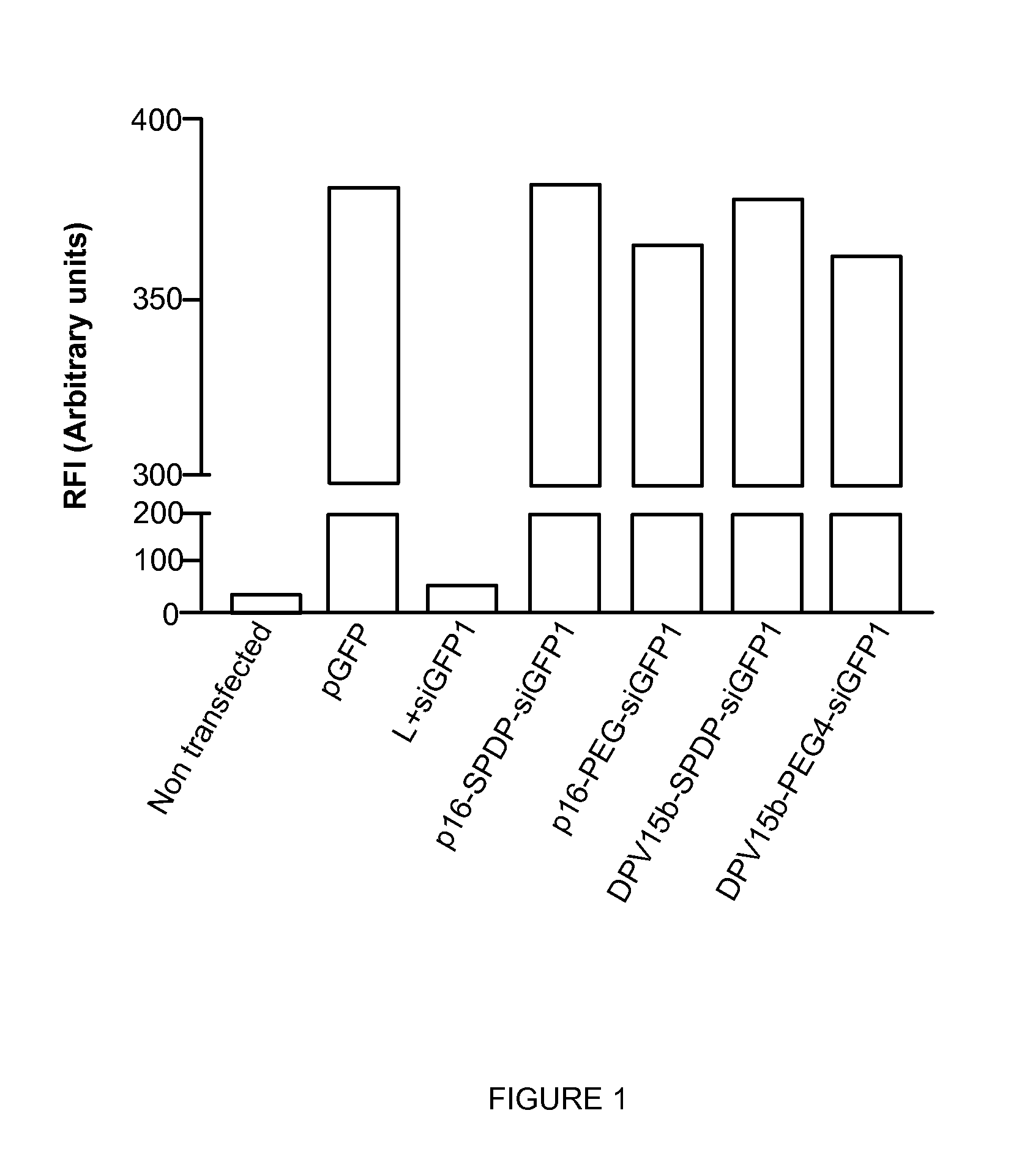
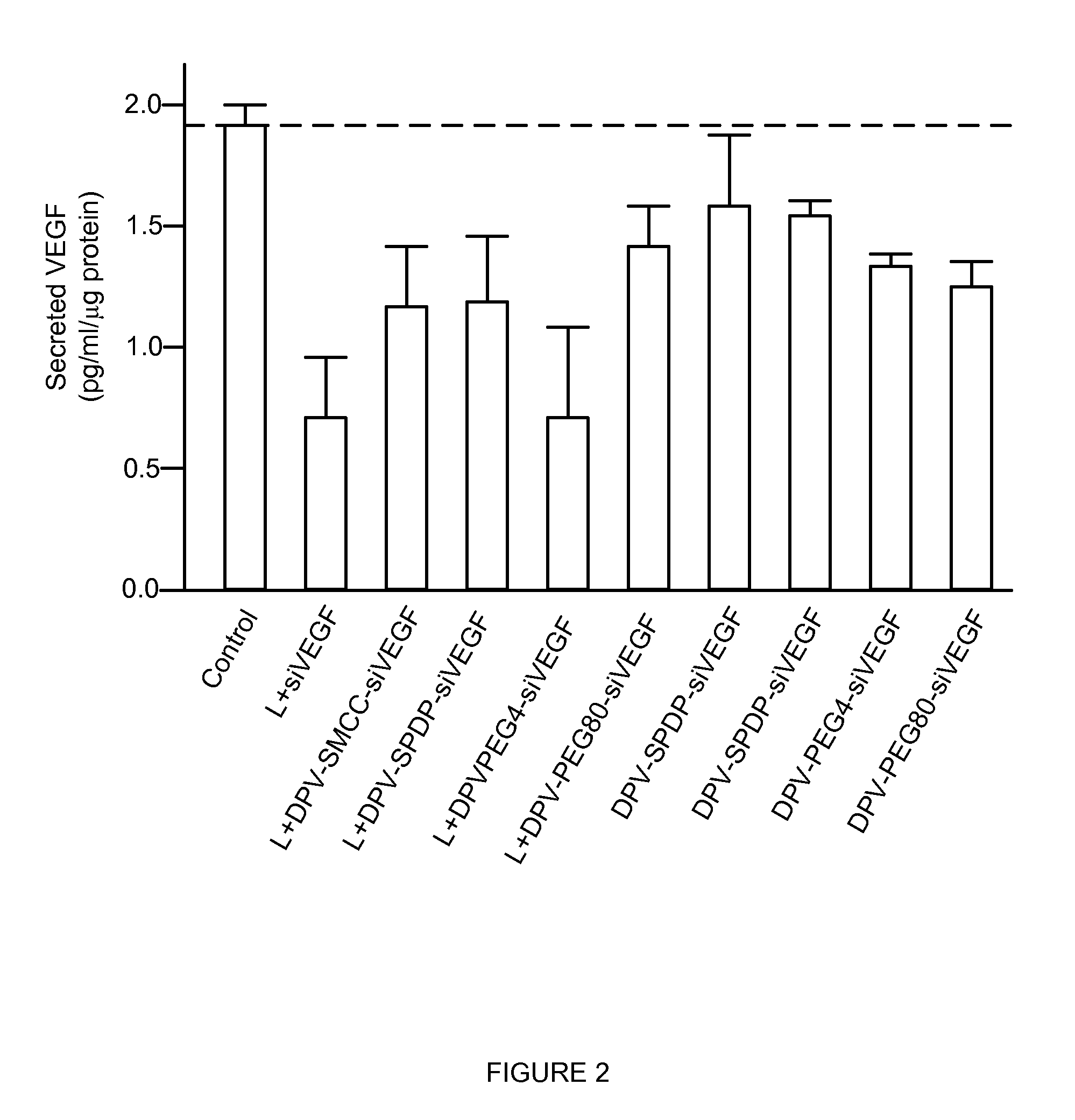
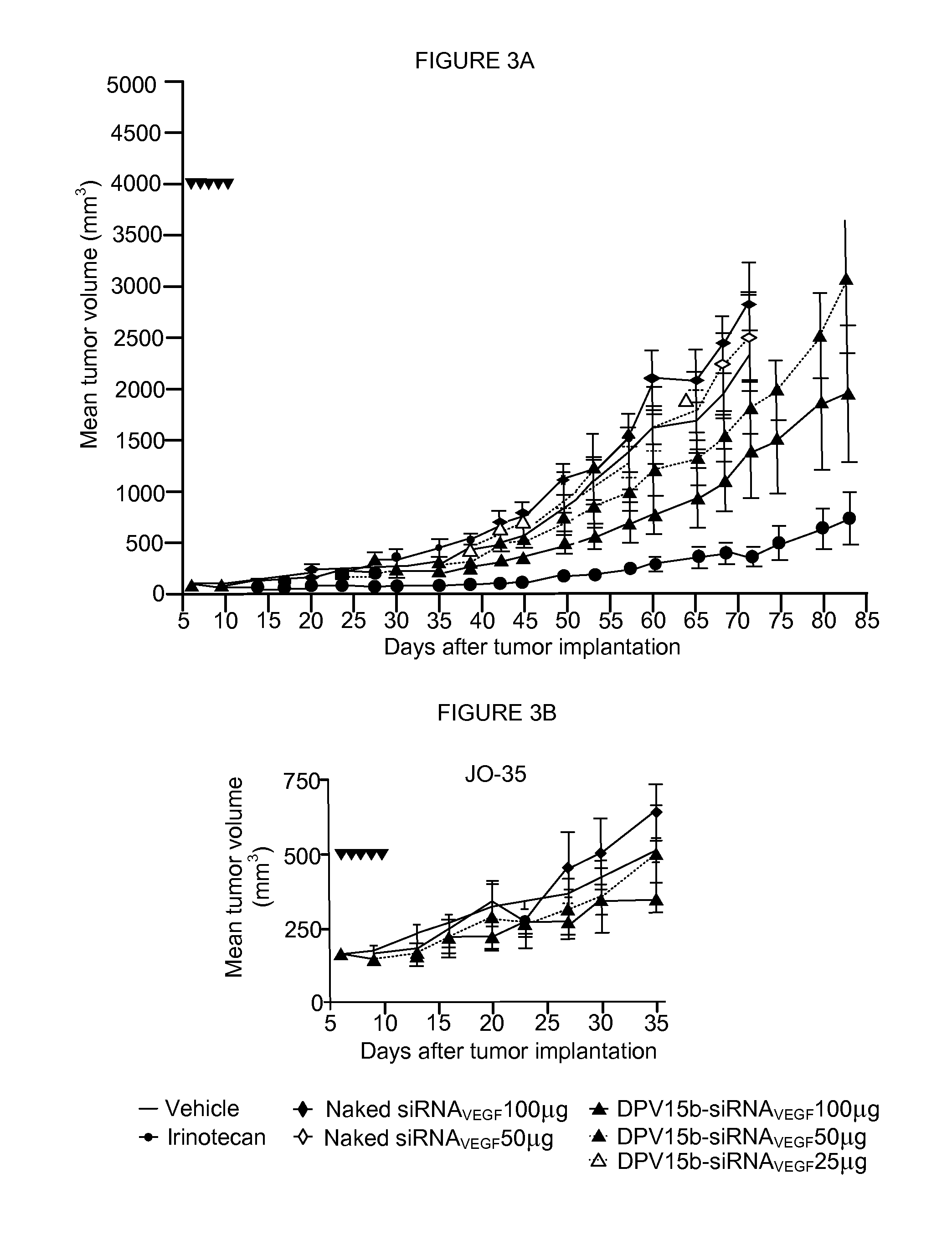
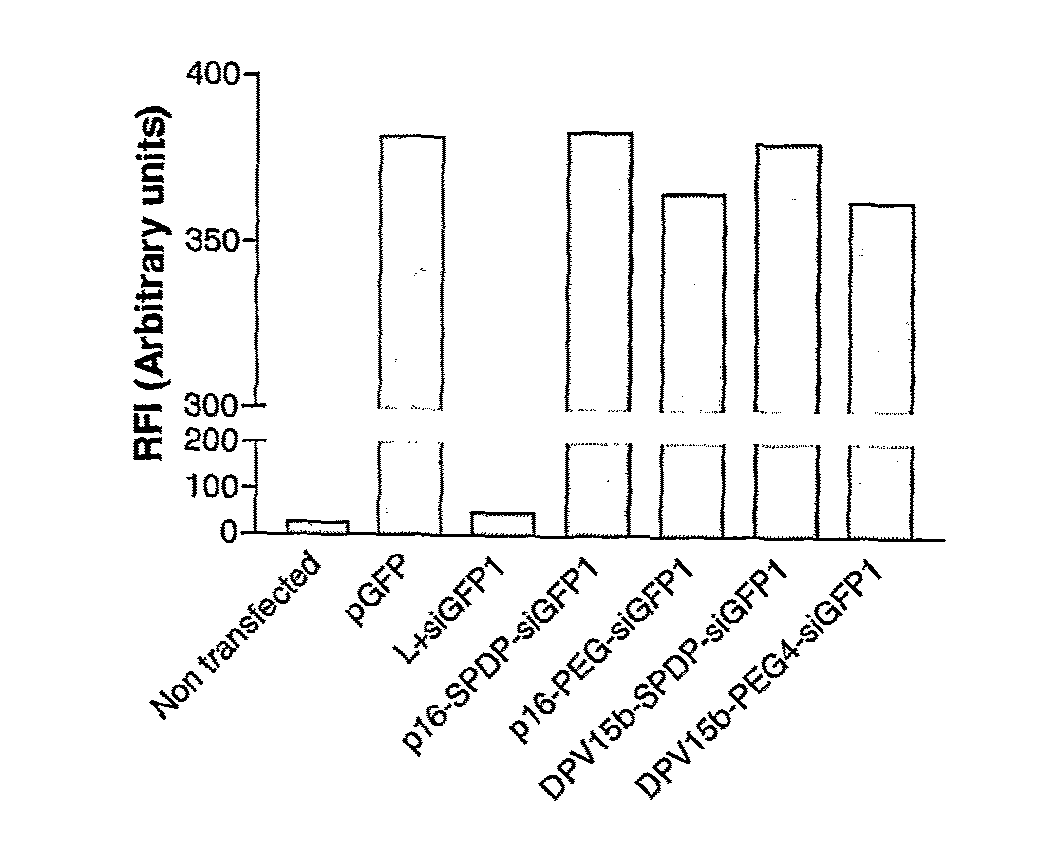
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31results about "Bactericidal/permeability-increasing protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
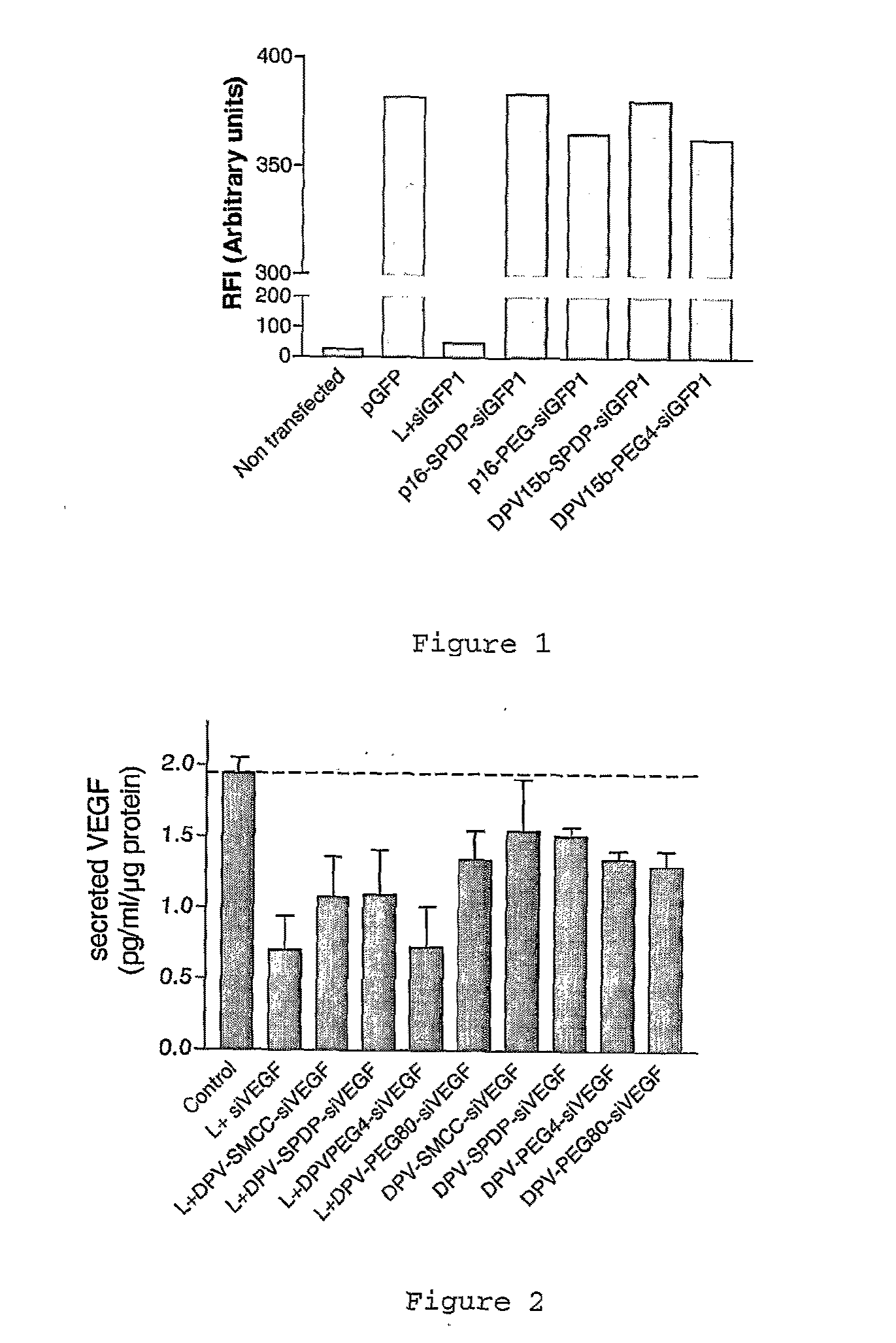
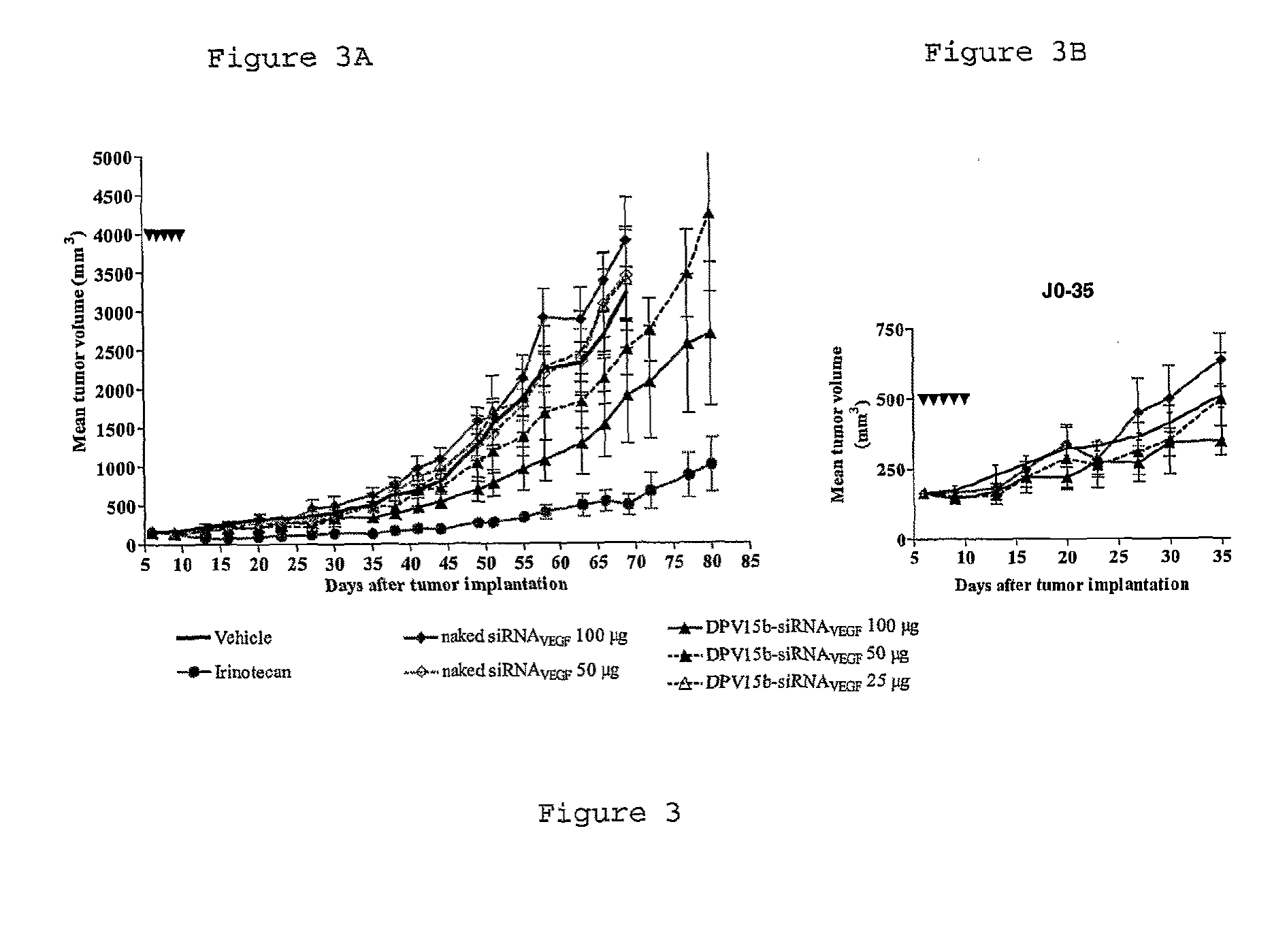
Cell penetrating peptide conjugates for delivering of nucleic acids into a cell
InactiveUS20130137644A1Good curative effectLarge indexNervous disorderAntipyreticHydrophilic polymersPolyethylene glycol
The invention provides cell penetrating peptide-nucleic acid conjugates having the formula P-L-N, wherein P is a cell penetrating peptide, N is a nucleic acid, preferably an oligonucleotide and more preferably a siRNA, and L is a hydrophilic polymer, preferably a polyethylene glycol (PEG)-based linker linking P and N together. Compositions, methods of use and methods for producing such conjugates are also disclosed.
Owner:CELLECTIS SA
Cell Penetrating Peptide Conjugates for Delivering of Nucleic Acids into a Cell
InactiveUS20090186802A1Improve propertiesGood curative effectNervous disorderAntipyreticPolyethylene glycolOligonucleotide
The invention provides cell penetrating peptide-nucleic acid conjugates having the formula P-L-N, wherein P is a cell penetrating peptide, N is a nucleic acid, preferably an oligonucleotide and more preferably a siRNA, and L is a hydrophilic polymer, preferably a polyethylene glycol (PEG)-based linker linking P and N together. Compositions, methods of use and methods for producing such conjugates are also disclosed.
