Method of modifying source chemicals in an ALD process
a technology of source chemicals and ald process, which is applied in the direction of crystal growth process, chemical vapor deposition coating, coating, etc., can solve the problems of difficult chemical vapor deposition methods, inability to obtain complete film coverage on the deep bottom of vias and trenches, and uneven deposition, etc., to achieve efficient film growth, short cycle time, and high growth rate of thin films
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
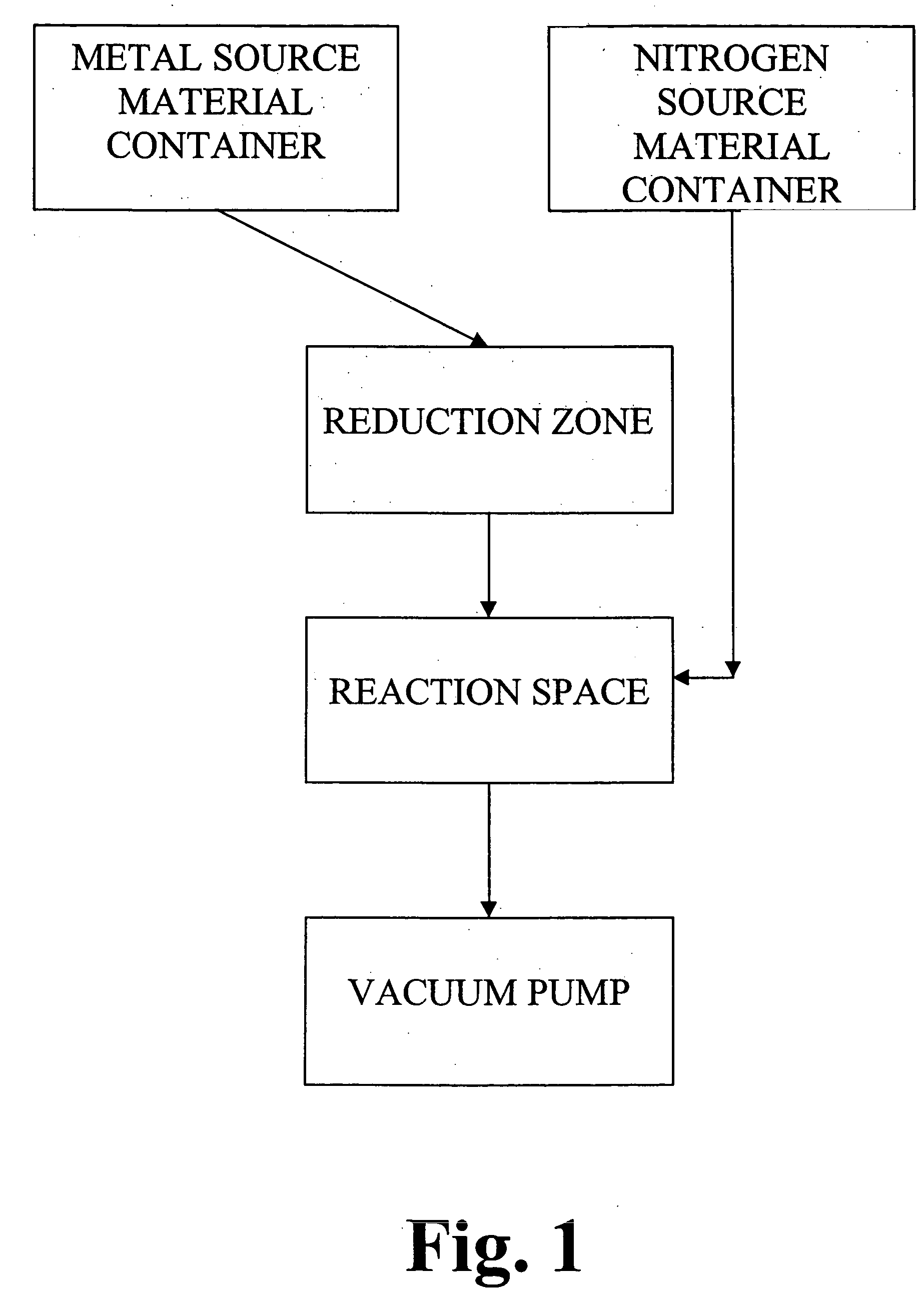
[0101] Experiments were performed with an F200 ALD reactor, as described in Finnish Patent No. 100409 of assignee by Suntola et al. Two series of titanium nitride samples were produced. One series of samples was made from TiCl4 and NH3. Heated reactor parts in contact with metal halides were passivated with amorphous aluminum oxide which protected the parts against corrosion. TiCl4 was evaporated from a source bottle at room temperature. The source gas was carried to the substrate chamber by nitrogen gas. Purging with nitrogen gas removed surplus TiCl4 molecules from the reactor. Then an NH3 gas pulse was introduced to the substrate chamber. The pulsing cycle was ended with a nitrogen purge which removed surplus NH3 and gaseous reaction byproducts from the reactor. The pulsing cycle was repeated 1000 times. The pressure of the substrate chamber was in the range of 200-1000 Pa. The resulting thin film on silicon wafer had metallic lustre with a yellowish hue. The growth rate at 400° ...
example 2
[0104] The same ALD reactor as in Example 1 was used for the reduction experiment. WF6 was evaporated from a source bottle at room temperature and carried towards the substrate chamber by nitrogen gas. In the gas flow channel near the substrate chamber there was tungsten metal foil heated to 400° C. WF6 flowed over tungsten metal, and the assumed reaction products (denoted with WFx) were carried to the substrate chamber by nitrogen gas. However, it was not possible to detect any visual signs of corrosion on W metal after the experiment. Theoretical calculations confirmed that at least simple reactions Eq. 3 and Eq. 4 are not favourable thermodynamically.
5WF6(g)+W→6WF5(g)ΔG(400° C.)=+1753 kJ (Eq. 3)
2WF6(g)+W→3WF4(g)ΔG(400° C.)=−79 kJ (Eq. 4)
theoretical example 3
[0105] Tungsten hexafluoride (WF6) is evaporated from the source bottle and carried towards the substrate chamber by nitrogen gas. Along the gas route there is a cartridge filled with tungsten silicide pieces. The cartridge is heated to 400° C. The reduction of WF6 into WF4 is thermodynamically favourable (Eq. 5). The pulsing sequence in the point of substrate is as follows: WFx vapor pulse / N2 gas purge / NH3 vapor pulse / N2 gas purge.
6WF6(g)+Wsi2(s)→7WF4(g)+2SiF4(g)ΔG(400° C.)=−737 kJ (Eq. 5)
[0106] It can be argued whether SiF4 disturbs the thin film growth process. However, experiments with ALE have shown that it is very difficult to grow any silicon oxide or silicon nitride film from silicon halides. SiF4 molecules may have low reactivity with NH3 gas and they can be purged away with nitrogen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com