Loratadine tablet and preparation technology thereof
A technology of loratadine and tablets, which is applied in the field of loratadine tablets and its preparation, can solve the problems of slow dissolution rate and incomplete dissolution, and achieve low friability, no solvent residue, and low energy consumption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3 and comparative example 1-4
[0037] The preparation of embodiment 1-3 and comparative example 1-4 loratadine sheet
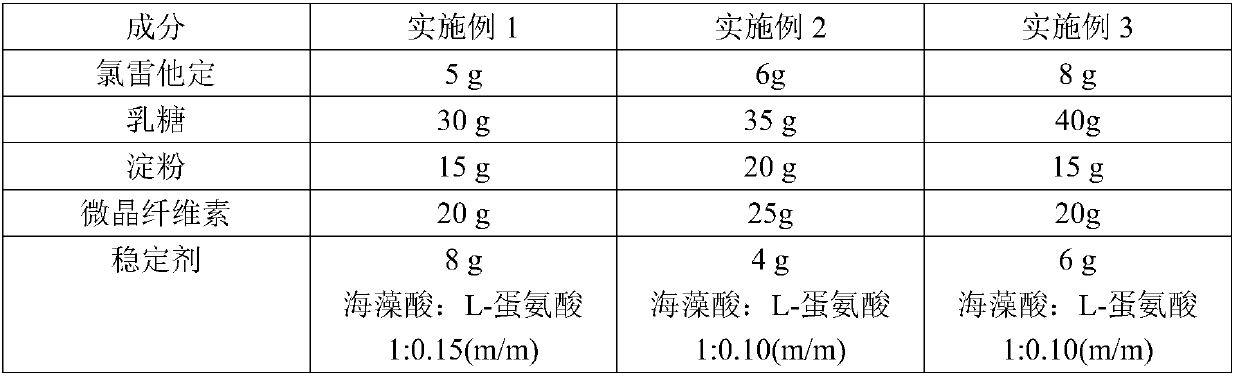
[0038] The composition of embodiment 1-3 loratadine tablet is shown in the table below:
[0039]
[0040]
[0041] The composition of comparative example 1-4 loratadine tablet is shown in the table below:
[0042]
Embodiment 1
[0043] The preparation of embodiment 1 loratadine tablet:
[0044](1) Loratadine is dissolved in absolute ethanol to make a solution with a concentration of 15% (m / v) to obtain solution A; sorbitol is dissolved in water to make a concentration of 25% (m / v) solution to obtain solution B;
[0045] (2) Add solution B to solution A under stirring conditions, stir for 10 minutes, heat the suspension, and evaporate to dryness to obtain material C;
[0046] (3) Mix material C and lactose according to the isovolumic incremental method, add starch, microcrystalline cellulose, and stabilizer to the wet granulator in turn, stir and mix evenly, add an appropriate amount of 10% starch slurry, stir for 90 seconds, cut Crushed for 60 seconds, made into soft materials and then granulated;
[0047] (4) Dry after making wet granules, set the air inlet temperature range ≤ 80°C, control the air inlet temperature range ≤ 87°C, control the material temperature ≤ 60°C, and the outlet air temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com