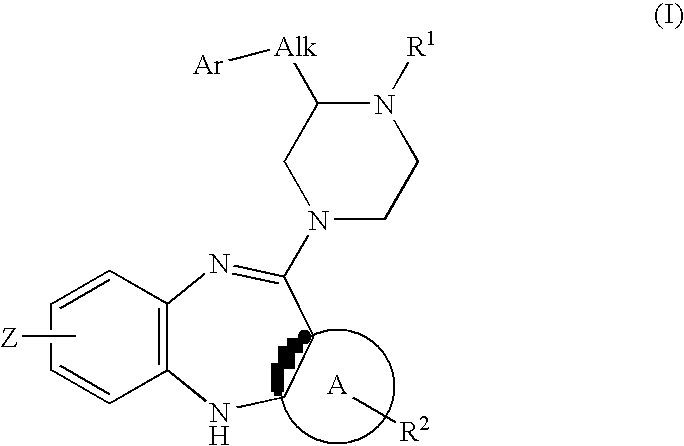
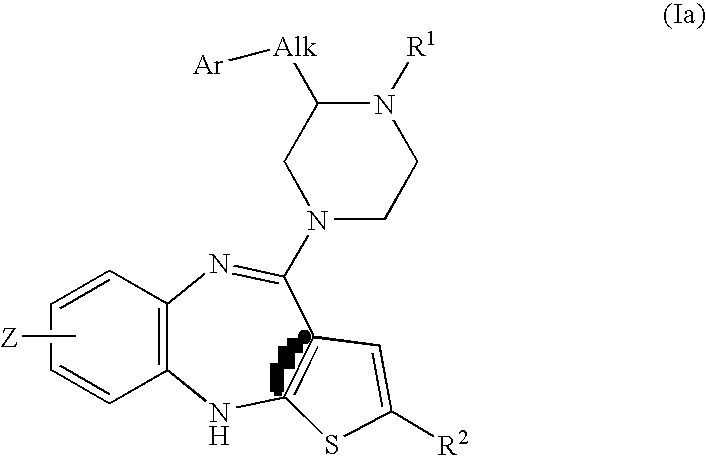
Piperazine substituted aryl benzodiazepines and their use as dopamine receptor antagonists for the treatment of psychotic disorders
Owner:ELI LILLY & CO
Domperidone oral disintegrating tablet and preparation process thereof
InactiveCN1449757AAvoid duplication of investmentSimple preparation processOrganic active ingredientsDigestive systemCelluloseCross-link
The present invention provides a domperiodone oral disitegrant tablet preparation. Its composition includes medicine active component, filling agent, corrective, disitegrant, lubricating agent and glidant. Its medicin active component includes dopamine receptor antagonist and gastroprokinetic, and its disitegrant agent is selected from cross-linked polyvinylpyrrolidone, carboxymethyl starch sodium and microcrystal cellulose or their mixture, and corrective is selected from aspartame. Said invention adopts direct addition of disitegrant in theprescription so as to attain the effect of quick disitegration. Its preparation process is simple, and it is convenient for taking in.
Owner:江西省药物研究所
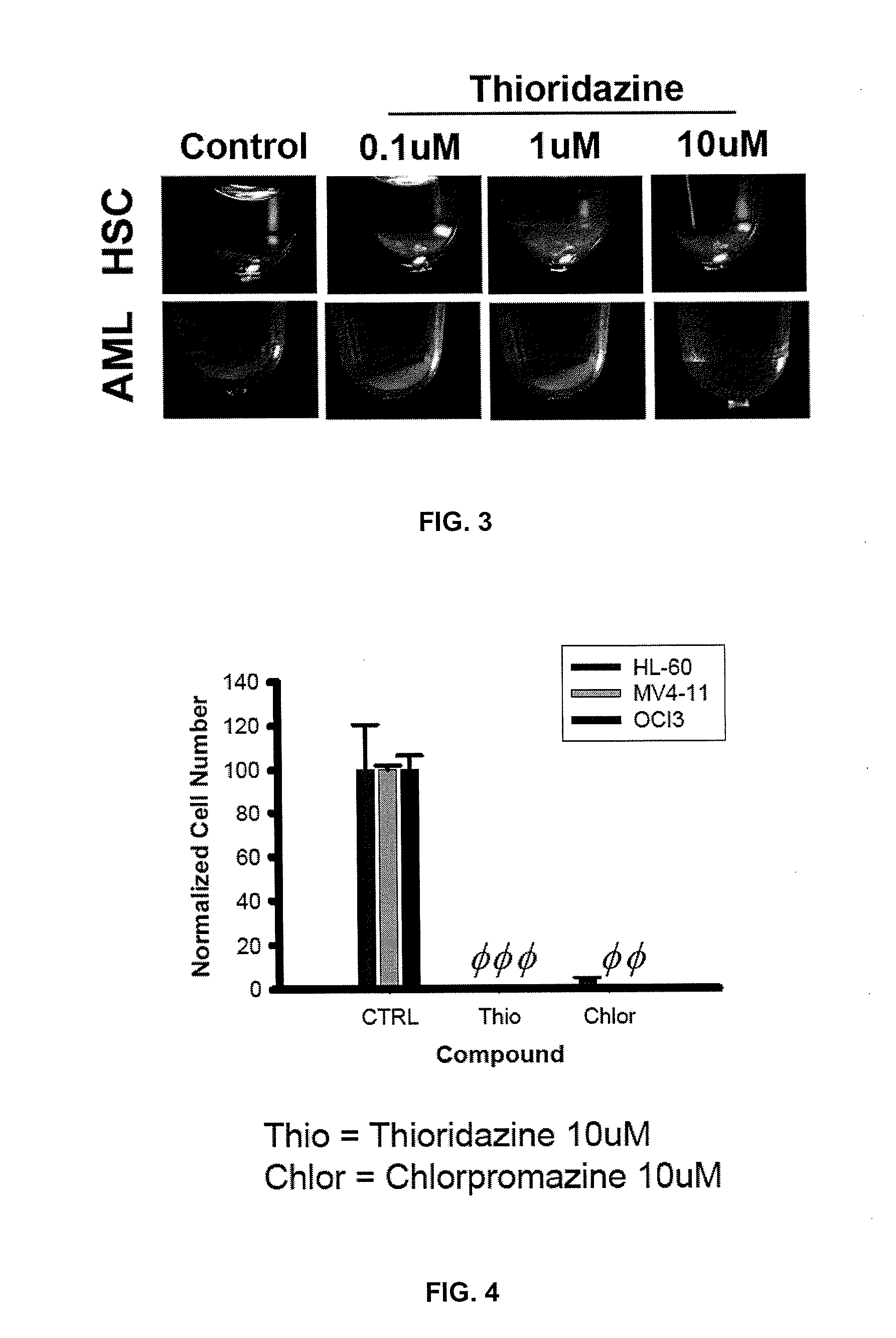
Treatment of Cancer WIth Dopamine Receptor Antagonists
InactiveUS20130331381A1Reduced activityReduce spreadCompound screeningApoptosis detectionMyeloid leukemiaThioridazine
Described are methods of treating a cancer comprising administering to a subject in need thereof an effective amount of a dopamine receptor (DR) antagonist. The DR antagonist may be a phenothiazine derivative, such as thioridazine or chlorpromazine. Optionally, the cancer is acute myeloid leukemia. Also described are methods for identifying subjects with cancer, methods for providing a prognosis for a subjects with cancer and / or subjects likely to be responsive to therapy with DR receptor antagonists. Methods for identifying cancer stem cells and chemotherapeutic compounds that are DR receptor antagonists as also provided.
Owner:MCMASTER UNIV
Methods of treating CNS disorders
The present invention relates to methods of treating various CNS disorders, e.g., mania, bipolar disorder and schizophrenia, by administering NMDA receptor antagonists, alone or in combination with dopamine receptor antagonists.
Owner:FOREST LAB HLDG LTD
Muscarinic receptor antagonists
