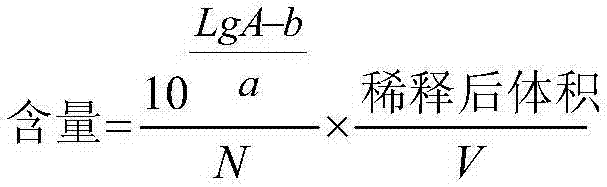Brucea javanica oil emulsion injection liquid quality control method
A quality control method and technology of bruceus brucei oil emulsion, which are applied to measurement devices, instruments, scientific instruments and other directions, can solve problems such as cumbersome steps, inaccurate results, etc., achieve high extraction rate, less mobile phase components, and increase peak area. The effect of ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
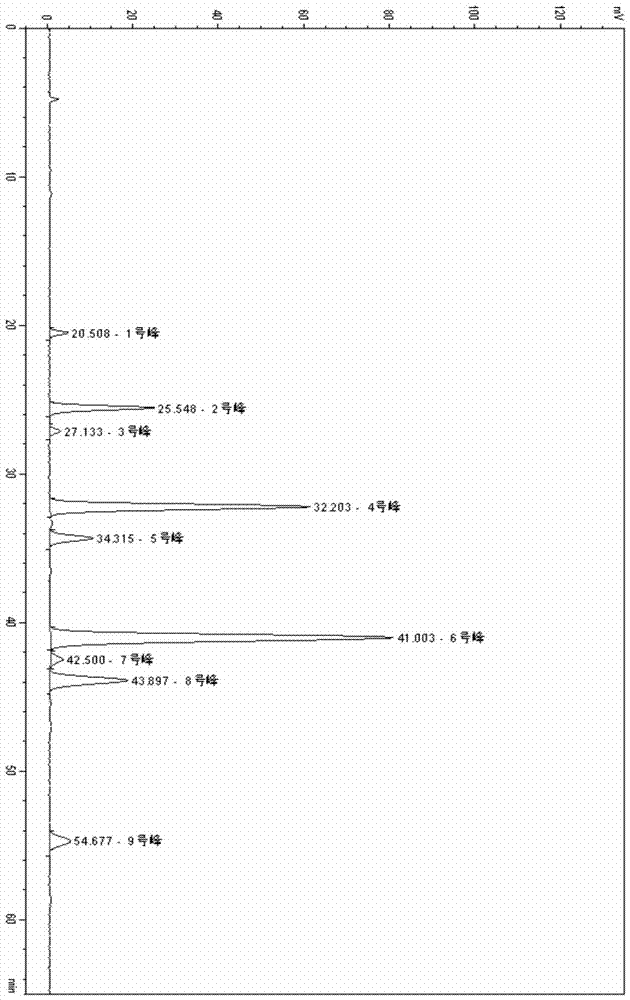
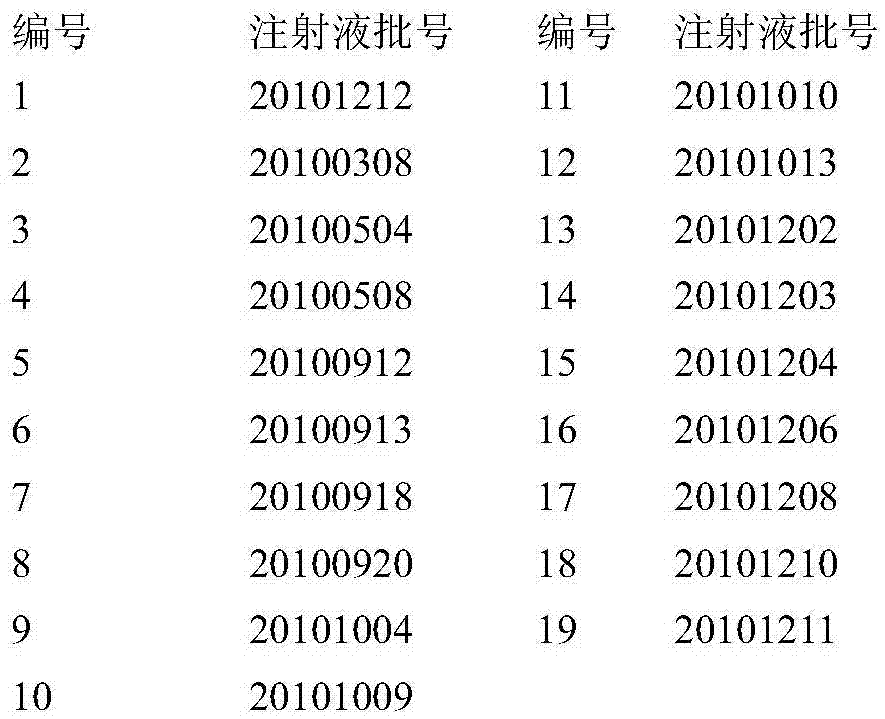
[0051] According to the preparation method of the above-mentioned test solution and the determined chromatographic conditions, the fingerprints of 19 batches of Brucea javanica oil emulsion injection were measured, and according to different production batch numbers, 10 batches of samples were selected to generate Brucea javanica oil emulsion injection. Liquid Standard Fingerprint
[0052] Determination of Fingerprint Samples of Brucea Brucea Oil Emulsion Injection
[0053] Using the standard fingerprints as a comparison, the similarity evaluation system for chromatographic fingerprints of traditional Chinese medicine 2.0 provided by the National Pharmacopoeia was used to measure the similarity of fingerprints of 19 batches of samples. The result is that 95% of the samples have a similarity greater than 0.998, and 100% of the samples have a similarity greater than 0.99.
[0054] Determination of trioleic acid ester content
[0055] The HPLC-ELSD method was used to simultaneo...
experiment example 1
[0057] Experimental Example 1: Fingerprint, determined with reference to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 Edition).
[0058] Chromatographic conditions and system suitability test
[0059] Octadecylsilane bonded silica gel is used as filler; acetonitrile / dichloromethane mixed solution (acetonitrile:dichloromethane is 60-70:40-30, preferably 65:35) is used as mobile phase; the flow rate is per minute 0.3-0.8ml, preferably 0.5ml; column temperature 30-40°C, preferably 35°C; evaporative light scattering detector; analysis time 40-130 minutes, preferably 65 minutes. The number of theoretical plates should be no less than 5000 based on the trioleate peak, and the resolution of peak No. 2 and peak No. 3 corresponding to the standard fingerprint in the test sample should be above 1.5.
[0060] Preparation of reference solution
[0061] Take 1,2-dilinoleic acid-3-oleic acid glyceride, 1,2-dioleic acid-3-linoleic acid glyceride and g...
Embodiment 2
[0182] Embodiment 2: content determination (triglyceride is example)
[0183] Determine according to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 edition).
[0184] The chromatographic conditions and system suitability test are the same as under [Fingerprint].
[0185] The preparation of the reference solution is the same as that of the reference solution I under [Fingerprint].
[0186] The preparation of the test solution is the same as under [Fingerprint].
[0187] Assay
[0188] Precisely draw 5 μl and 10 μl of the reference substance solution and 5-10 μl of the test solution, inject it into the liquid chromatograph, measure its spectrum, and calculate it with the logarithmic equation of the external standard two-point method.
[0189] LgA=aLg(NC)+b
[0190]
[0191] In the formula:
[0192] A is the peak area
[0193] N is the injection volume, μl;
[0194] C is concentration, mg / ml;
[0195] V is the sampling volume, ml; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com