Treatment of Cancer WIth Dopamine Receptor Antagonists
a technology of dopamine receptor and dopamine receptor, which is applied in the direction of drug compositions, instruments, library screening, etc., can solve the problems that dopamine receptor antagonists at toxic concentrations to cancer cells have a relatively limited effect on normal stem cells, and achieve the effect of less expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Thioridazine is Cytotoxic to Leukemic Cell Lines
[0078]The effect of Thioridazine on normalized cell number was evaluated in 3 leukemic cells lines: HL-60, MV4-11 and OCI-AML3. All three lines are leukemic cell lines. HL-60 was derived from promyelocytic AML whereas MV 4-11 and OCI-AML3 are representative of AML. Each compound was incubated with the cells for 72 h. The control was DMSO (ie the vehicle used for the compound) for 72 h. Each condition had three replicates.
[0079]As shown in FIG. 1, doses of 0.1 μM and 1 μM thioridazine had little effect on normalized cell number, while at 10 μM the normalized cell number was reduced to almost zero.
example 2
Differential Activity of Thioridazine on AML Blast-Forming Potential and Colony Forming Potential of Normal Stem Cells
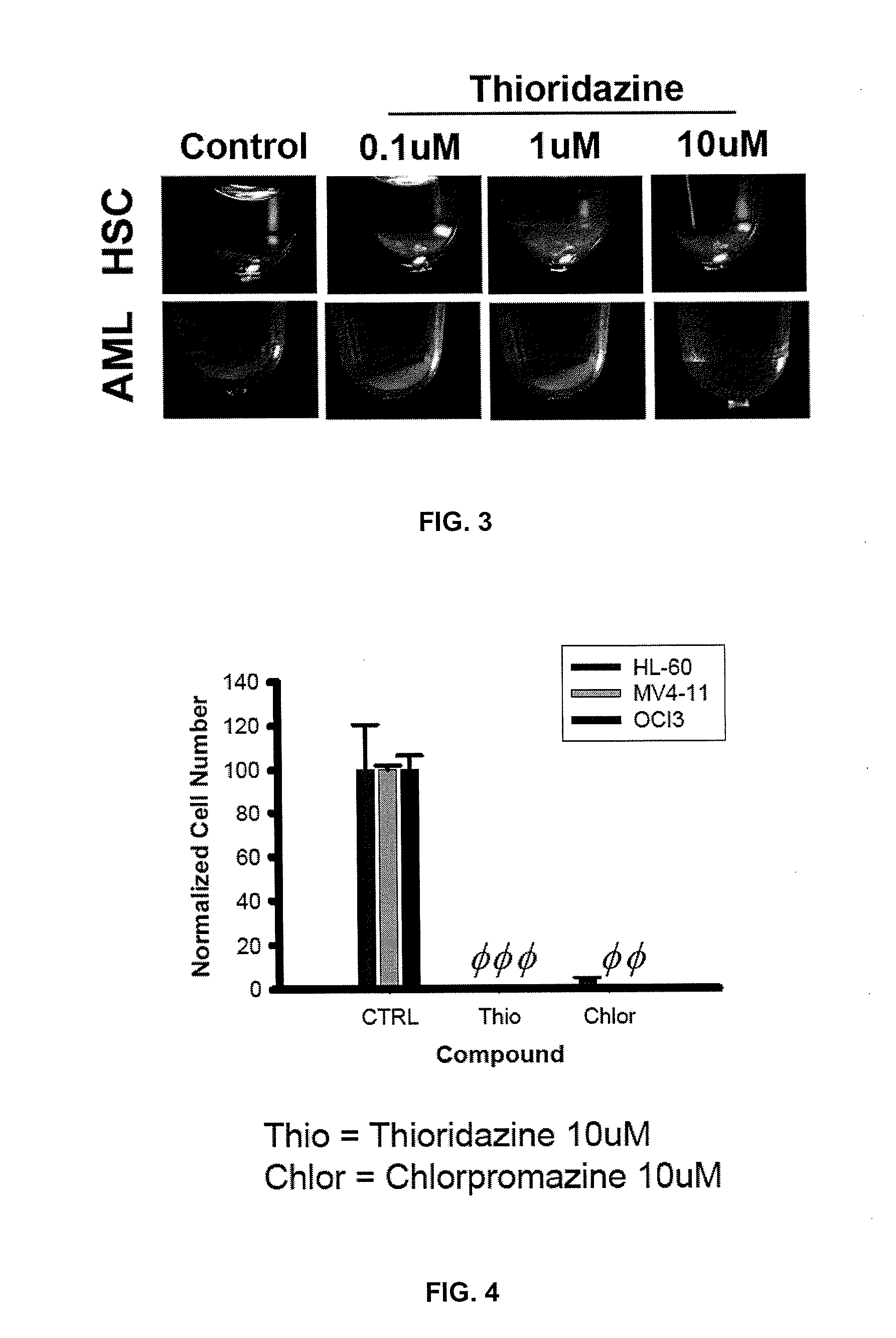
[0080]The effects of thioridazine on blast formation in an AML cell line was compared to the effect of thioridazine on colony formation in normal human stem cells.
[0081]Normal HSCs and progenitors were sourced from either mobilized peripheral blood or umbilical cord blood of health patients. Primary AML cells were taken from patients diagnosed with AML. Both normal HSCs and primary AML cells were cultured under standard in vitro methocellulose assay conditions (see http: / / www.stemcell.com / en / Products / All-Products / MethoCult-H4434-Classic.aspx as well as Clinton Campbell et al. The human stem cell hierarchy is defined by a functional dependence on Mcl-1 for self-renewal capacity. Blood 116 (9) 1433-1442 (Jun. 4, 2010), hereby incorporated by reference) for at least 14 days before the number of colonies were recorded. As shown in FIG. 2, 10 μM thioridazine has a differe...
example 3
Chlorpromazine is Toxic to AML Cell Lines
[0083]The dopamine receptor antagonist and phenothiazine-related compound chlorpromazine was also investigated for effects on the AML cell lines HL-60, MV4-11 and OCI-AML3. Testing was performed as set out in Example 1. As shown in FIG. 4, 10 μM Chlorpromazine is toxic to AML cell lines.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| fluorescence activated cell sorting | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com