A kind of photosensitive resin, positive photoresist and application
A positive photoresist and photosensitive resin technology, applied in the field of photolithography, can solve the problems of turbidity, limited improvement, and difficult to peel off the photoresist, and achieve the effect of high heat resistance stability and good photosensitive performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
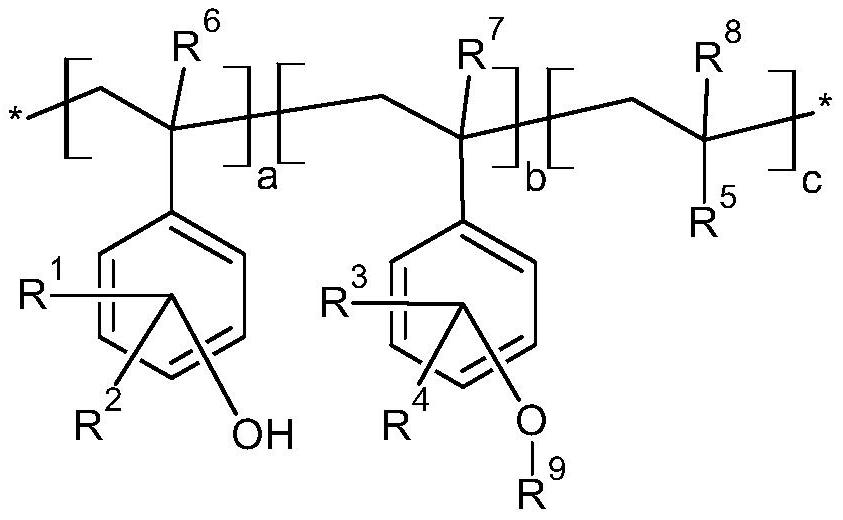
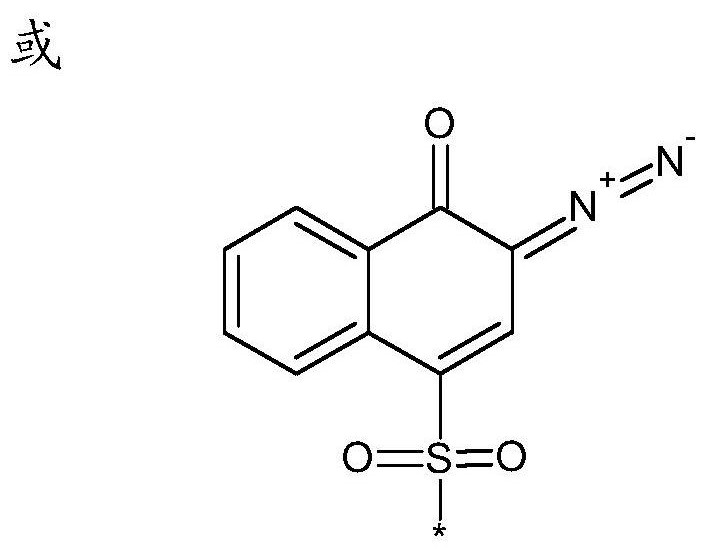
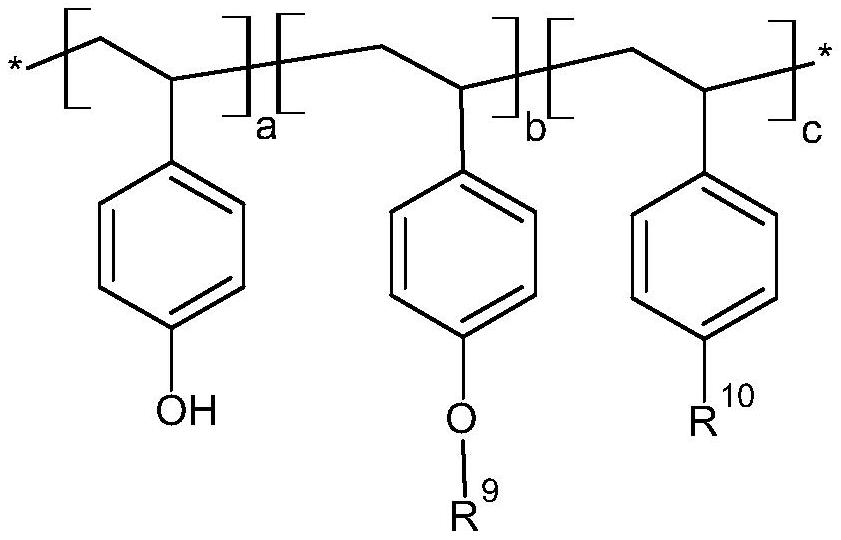
[0046] During the preparation of the photosensitive resin, the above-mentioned vinyl phenolic monomers can be used for free radical polymerization, but there are many side reactions of phenolic free radical polymerization. The side reaction of deprotonation of phenolic hydroxyl group will occur in anionic polymerization, and the side reaction of electrophilic addition of benzene ring will easily occur in cationic polymerization. Therefore, the embodiments of the present invention preferably use acetyl groups or other groups to protect vinylphenol monomers, and carry out free radical, active free radical, anionic, or cationic polymerization reactions, and then remove the protective groups to obtain the desired polyhydroxybenzene Ethylene homopolymer or copolymer. In principle, various polyhydroxystyrene homopolymers or copolymers prepared from the above-mentioned monomers can utilize diazonaphthoquinone sulfonyl chloride, such as 2-diazo-1-naphthoquinone-5-sulfonyl chloride or ...
Embodiment 1
[0063] This example provides three kinds of p-hydroxystyrene-styrene copolymers, the preparation method of which is as follows: add 8.0 kg of propylene glycol mono-n-propyl ether and 2.0 kg of p-acetylstyrene into a 20-liter reactor, mix well Finally, 0.020 kg of benzoyl peroxide was added as an initiator, 0.020 kg of dimer-methylstyrene was used as a chain transfer catalyst, and the temperature was raised to 80° C. and kept at a temperature of 3 hours for polymerization. Cool to room temperature, quickly disperse and precipitate in 8 kg of methanol, and filter to separate the resin. The resin is dispersed in 4 kg of methanol, and 1.2 kg of ammonia water with a mass fraction of 25-28% is slowly added for ammonolysis. Stir at room temperature until the resulting polyparaacetylstyrene resin precipitate dissolves. Heat to 80°C and keep it warm until the ester carbonyl group disappears in the infrared spectrum. Under high-speed stirring, the resin was dissolved and dispersed in ...
Embodiment 2
[0067] This example provides a p-hydroxystyrene-p-acetoxystyrene copolymer, the specific preparation method of which is as follows: 8 kg of propylene glycol mono-n-propyl ether and 2 kg of p-acetyl styrene. After mixing evenly, add 0.10 kg of benzoyl peroxide as an initiator, 0.05 kg of dimer-methylstyrene (i.e. methylstyrene dimer) as a chain transfer catalyst, heat up to 80°C and keep it warm for 3 hours for polymerization reaction. After cooling to room temperature, the reaction solution was dispersed in 40 kg of deionized water under slow and then high-speed stirring, then vacuum filtered, and washed with a small amount of anhydrous methanol. Disperse the obtained poly(p-acetoxystyrene) powder in 8 kg of anhydrous methanol, add 20 g of p-toluenesulfonic acid monohydrate, and reflux under a nitrogen atmosphere. As the acetyl group is gradually alcoholysed, the system polymer Gradually dissolve methanol to form a uniform solution, take samples, and use Fourier transform in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com