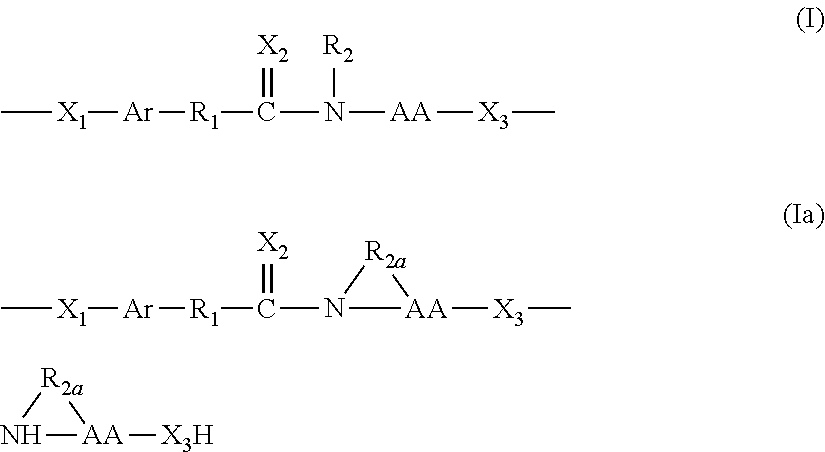Bioresorbable polymers synthesized from monomer analogs of natural metabolites
a bioresorbable polymer and monomer technology, applied in the field of new bioresorbable polymers synthesized from monomer analogs of natural metabolites, can solve the problems of slow degradation rate, limited resulting homopolymer to fully aromatic backbone structure, and many limitations of monomers, and achieves the effects of increasing poly(alkylene), increasing the amount of pendent free carboxylic acid groups, and promoting cellular attachmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Ethyl Ester of Amino Acids
[0159]Ethyl esters of amino acids were prepared by reaction of the amino acid with ethanol and thionyl chloride as described in a literature procedure (Bodanszky, Practice of Peptide Synthesis (Springer-Verlag, New York 1984). The products were characterized using HPLC, 1H NMR, elemental analysis and melting point. In most cases esters were used as the hydrochloride salt with in situ free base generation with triethylamine Free bases of the esters were also prepared and isolated in some cases by treating with 5M aqueous potassium carbonate. When available esters were obtained from commercial sources.
example 2
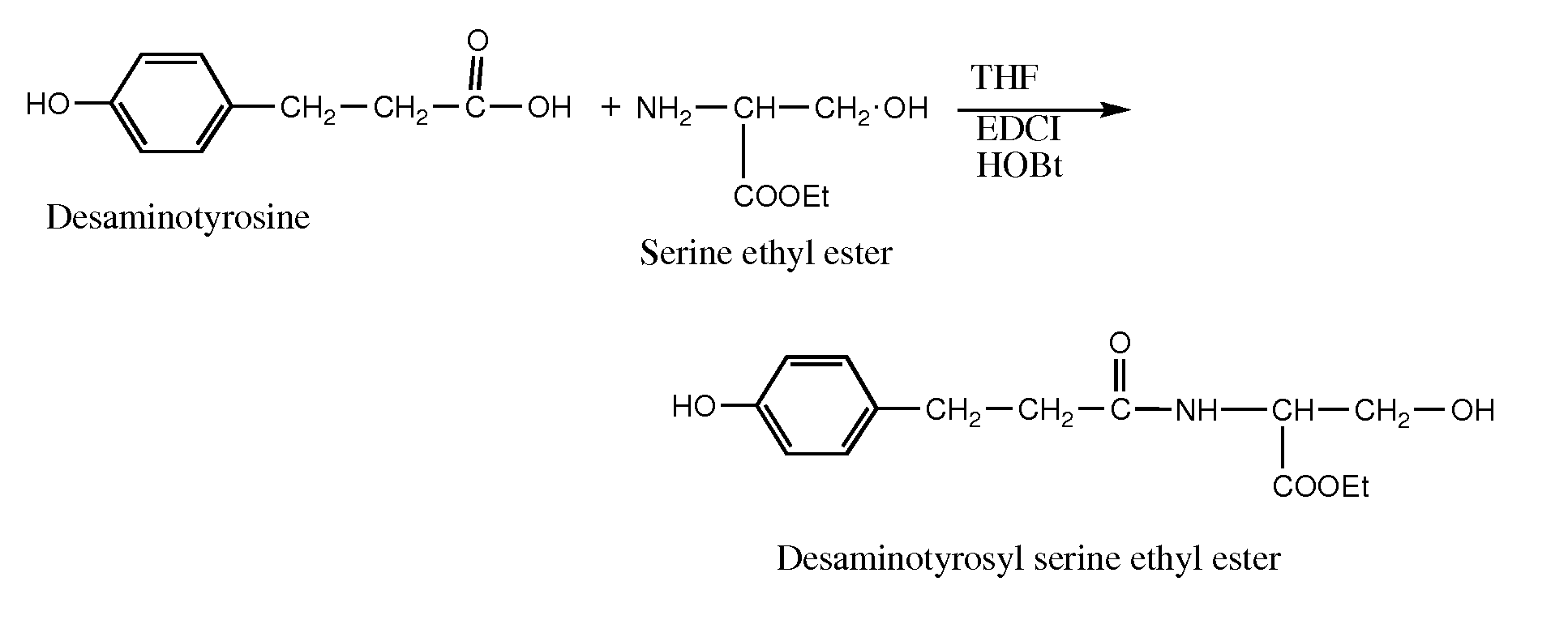
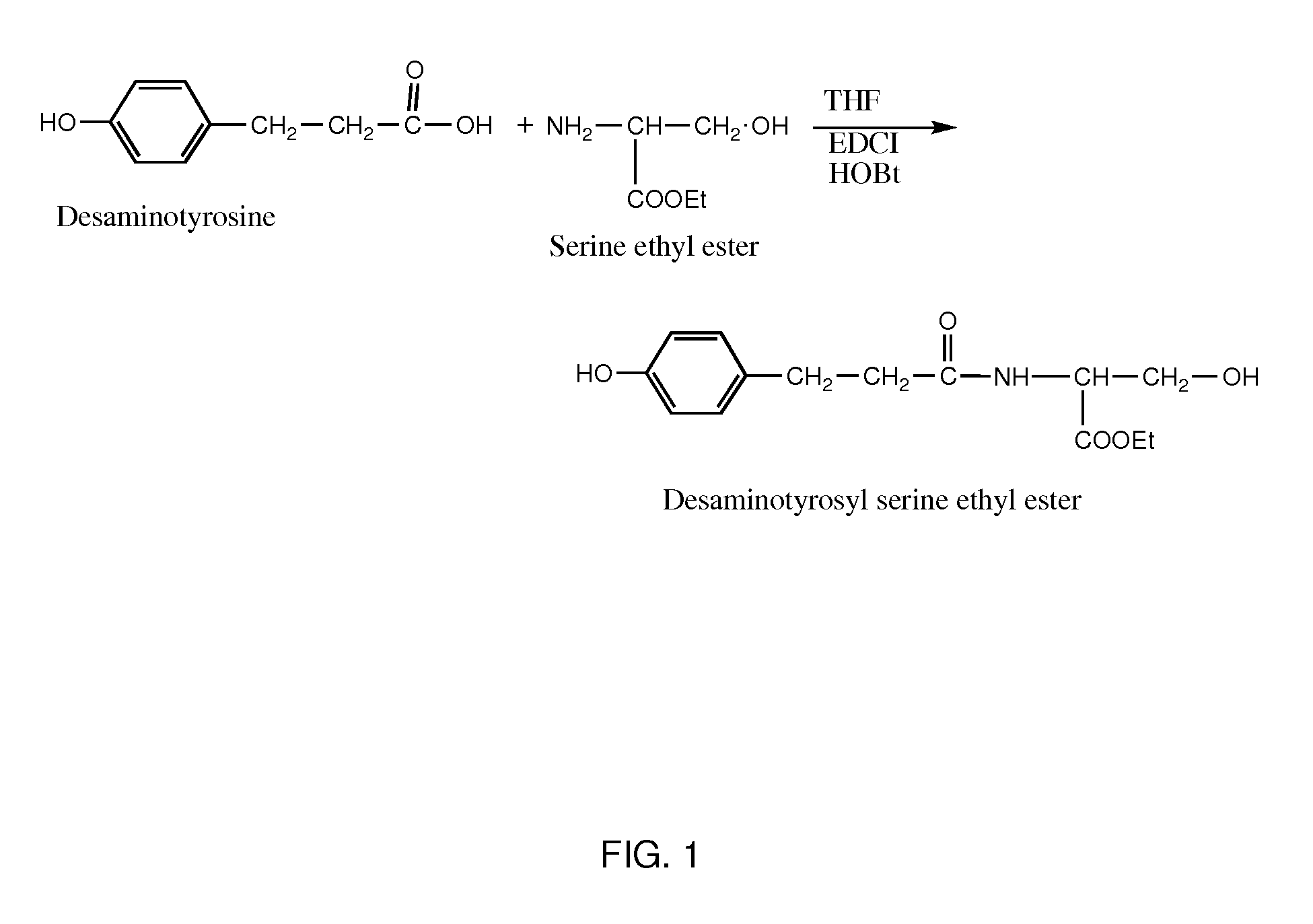
Synthesis of Desaminotyrosyl Serine Ethyl Ester
