An anion-doped fluorite type tungstic acid-based mixed conductor hydrogen permeable membrane material and its preparation method and application
A mixed conductor and fluorite-type technology, which is applied in the field of fluorite-type tungstic acid-based mixed conductor hydrogen permeable membrane material and its preparation, can solve the problem of hydrogen permeable membrane material hydrogen permeability and stability. Hydrogen permeable membrane technology is difficult to achieve large-scale For industrial applications, it is difficult to meet the requirements of hydrogen flux and demanding mechanical properties, etc., to achieve the effect of increasing hydrogen permeation, high operational stability, and increasing the concentration of oxygen holes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A fluorite-type tungstic acid-based mixed conductor hydrogen permeable membrane material La 5.2 W 0.8 Mo 0.2 o 10.3-δ F(LWMF 1.0 ) and the hydrogen permeable membrane material La without fluoride ion 5.2 W 0.8 Mo 0.2 o 11.25-δ (LWM02) preparation method, wherein δ=0~1, specifically comprises the following steps:
[0043] (1) Synthetic LWMF 1.0 : Weigh 25.84g La respectively 2 o 3 , 2.285g LaF 3 , 6.491g WO 3 , 1.007g MoO 3 , after preliminary mixing, add 30ml of ethanol, ball mill at a speed of 300r / min, take it out after 24h and dry it naturally;
