Method for determining phenylhydrazine and phenylhydrazine derivative in medicine or synthetic intermediate by derivatization HPLC-DAD method
A derivative and derivatization technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as low sensitivity, many interferences, and large baseline noise
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] 1.3. Solution preparation
[0043] 1.3.1 Phenylhydrazine stock solution:
[0044] Take about 10 mg of phenylhydrazine, weigh it accurately, place it in a 10 mL measuring bottle, dilute to the mark with acetonitrile, and shake well. Precisely measure 100 μL of the above solution, place it in a 10 mL measuring bottle, dilute to the mark with acetonitrile, and shake well. The concentration of phenylhydrazine in the stock solution is 10 μg / mL.
[0045] 1.3.2 p-sulfonamidophenylhydrazine stock solution:
[0046] Take about 10 mg of p-sulfonamidophenylhydrazine (CAS No. 4392-54-5), weigh it accurately, place it in a 10 mL measuring bottle, dilute to the mark with acetonitrile, and shake well. Precisely measure 100 μL of the above solution, place it in a 10 mL measuring bottle, dilute to the mark with acetonitrile, and shake well. The concentration of p-sulfonamidophenylhydrazine in the stock solution is 10 μg / mL.
[0047] 1.3.3 Derivatization test solution:
[0048] 2-nit...
Embodiment 1
[0055] The comparison of embodiment 1 derivatization reagent
[0056] Precisely pipette 1mL of 10μg / mL phenylhydrazine stock solution, put it in a 10mL volumetric flask, add 1mL of benzaldehyde derivatization test solution, dilute to the mark with acetonitrile-water (80:20, v / v), shake uniform. After reacting at room temperature for 45 minutes, 20 μL was injected into HPLC-DAD for analysis.
[0057] HPLC-DAD conditions: Shimadzu LC 20AT liquid chromatograph (including online vacuum degasser, binary gradient pump, autosampler, column oven, DAD detector and LC-solution chromatographic workstation); chromatographic column adopts 250mm× 4.6mm, 5μm Diamonsil TM C18 column; injection volume: 20 μL; mobile phase: acetonitrile-0.1% phosphoric acid aqueous solution (70:30, v / v), isocratic elution; flow rate: 1.0mL / min; column temperature: 30°C; detection wavelength: 416nm . Record the spectrum and chromatogram of the product, and use the maximum absorption wavelength and absorptio...
Embodiment 2
[0060] Example 2 Derivatization condition optimization
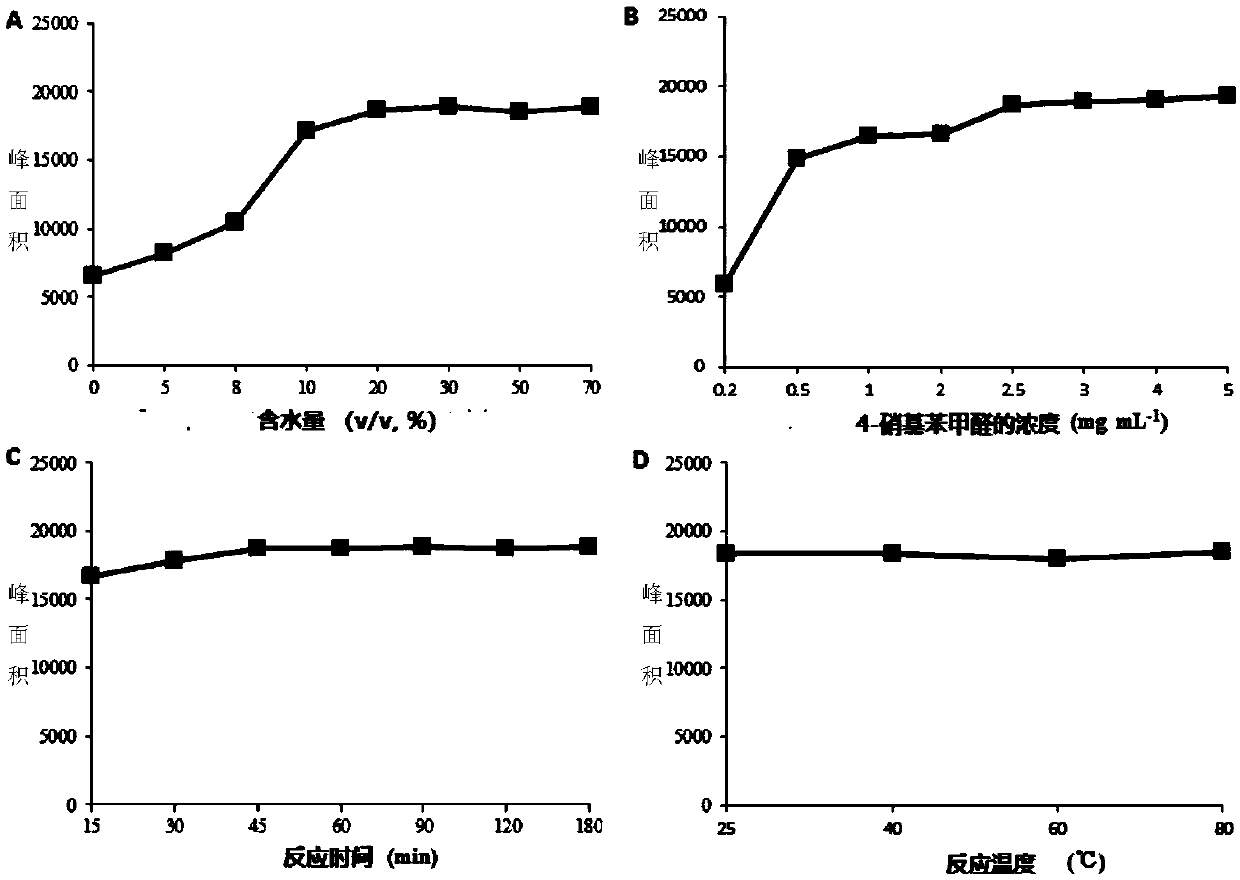
[0061] In order to ensure the efficiency of the derivatization reaction, the concentration of the derivatization reagent in the system, the reaction time, the reaction temperature and the proportion of the organic phase were investigated. Taking the peak area of the derivative product of phenylhydrazine and 4-nitrobenzaldehyde as an index, the water content of the reaction system (1, 5, 8, 10, 20, 30, 50, 70%, v / v ), 4-nitrobenzaldehyde concentration (0.2,0.5,1.0,2.0,2.5,3.0,4.0,5.0mg / ml), reaction time (15,30,45,60,90,120,180min) and reaction Effect of temperature (25, 40, 60, 80°C) on derivatization reaction efficiency, the results are as follows image 3 Shown; In the experiment, the concentration of phenylhydrazine in the system was 1 μg / mL.
[0062] Depend on image 3 In A, it can be seen that adding a certain volume of water in the reaction system is beneficial to the derivatization reaction, and the peak area...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com