[0160]To a single-necked 500 mL round-bottomed flask equipped with an addition funnel and a magnetic stirrer was added 3-(4-hydroxyphenyl)propionic acid (10.0 g, 60.2 mmol), serine-ethyl ester hydrochloride (10.7 g, 63.2 mmol), hydroxybenzotriazole hydrate (0.81 g, 6.0 mmol), and tetrahydrofuran (50 mL). The flask was cooled in an ice-water bath and triethylamine (8.85 mL, 63.4 mmol) was added drop wise over a period of 10 minutes and the reaction mixture was stirred for 30 more minutes and then 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (12 g, 50 mmol) was added and stirred at ice-water bath temperature for 1 hour.
[0161]The reaction mixture was further stirred at room temperature for 4 hours. Distilled water (150 mL) was added to the reaction flask and was transferred to a separatory funnel and extracted twice with 100 mL ethyl acetate. The combined extract was washed twice with 0.2 M hydrochloric acid solution (100 ...
examples 3 and 4
Synthesis of DAT-hydroxyproline ethyl ester and DAT-threonine ethyl ester
[0162]Using the procedure of Example 2, ethyl esters of trans-hydroxyproline and threonine were coupled to desaminotyrosine. The resulting monomers were characterized as in Example 1 by elemental analysis, 1H NMR spectroscopy and HPLC.
Table of reagents used for the preparationof monomers (DAT-AA ethyl ester)AminoAA-ethylDATHOBtTriethyl-EDCIYieldacidester•HCl, (g)(g)(g)amine (g)(g)(%)Serine21.43201.6312.825.449.3Threonine5.224.50.372.885.7154.0tHyp9.898.00.655.1210.243.35-HTrp5.232.90.241.863.6969.8Thyronine0.65*0.340.03—0.4361.2*thyronine ethyl ester free base was used
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com