The present invention relates generally to muscarinic receptor antagonist, which are useful, among other uses, for the treatment of various diseases of the respiratory, urinary and gastrointestinal systems mediated through muscarinic receptors. The invention also relates to the process for the preparation of disclosed compounds, pharmaceutical compositions containing the disclosed compounds and the method for treating diseases mediated through muscarinic receptors. Also provided herein are pharmaceutical composition comprising one or more muscarinic receptor antagonists and at least one other active ingredients include, but are not limited to, corticosteroids, beta agonists, leukotriene antagonists, 5-lipoxygenase inhibitors, anti-histamines, antitussives, dopamine receptor antagonists, chemokine inhibitors, p38 MAP Kinase inhibitors, and PDE-IV inhibitors.
Owner:RANBAXY LAB LTD
Piperazine substituted aryl benzodiazepines and their use as dopamine receptor antagonists for the treatment of psychotic disorders
Described herein are antipyschotic compounds of formula (I) wherein, A is an optionally benzo-fused five or six member aromatic ring having zero to three hetero atoms independently selected from N, O, and S; Alk is (C1-4) alkylene optionally substituted with OH, methoxy, ethoxy, or F; Ar is optionally substituted phenyl, naphthyl, monocyclic heteroaromatic, or bicyclic heteroaromatic; R1 is hydrogen or (C1-4) alkyl optionally substituted with OH, OR3, or OCH2CH2OH, wherein R3 is (C1-2) alkyl; R2 is H, (C1-6) alkyl, halogen, fluorinated (C1-6) alkyl, OR4, SR4, NO2, CN, COR4, CONR5R6, SO2NR5R6, NR5R6, NR5COR4, NR5SO2R4, or optionally substituted phenyl, wherein R4 is hydrogen, (C1-6) alkyl, fluorinated (C1-6) alkyl, benzyl, or optionally substituted phenyl, R5 and R6 are independently hydrogen, (C1-6) alkyl, or optionally substituted phenyl; Z is one or two substituents independently selected from hydrogen, halogen, (C1-6) alkyl, fluorinated (C1-6) alkyl, OR7, SR7, NO2, CN, COR7, CONR8R9, SO2NR8R9, NR8SO2R7, NR8R9, or optionally substituted phenyl, wherein R7 is hydrogen, (C1-6) alkyl, fluorinated alkyl, benzyl, or optionally substituted phenyl, R8 and R9 are independently hydrogen, (C1-6) alkyl, or optionally substituted phenyl; and salts, solvates, and crystal forms thereof. Also described are the use of the compounds of formula (a) as antagonists of the dopamine D2 receptor and as agents for the treatment of psychosis and bipolar disorders, and pharmaceutical formulations of the compounds of formula (I). Also described are compounds useful as intermediates for the synthesis of the compounds of formula (I).
