Human varicella virus inactivated vaccine and preparation method thereof
A technology for varicella virus and inactivated vaccines, applied in biochemical equipment and methods, viruses, antiviral agents, etc., can solve problems affecting the quality of vaccines, and achieve economic benefits, good immunogenicity, and thorough inactivation effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 preparation method of human varicella virus inactivated vaccine
[0037] (1) MRC-5 cells are subcultured to 28 generations in cell culture flasks and cell factories;
[0038](2) When the cells are amplified to a confluence of more than 90%, the virus is inoculated into the cells for infection, and the M.O.I. is 0.01-0.1;
[0039] (3) Add MEM medium containing 2% to 5% fetal bovine serum, and place in a cell incubator at 34 to 37°C for cultivation;
[0040] (4) observe the degree of cell lesion every day, and when the CPE of the cells reaches more than 50%, discard the culture medium and wash the cells with PBS;
[0041] (5) Add 0.03% EDTA solution to elute the infected cells, collect the cell suspension in a centrifuge tube, centrifuge at 4000rpm for 10 minutes at 4°C, remove the supernatant, add a stabilizer to combine, mix well, and take a sample from a single bottle. Bacteria detection, frozen at -80°C;
[0042] (6) Place the sample to be tested for s...
Embodiment 2
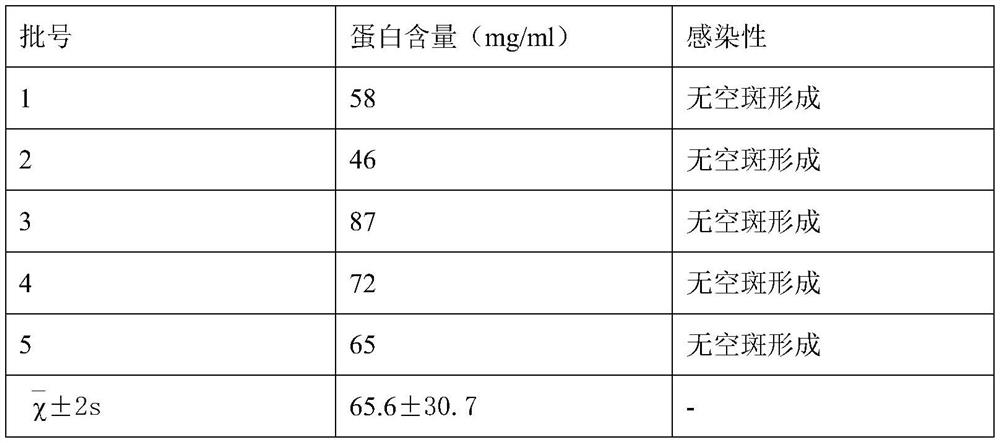
[0049] The selection of embodiment 2 virus concentrated liquid protein concentration
[0050] (1) Prepare 5 batches of inactivated virus liquids according to steps (1)-(10) of the preparation method of human varicella virus inactivated vaccine;
[0051] (2) The measured concentration of the virus concentrate and the infectivity after inactivation are shown in the following table:
[0052]
[0053] According to the results in the above table, we chose to inactivate the virus concentrate with a protein content of 35-95 mg / ml.
Embodiment 3
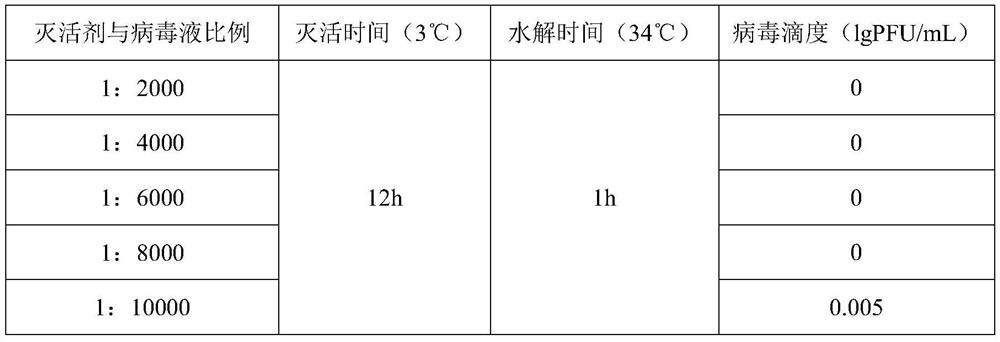
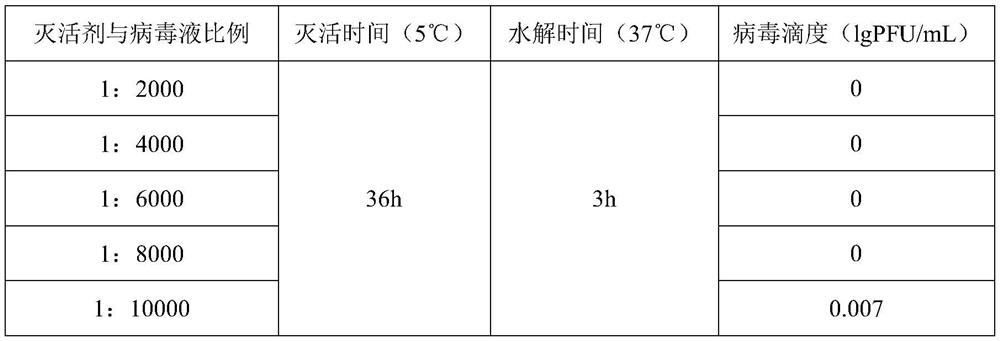
[0054] Embodiment 3 plaque method measures the infectivity of varicella virus inactivated vaccine
[0055] Spread MRC-5 cells on a 6-well plate, inoculate 3*10 per well 5 cells, 5% CO 2 After incubating in an incubator at 34-37°C for 3-4 days, the titer of varicella virus can be measured after the cells grow into a monolayer. Aspirate the medium, dilute the inactivated varicella virus vaccine to an appropriate concentration to infect the cells, and in 5% CO 2 Adsorb in an incubator at 34-37°C for 1-2 hours, then add 3 mL of virus culture solution to each well, and store in 5% CO 2 Cultivate in an incubator at 34-37°C. Staining was carried out after 7-10 days of culture. Take the average value of the counts, multiply it by the dilution factor and the inoculation volume correction factor, and obtain the titer of the inactivated varicella virus vaccine, which is expressed in lg PFU / mL. The titer results of the inactivated varicella virus inactivated vaccine are all empty spo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com