Multistage response injectable hydrogel loaded with gambogic acid and application thereof
A technology of hydrogel and gambogic acid, which is applied in the field of multi-level response injectable hydrogel loaded with gambogic acid, which can solve the problem of low water solubility of gambogic acid, strong vascular irritation, and limitations of gambogic acid in clinical applications, etc. problem, to achieve the effect of improving drug loading capacity, less toxic and side effects, and good injectability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the preparation of the hydrogel of the present invention
[0046] 1. Synthesis and purification of polyethylene glycol-modified gambogic acid (GM)
[0047] (1) Gambogic acid, 1-ethyl-(3-dimethylaminopropyl)carbodiimide (EDC) and 4-dimethylaminopyridine (DMAP) dissolved in CH 2 Cl 2 In an ice-water bath, polyethylene glycol (weight average molecular weight 2000) was added. The resulting mixture was stirred at room temperature overnight.
[0048] (2) Wash with HCl, water, saturated brine, and use Mg 2 SO 4 Dry and concentrate in vacuo. The precipitate was stirred in ether to remove free gambogic acid. The solution was filtered to give a yellow precipitate.
[0049] (3) The crude product was further purified by silica gel column chromatography, in CH 2 Cl2 The free polyethylene glycol was removed by a step gradient of methanol, and the polyethylene glycol-modified gambogic acid (GM) was obtained as a light yellow solid.
[0050] 2. Preparation of Gam...
experiment example 1
[0063] Experimental example 1. Investigation on the gelation time of the hydrogel
[0064] 1. Experimental method
[0065] GA@LPS was prepared by referring to the preparation method of Example 1, and fibrinogen was dissolved in physiological saline to obtain four different concentrations of fibrinogen solutions (2 mg / ml, 5 mg / ml, 10 mg / ml and 20 mg / ml). Then, different concentrations of fibrinogen were mixed with an equal volume (1:1 volume ratio) of thrombin solution (10 NIH U / mL and 40 μmol / mL of calcium hydrogen phosphate nanowires) respectively, and three parallel experimental groups were set up for each concentration. The turbidity change of each group of solutions was measured immediately at a wavelength of 550 nm using a VersaMax UV-Vis spectrophotometer (Molecular Devices, Sunnyvale, CA), and the gelation time was defined as the time when the absorbance curve reached a maximum value.
[0066] 2. Experimental results
[0067] The result is as figure 1 As shown, it ca...
experiment example 2
[0068] Experimental example 2. Research on the synergistic effect of hydrogel gambogic acid and calcium hydrogen phosphate of the present invention
[0069] 1. Experimental method
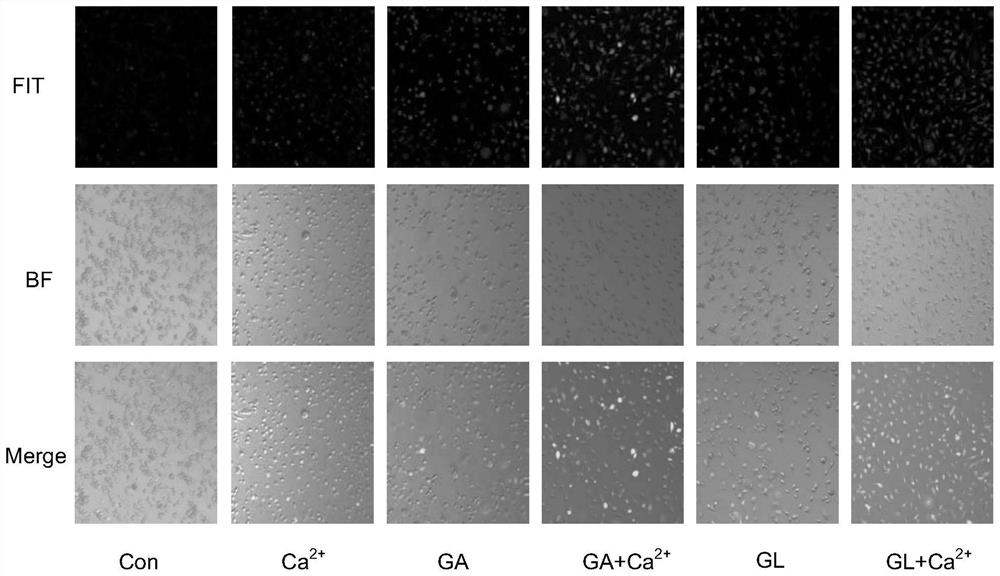
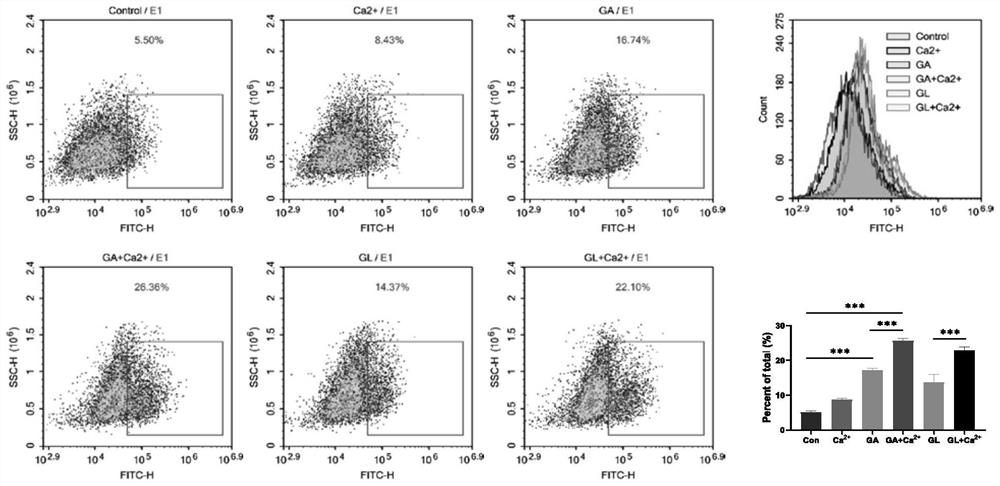
[0070] Since calcium hydrogen phosphate nanowires have certain problems in the dispersion in aqueous solution and are easy to settle, the hydrogel of the present invention adopts the method of in situ administration of tumors, and calcium hydrogen phosphate is degraded in the slightly acidic environment of tumors to release Ca. 2+ , so in the systematic study of gambogic acid and calcium hydrogen phosphate in cell experiments, CaCl was used. 2 Substitute calcium hydrogen phosphate (CP) as calcium source.
[0071] Specifically, carried out:
[0072] (1) Calcium ion staining experiment:
[0073] The experiment was carried out when the K7M2 cells grew well and the density was above 85%. First, K7M2 cells were digested, resuspended, and counted, and then seeded in 6-well plates, 2 × 10 cells per we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com