Owner:ELI LILLY & CO
Dopamine d3 receptor antagonists having a bicyclo moiety
ActiveUS20180222918A1Organic active ingredientsNervous disorderAmine receptorDopamine receptor antagonist
Owner:INDIVIOR UK
Anti-Emetic Substance
InactiveUS20120101089A1Effective controlPatient compliance is goodDigestive systemHeterocyclic compound active ingredientsDopamine receptor antagonistDrug
The present invention provides a therapeutic solution for effective control of symptoms related to nausea and vomiting. The therapeutic solution is a pharmaceutical composition which combines anti-emetics of different classes. These classes include dopamine receptor antagonists, serotonin receptor antagonists, butyrphenones, and neurokinin receptor antagonists. The combination of different anti-emetics mitigates adverse affects of a single anti-emetic alone while increasing drug efficacy and decreasing cost of administration to the individual patient.
Owner:AGARWAL ASHWANI +1
Piperazine substituted aryl benzodiazepines
Described herein are compounds of formula (I) wherein: is an optionally benzo-fused five or six member aromatic ring having zero to three hetero atoms independently selected from N, S, and O; Alk is (C1-4) alkylene or hydroxy substituted (C1-4) alkylene; X is oxygen or sulfur; R1 is hydrogen, (C1-6) fluroalkyl, (C3-6) cycloalkyl, or (C1-4) alkyl, wherein the (C1-4) alkyl is unsubstituted or substituted with hydroxy, methoxy, ethoxy, OCH2CH2OH, or —CN; R2 is H, halogen, (C1-6) fluoroalkyl, (C1-6) cycloalkyl, OR4, SR4, NO2, CN, COR4, C(O)OR4, CONR5R6, NR5R6, SO2NR5R6, NR5COR4, NR5SO2R4, optionally substituted aromatic, or (C1-6) alkyl, wherein (C1-6) alkyl is unsubstituted or substituted with a hydroxy group; R3 is hydrogen (C1-6) fluoroalkyl, (C2-6) alkenyl, Ar, (C1-4)alkyl-Ar, or (C1-4) alkyl wherein (C1-4) alkyly is unsubstituted or substituted with a phenyl; R4 is hydrogen, (C1-6) alkyl, (C1-6) fluoroalkyl, or optionally substituted aromatic; R5 and R6 are independently hydrogen, (C1-6) alkyl, or optionally substituted aromatic, R7 is hydrogen, (C1-6) alkyl, (C1-6) fluoroalkyl, or optionally substituted aromatic; R8 and R9 are independently hydrogen, (C1-6) alkyl, or optionally substituted aromatic; Ar is optionally substituted phenyl, napthyl, monocyclic heteroaromatic or bicyclic heteroaromatic; Z1 and Z2 are independently selected from hydrogen, halogen, (C1-6) alkyl, (C1-6) fluoroalkyl, OR7, SR7, NO2, CN, COR7, CONR8R9, NR8R9, and optionally substituted aromatic; and all salts, solvates, optical and geometric isomers, and crystalline forms thereof. Also, described are the use of the compounds of formula (I) as antagonists of the dopamine D2 receptor and as agents for the treatment of psychosis and bipolar disorders, and pharmaceutical formulations of the compounds of formula (I)
Owner:ELI LILLY & CO
Haloperidol analogs
InactiveUS20060052363A1Symptoms improvedEffective treatmentBiocideNervous disorderReceptor subtypeCompound (substance)
Haloperidol analogs that conforms to the structural formulae: wherein: R is H, or —(CH2)n—OH, n is an integer from 0 to 2, and A is a heterocyclic bridging group, consisting essentially of carbon and at least one nitrogen atom, which effectively maintains the distance between the moieties connected thereby such that the compound (1) is incapable of metabolizing to BCPP+ like species, (2) has an affinity for the D2 receptor subtype of 15<D2<250 and (3) functions as a dopamine receptor antagonist, or the structural formulae: wherein: R1 is H, or —(CH2)n—OH, n is an integer from 0 to 2, B is an aza- or diaza-bicyclo group, which effectively maintains the distance between the moieties connected thereby such that the compound is incapable of metabolizing to BCPP+ like species; and Z is —CH— or N; and pharmaceutically acceptable salts, esters, derivatives, metal complexes, conjugates and prodrugs thereof.
Owner:FLORIDA A&M UNIVERSITY
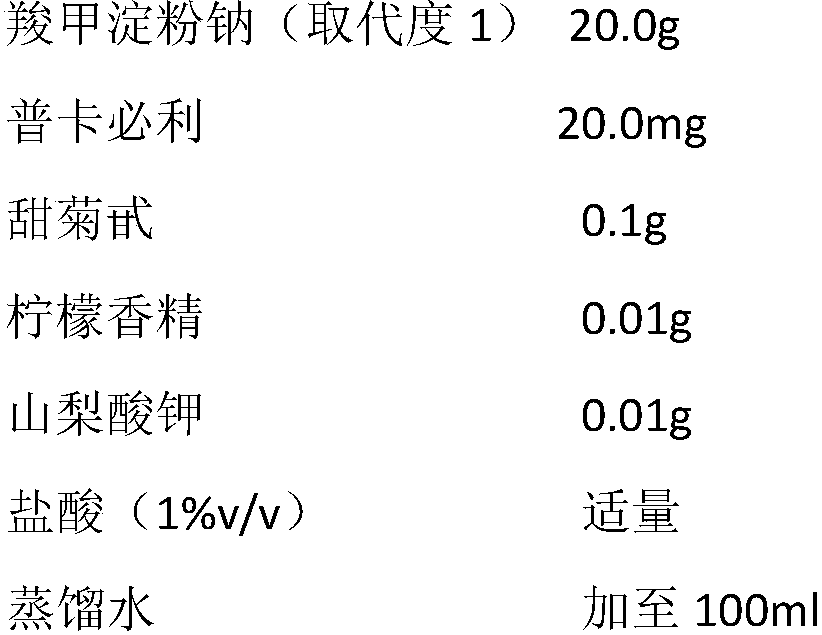
Pharmaceutical composition for treating constipation and application thereof
InactiveCN108904808AIncrease the average daily defecation volumeIncrease the number ofElcosanoid active ingredientsPeptide/protein ingredientsOral suspensionsMotility
The invention discloses the combined application of a pharmaceutical composition composed of water-soluble starch salt modified by carboxylic acid groups and gastrointestinal motility promoting drugsor secretion promoting drugs in the prevention and treatment of constipation, the pharmaceutical composition can be suitable for various functional constipation, especially has good curative effects on old age refractory constipation, chronic constipation, habitual constipation, drug-induced constipation and the like, has slight adverse reactions, and can be suitable for various crowds. The representative drug of the water-soluble starch salt modified by the carboxylic acid groups is sodium carboxymethyl starch, and the gastrointestinal motility promoting drugs or secretion promoting drugs areselected from dopamine receptor antagonist, 5-HT4 receptor agonist, motilin receptor agonist, chloridion channel activator, guanylate cyclase agonist. The two components are combined according to a suitable weight ratio and can be prepared into any oral pharmaceutical dosage form including oral solution, oral suspension, granule, powder, tablet, capsule and the like.
