Benzoazepine compound, preparation method and application thereof
A technology of benzonitrogen and compounds, applied in the fields of drug addiction, medicinal chemistry, and treatment of neurological diseases, can solve problems such as addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
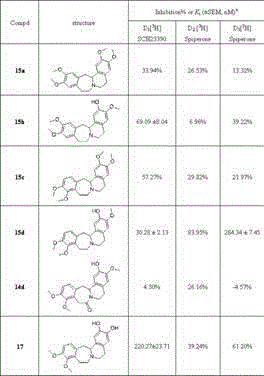
Image
Examples
Embodiment 1
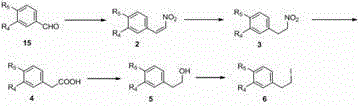
[0087] Example 1: 1-benzyl-2-methoxy-4-(2-nitrovinyl)benzene ( 2 )
[0088] Add vanillin (50 g, 0.33mol) at room temperature, K 2 CO 3 (68.1g, 0.50mol) in methanol (200mL) solution was added dropwise benzyl chloride (49.2mL, 0.43mol), stirred and refluxed for 8 h after the dropwise addition, after the reaction was detected by TLC, filtered and evaporated under reduced pressure to remove the solvent. Then add water (100mL), extract with ethyl acetate (3×100L), wash the organic phase twice with 15% sodium hydroxide and water, anhydrous magnesium sulfate, filter and concentrate to obtain white solid benzyl ether (75g. 93.8%). 4-Benzyloxy-3-methoxyvanillin (50g, 0.21mol) and ammonium acetate (32.4g, 0.42mol) were added to nitromethane (23ml, 0.42mol), then 80 o C for 1.5 h. After the reaction was detected by TLC, it was cooled to room temperature, and a mixture of ice and water was added, a large amount of yellow solid precipitated, filtered, the solid was washed with water ...
Embodiment 2
[0089] Example 2: 1-benzyl-2-methoxy-4-(2-nitroethyl)benzene ( 3 )
[0090] at 0°C to contain KBH 4 (4.73g, 87.6mmol) in methanol solution (50ml) was added dropwise the compound dissolved in 80mlTHF 2 (10 g, 35.1 mmol), stirred at 50 °C for 3-4 h, distilled off the solvent under reduced pressure, extracted with ethyl acetate (3 × 50 mL), washed the organic phase three times with brine, anhydrous Na 2 SO 4 Drying, filtration, concentration, and column chromatography (petroleum ether: ethyl acetate = 5:1) gave a white solid 3 (6.1 g, yield 60.5%).
Embodiment 3
[0091] Example 3: 2-(4-benzyl-3-methoxyphenyl)acetic acid ( 4a )
[0092] compound 3 (7.2g, 25.1mmol) was dissolved in a mixed solution of acetic acid (14ml) and DMSO (50ml), added sodium nitrite (5.2g, 75.2mmol), and stirred at 45°C for 12h. After the reaction was detected by TLC, acidify with 10% hydrochloric acid, add 50ml of water, extract with ethyl acetate (3 × 50ml), wash the organic phase with brine three times, and anhydrous Na 2 SO 4 Drying under low temperature, filtration, concentration, and column chromatography (petroleum ether: ethyl acetate = 5:1) gave a white solid 4a (6.1 g, yield 90%). 1 H NMR (400 MHz, CDCl 3 ) δ 7.43 (d, J = 7.4 Hz, 2H), 7.36 (t, J = 7.4 Hz, 2H), 7.29 (t, J = 7.2 Hz, 1H), 6.83 (dd, J = 5.1, 3.1 Hz, 2H), 6.75 (dd, J = 8.2, 1.9 Hz, 1H), 5.14 (s, 2H), 3.88 (s, 3H), 3.57 (s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com