[0044] (2) Synthesis of LWM02: Weigh 29.65g La 2 o 3 , 6.491g WO 3 , 1.007 g MoO 3 , after preliminary mixing, add 30ml of ethanol, ball mill at a speed of 300r / min, take it out after 24h and dry it naturally;
[0045] (3) LWMF obtained by ball milling 1.0 The raw material powder mixed evenly with LWM02 is placed in a high-temperature muffle furnace to raise the temperature to 1000°C at a heating rate of 2...
Embodiment 2
[0049] A fluorite-type tungstic acid-based mixed conductor hydrogen permeable film material Nd doped with fluorine ions in this embodiment 5.5 WO 11-δ f 0.5 (NWMF 0.5 ) preparation method, wherein δ=0~1, specifically comprises the following steps:
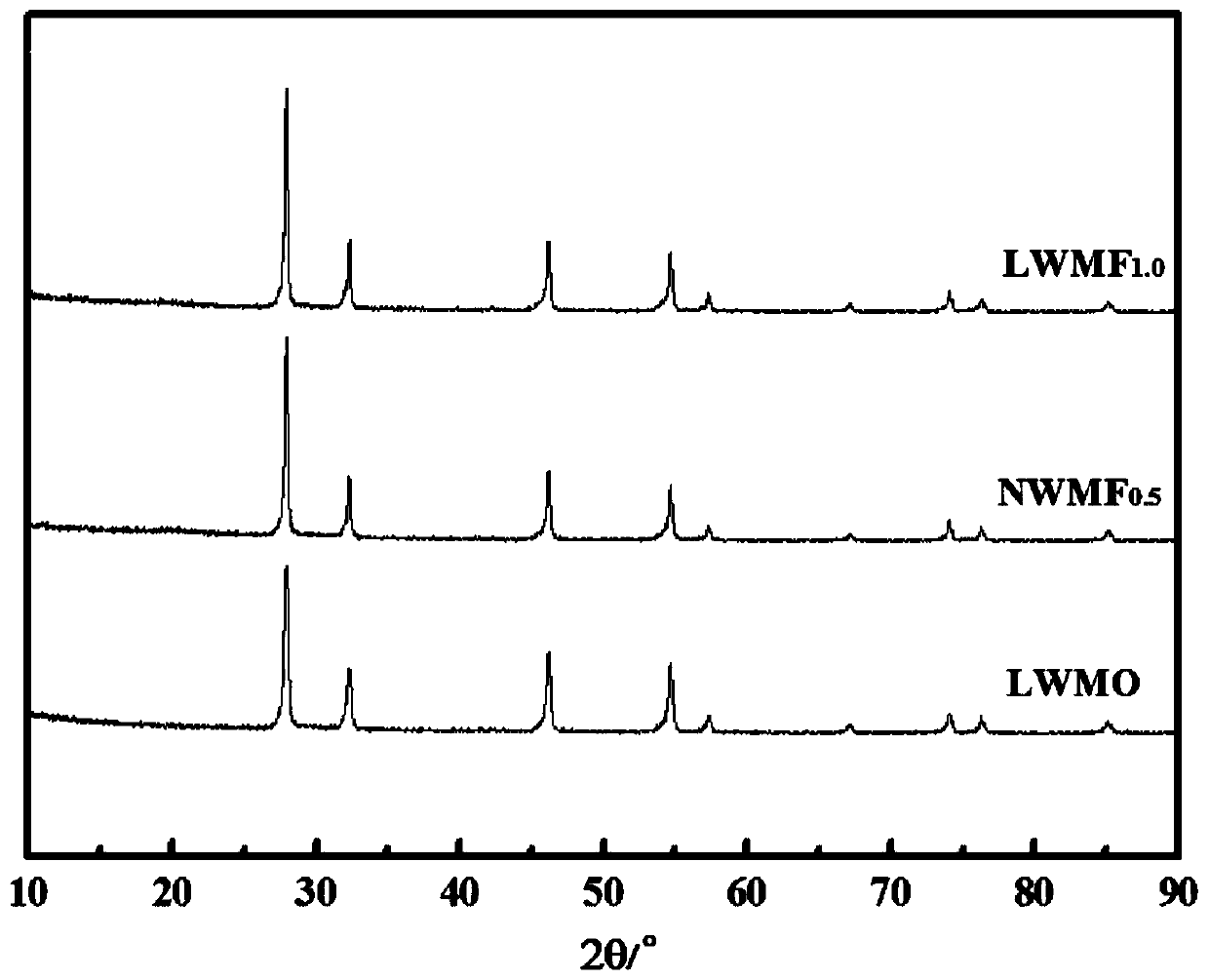
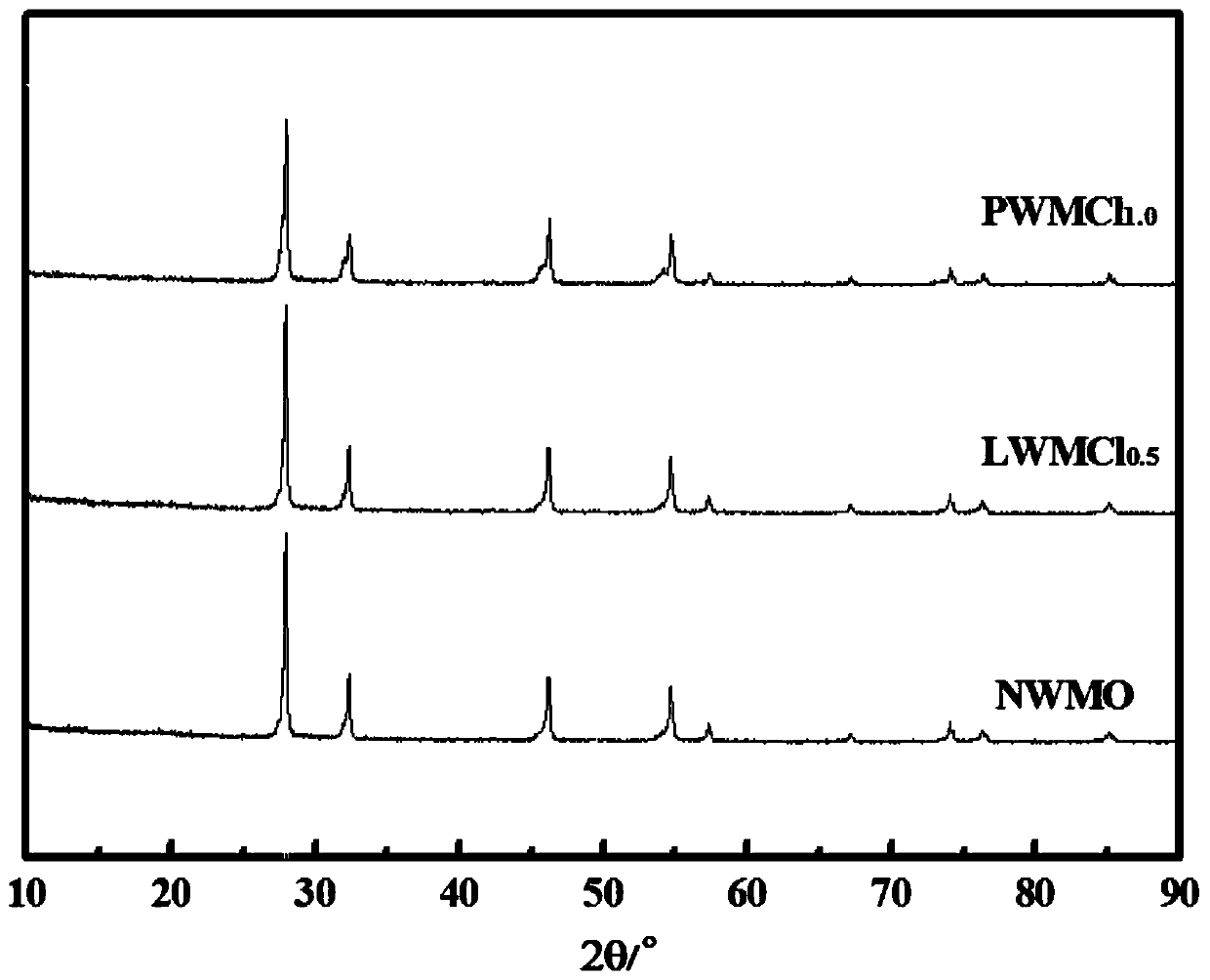
[0050] (1) Weigh 30.42g Nd respectively 2 o 3 , 1.174g NdF 3 , 8.114g WO 3 After preliminary mixing, 20ml of ethanol was added, ball milled at a speed of 300r / min, taken out and dried naturally after 24h; then placed in a high-temperature muffle furnace to raise the temperature to 850°C at a heating rate of 1°C / min, and kept for 10h, and then The rate of 1°C / min was lowered to room temperature to obtain a phase-formed powder. The obtained powder was subjected to phase analysis, and the results were as follows: figure 1 As shown, the material maintains the original fluorite structure after doping with F element, and no other impurity phases are formed.
[0051] (2) Weigh 1.0g of the powder after grinding the phase-formed po...
Embodiment 3
[0053] The non-doped anion-free fluorite-type tungstic acid-based mixed conductor hydrogen permeable membrane material La 5.8 W 0.6 Mo 0.4 o 11.25-δ (LWMO) preparation method, wherein δ=0~1, specifically comprises the following steps:
[0054] (1) Weigh 18.89g La respectively 2 o 3 , 2.782g WO 3 , 1.151 g MoO 3 After initial mixing, 10ml of ethanol was added, ball milled at a speed of 300r / min, taken out and dried naturally after 24h; then placed in a high-temperature muffle furnace to raise the temperature to 700°C at a heating rate of 5°C / min, and kept for 5h, and then The rate of 1°C / min was lowered to room temperature to obtain a phase-formed powder. The obtained powder was subjected to phase analysis, and the results were as follows: figure 1 As shown, the material maintains the original fluorite structure after doping with F element, and no other impurity phases are formed.
[0055] (2) Grind the phase-formed powder for a while, weigh 2.0g of the powder, place i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com