Owner:XIAN LIBANG PHARMA TECH
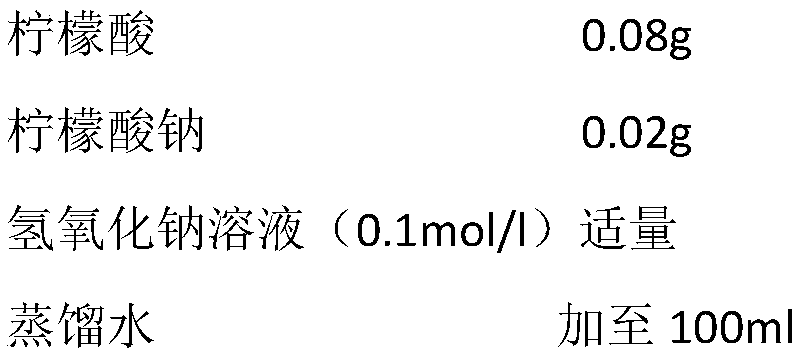
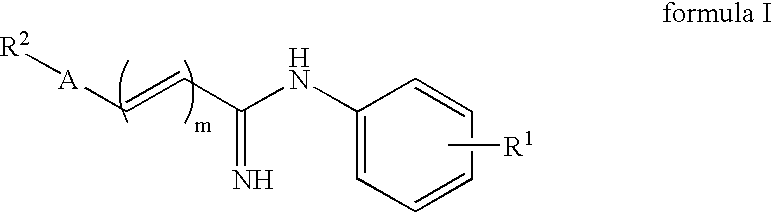
Substituted n-aryl amidines as selective D1 dopamine receptor antagonists for the treatment of obesity and CNS disorders
The present invention provides compounds, which, are novel antagonists for D1 receptors as well as methods for preparing such compounds. In another embodiment, the invention provides pharmaceutical compositions comprising such D1 receptor antagonists as well as methods of using them to treat CNS disorders, obesity, metabolic disorders, eating disorders such as hyperphagia, and diabetes.
Owner:MERCK SHARP & DOHME CORP
Methods of treating CNS disorders
The present invention relates to methods of treating various CNS disorders, e.g., mania, bipolar disorder and schizophrenia, by administering NMDA receptor antagonists, alone or in combination with dopamine receptor antagonists.
Owner:PAPADAKIS KELLY +2
Benzoazepine compound, preparation method and application thereof
InactiveCN104031042ANervous disorderOrganic chemistryPharmaceutical drugDopamine receptor antagonist
Belonging to the field of pharmaceutical chemistry, the invention relates to a benzoazepine compound, a preparation method and application thereof, in particular to the benzoazepine compound, its preparation method and application in the field of treatment of nervous system diseases, especially application in the field of drug addiction related to dopamine D1 and D3 receptors. The benzoazepine compound and pharmacologically acceptable inorganic or organic salts thereof have a structure shown as formula (I). Results of drug experiments carried out by the invention show that the benzoazepine compound and its pharmacologically acceptable inorganic or organic salts have high antagonistic activity on dopamine D1 and D3, can be used as drug leads for further development of dopamine receptor antagonists with good selectivity and high activity, and can be used as potential drugs for treatment of drug addiction by dopamine D1, D3 receptor antagonists. (formula (I)).
Owner:FUDAN UNIV
Synergy of dopamine d2 and adenosine a2 receptors activates pka signaling via beta/gamma dimers
InactiveUS20090137662A1Easy to addImprove stabilityBiocideMicrobiological testing/measurementDopamine receptor antagonistPsychiatry
This invention pertains to the discovery that a dopamine receptor agonist can activate PKA signaling and / or can act synergistically with an adenosine receptor to activate such signaling. In various embodiments, this invention exploits the synergy between the dopamine receptor pathway and an adenosine receptor pathway to provide methods of mitigating one or more symptoms produced by the chronic consumption of a substance of abuse or to mitigate one or more physiological and / or behavioral symptoms associated with cessation of chronic consumption of a substance of abuse. In certain embodiments, the methods involve administering to the mammal an effective amount of an adenosine receptor antagonist; and an effective amount of a dopamine receptor antagonist; where the effective amount of the adenosine receptor antagonist is lower than the effective amount of an adenosine receptor antagonist administered without said dopamine receptor antagonist.
Owner:RGT UNIV OF CALIFORNIA
Co-Administration of Dopamine-Receptor Binding Compounds
Methods for treating a patient having neurological, psychotic, and psychiatric disorders are described comprising the steps of administering to the patient an effective amount of a partial and / or full dopamine D1 receptor agonist, and administering to the patient an effective amount of a dopamine D2 receptor antagonist, Pharmaceutical compositions comprising a dopamine D1 receptor agonist and a dopamine D2 receptor antagonist are also described. The D1 dopamine receptor agonist and the D2 dopamine receptor antagonist can be administered to the patient in the same or in a different composition or compositions.
Owner:DARPHARMA INC
Use of dopamine receptor antagonists in palliative tumor therapy
The side effect of decrease in body weight caused by the alkylphosphocholines such as miltefosine can be antagonized by certain acetylcholine receptor antagonists such as domperidone and pimozide. The combination of alkylphosphocholine plus the antagonist does not have any effect on the anti-tumor action of the alkylphosphocholine. The combination also caused no new side effects in the animals.
Owner:AETERNA ZENTARIS GMBH
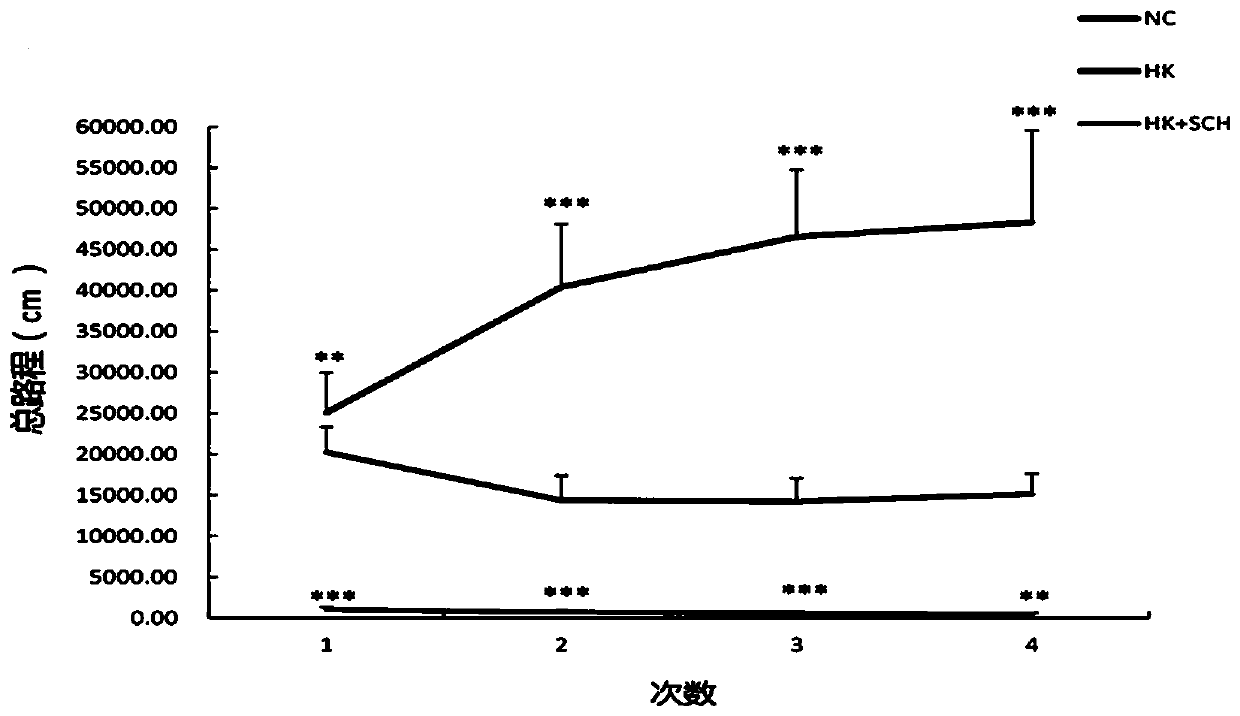
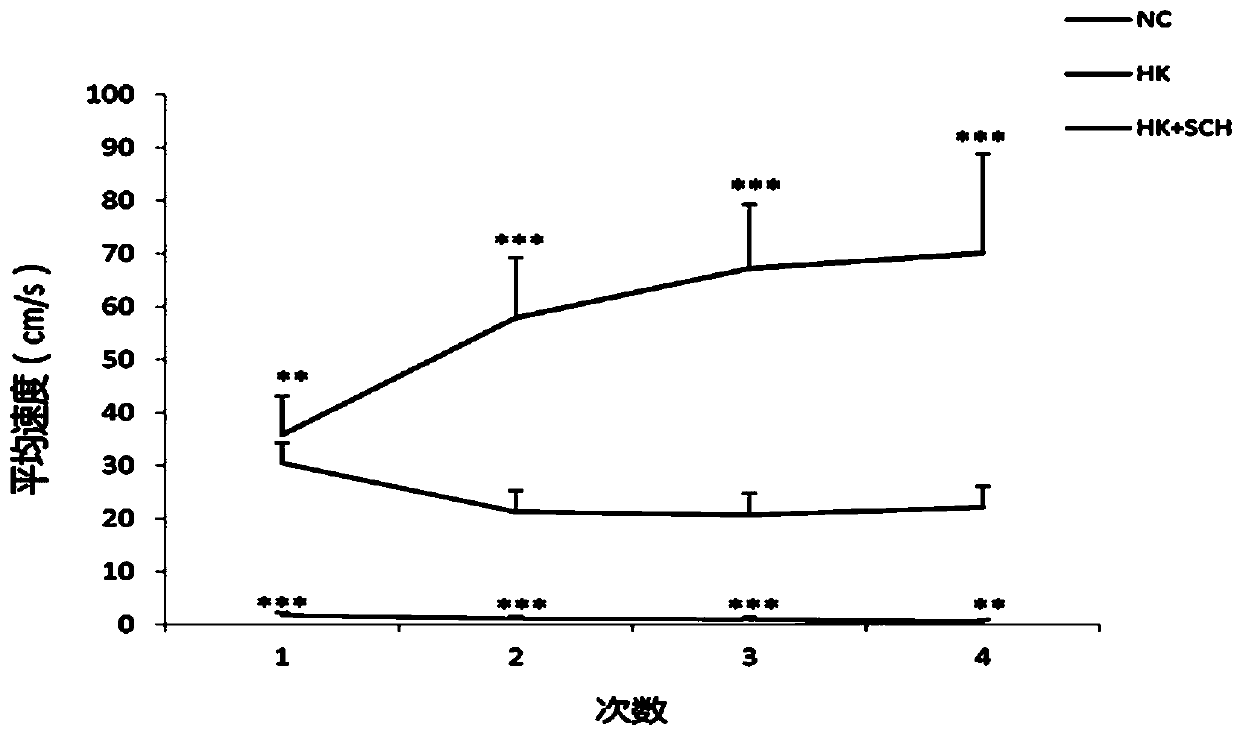
Application of dopamine receptor 1 antagonist in preparing drugs for treatment of ketamine-induced schizoid symptoms in mice
InactiveCN110833621ANervous disorderHeterocyclic compound active ingredientsPharmaceutical drugAmine receptor
The invention belongs to the technical field of biomedicine and particularly relates to a novel pharmaceutical use of a dopamine receptor 1 antagonist, and to a novel use of the dopamine receptor 1 antagonist in preparing drugs for the treatment of ketamine-induced schizoid symptoms in mice. The dopamine receptor 1 antagonist is selected from SCH23390 having a molecular formula of C17H18ClNO.HCl,and is one or only one effective ingredient to the drugs. A drug for the treatment of ketamine-induced schizoid symptoms in mice comprises the dopamine receptor 1 antagonist having the effective amount (5 mg / kg). The dopamine receptor 1 antagonist is used as one means to treat ketamine-induced schizoid behaviors of mice, and scientific basis is provided for the research and development of a methodto effectively relieve schizoid symptoms in mice.
Owner:中国医科大学
Combination medicine for treatment of depression
InactiveUS20140107104A1Convenient treatmentImprove actionBiocideNervous disorderPharmaceutical drugAgonist
The present invention provides a combination medicine for treatment of depression, comprising a combination of (A1) an antidepressant and either (B1) a dopamine D1 receptor agonist or (C1) a dopamine D1 receptor antagonist; and a method for screening for an antidepressant that in combination with a dopamine D1 receptor agonist provides an improvement in treatment of depression, the method comprising the steps of: administering, to a mammal, (A2) a compound having an antidepressant action and (B1) a dopamine D1 receptor agonist, and detecting a greater increase in depression-related gene expression, dopamine D1 receptor expression and / or dopamine D1 receptor signaling in comparison with the case where (A2) the compound having an antidepressant action or (B1) the dopamine D1 receptor agonist is administered.
Owner:NIPPON MEDICAL SCHOOL FOUND +3
Application of dopamine receptor antagonist chlorprothixene in treatment of acute myeloid leukemia
ActiveCN110585197ADoes not affect normal physiological stateGrowth inhibitionOrganic active ingredientsAntineoplastic agentsApoptosisAlternative strategy
The invention discloses an application of a dopamine receptor antagonist chlorprothixene in treatment of acute myeloid leukemia, develops a new application of the dopamine receptor antagonist chlorprothixene as a drug, also provides a new way for treating acute myeloid leukemia, and uses the action mechanism between different fusion proteins and the existing APL theoretical model as support. Related in-vitro cell experiments and in-vivo animal experiments prove that: the chlorprothixene can induce cell apoptosis and autophagy pathways to promote death of AML cells, and can induce the decreaseof the expression quantity of fusion proteins PML-RARA and AML1-ETO, so that the chlorprothixene is a potential drug for inducing AML cell death, and provides a new visual angle for exploring alternative strategies of various AML subtype treatments.
Owner:SHANGHAI JIAO TONG UNIV +1
Use of chromone derivatives as dopamine d3 receptor antagonists for the treatment of autism spectrum disorders
Owner:PIERRE FABRE MEDICAMENT SAS
Co-administration of dopamine-receptor binding compounds
Methods for treating a patient having neurological, psychotic, and psychiatric disorders are described comprising the steps of administering to the patient an effective amount of a partial and / or full dopamine D1 receptor agonist, and administering to the patient an effective amount of a dopamine D2 receptor antagonist. Pharmaceutical compositions comprising a dopamine D1 receptor agonist and a dopamine D2 receptor antagonist are also described. The D1 dopamine receptor agonist and the D2 dopamine receptor antagonist can be administered to the patient in the same or in a different composition or compositions.
Owner:DARPHARMA INC
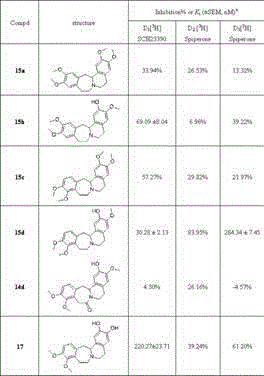
Dopamine D1 receptor antagonist and application thereof
The invention provides a dopamine D1 receptor antagonist and an application thereof, and relates to the field of medicinal chemistry. The invention relates to the field of medicine, in particular to adopamine D1 receptor antagonist shown in a structural formula (I), pharmaceutically acceptable salt of the dopamine D1 receptor antagonist, a pharmaceutical composition containing the dopamine D1 receptor antagonist, and an application of a compound medicine to preparation of medicines for treating or preventing related diseases or symptoms caused by high expression or over-high activity of a dopamine D1 receptor.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Dopamine receptor antagonist and application thereof
ActiveCN111184719AInhibition of cellular calcium ion influxGood analgesic effectOrganic active ingredientsNervous disorderAmine receptorDopamine receptor antagonist
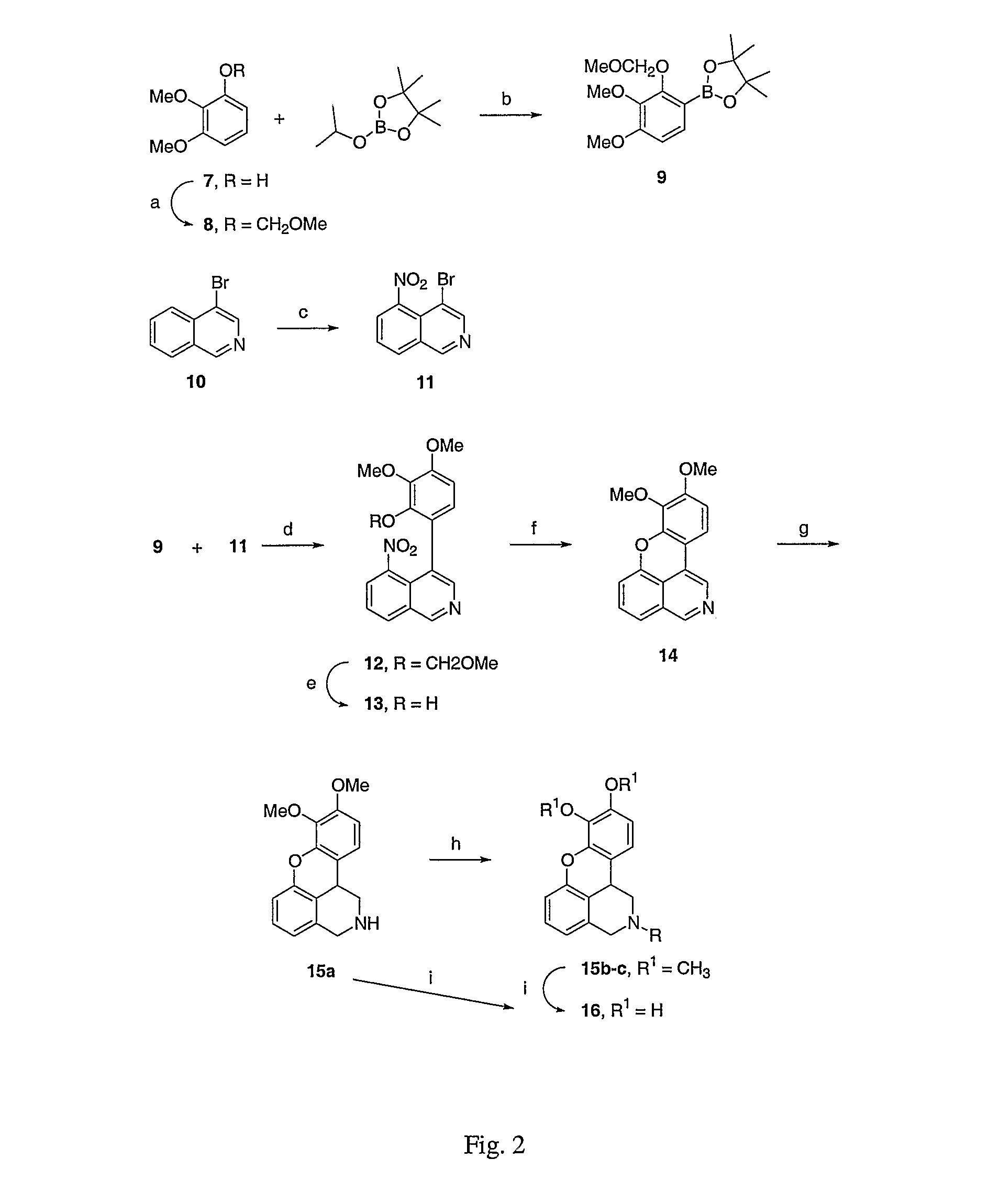
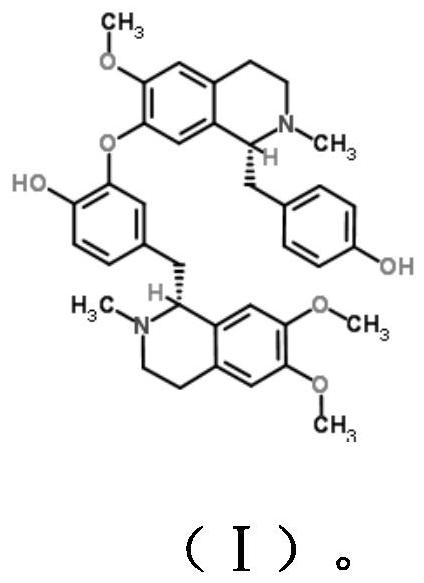
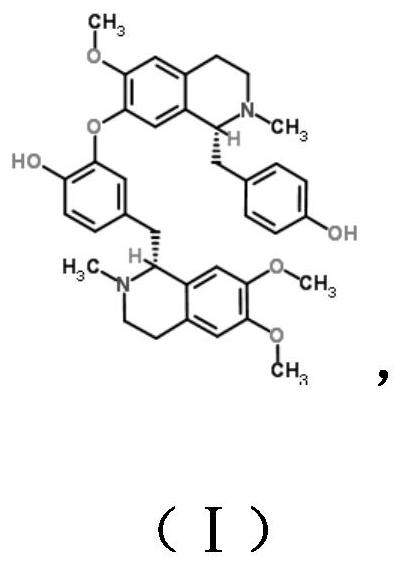
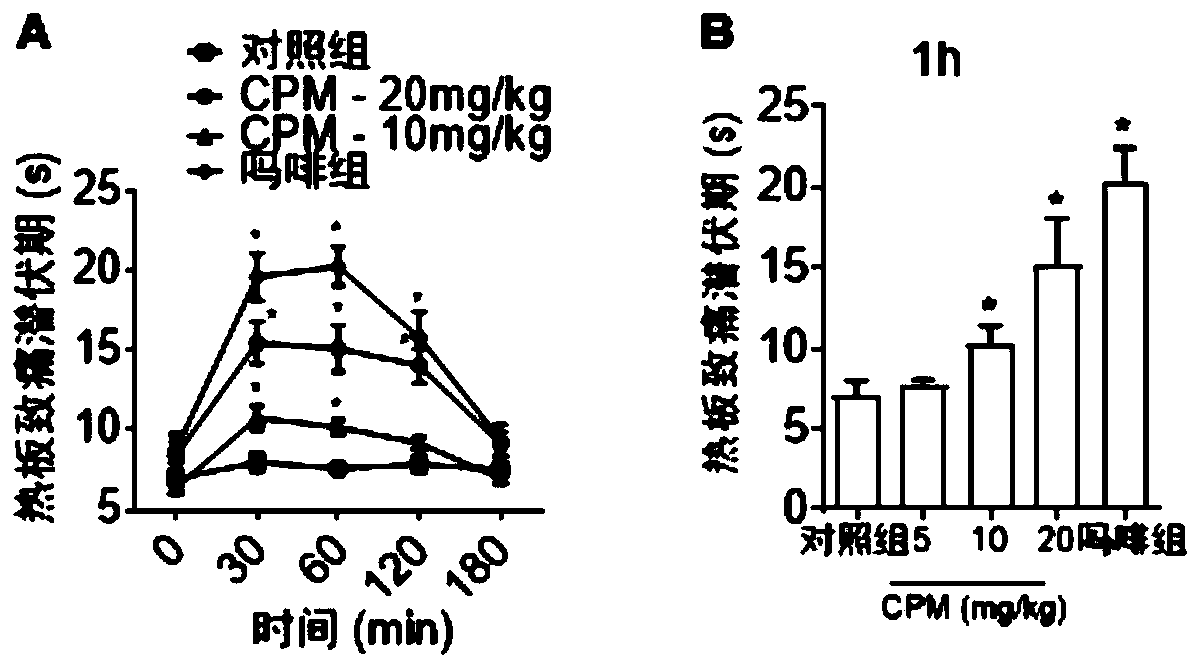
The invention discloses a dopamine receptor antagonist and an application thereof. The dopamine receptor antagonist includes 2,9,10-trimethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinolin-3-ol and a pharmaceutically acceptable salt or precursor compound. The dopamine receptor antagonist can antagonize a calcium influx caused by activation of a dopamine receptor, widen the application range of analgesia for a ligand of the dopamine receptor, and find a novel idea for development of a novel drug. Animal experiments provided by the invention prove that the 2,9,10-trimethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinolin-3-ol has a good analgesic effect on acute pain, inflammatory pain and bone cancer pain as the dopamine receptor antagonist.
Owner:SHANGHAI JIAO TONG UNIV
Treatment and management of augmentation in restless legs syndrome
ActiveUS10751327B2Reduce and revert augmentationAvoid side effectsBiocidePharmaceutical delivery mechanismDopamine receptor antagonistDopamine
The present invention provides methods of treating Restless Legs Syndrome (RLS) including administering to a subject an effective amount of a dopamine D1 receptor antagonist. Compositions and kits useful for treating RLS are also provided.
Owner:EAST CAROLINA UNIVERISTY +1
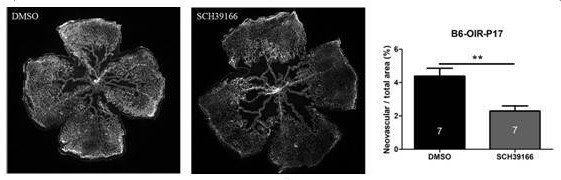
Application of dopamine d1 receptor antagonist sch39166 as a drug for the treatment of ocular pathological angiogenesis
ActiveCN110327350BNeonatal inhibitionSenses disorderHeterocyclic compound active ingredientsChoroid membraneOcular angiogenesis
Owner:WENZHOU MEDICAL UNIV
Dopamine d3 receptor antagonist compounds
ActiveUS20170334895A1Organic active ingredientsGroup 4/14 element organic compoundsDopamine receptor antagonistDopamine
Owner:INDIVIOR UK
Substituted n-aryl amidines as selective D1 dopamine receptor antagonists for the treatment of obesity and CNS disorders
The present invention provides compounds, which, are novel antagonists for D1 receptors as well as methods for preparing such compounds. In another embodiment, the invention provides pharmaceutical compositions comprising such D1 receptor antagonists as well as methods of using them to treat CNS disorders, obesity, metabolic disorders, eating disorders such as hyperphagia, and diabetes.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com