Owner:CELLECTIS SA
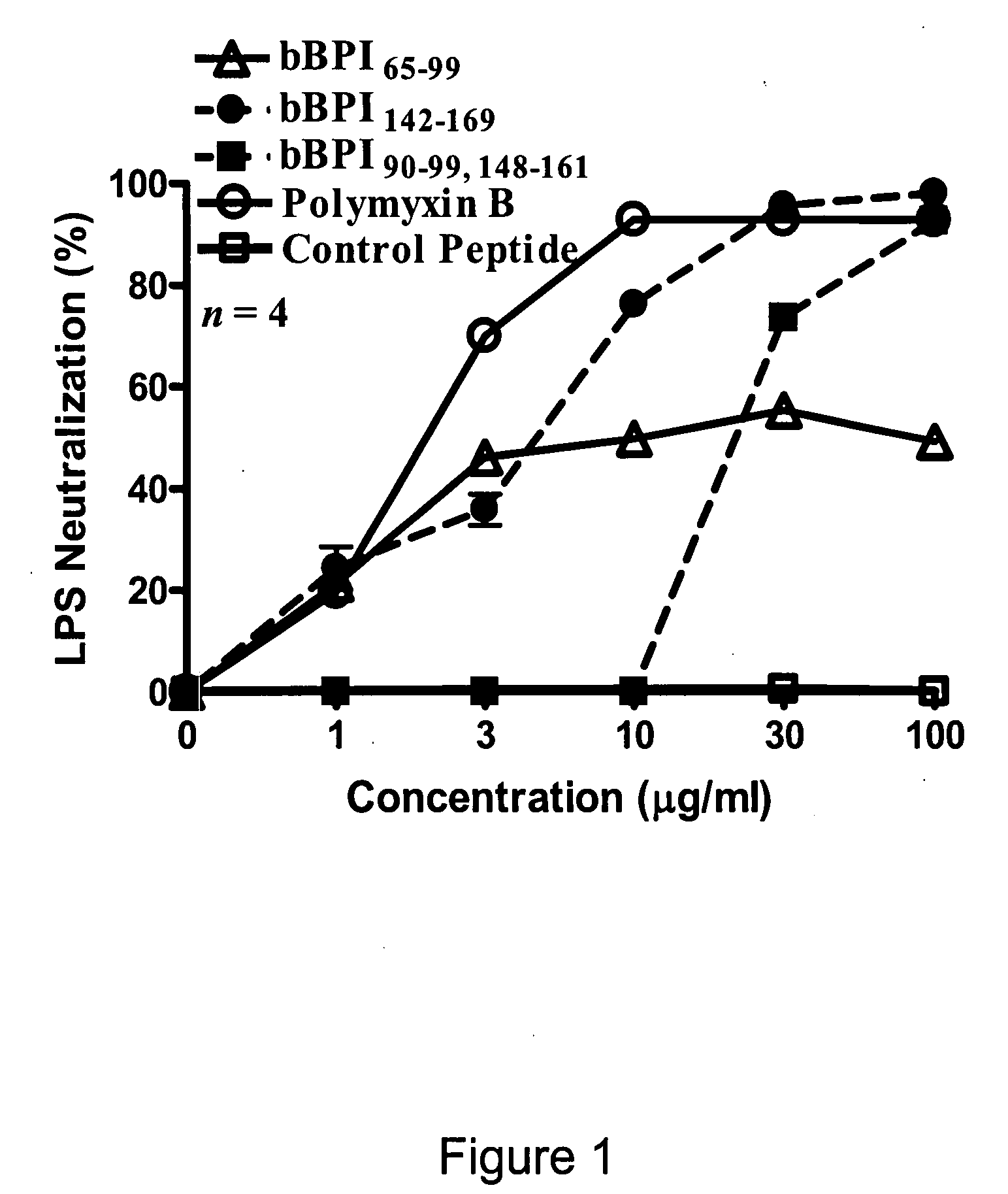
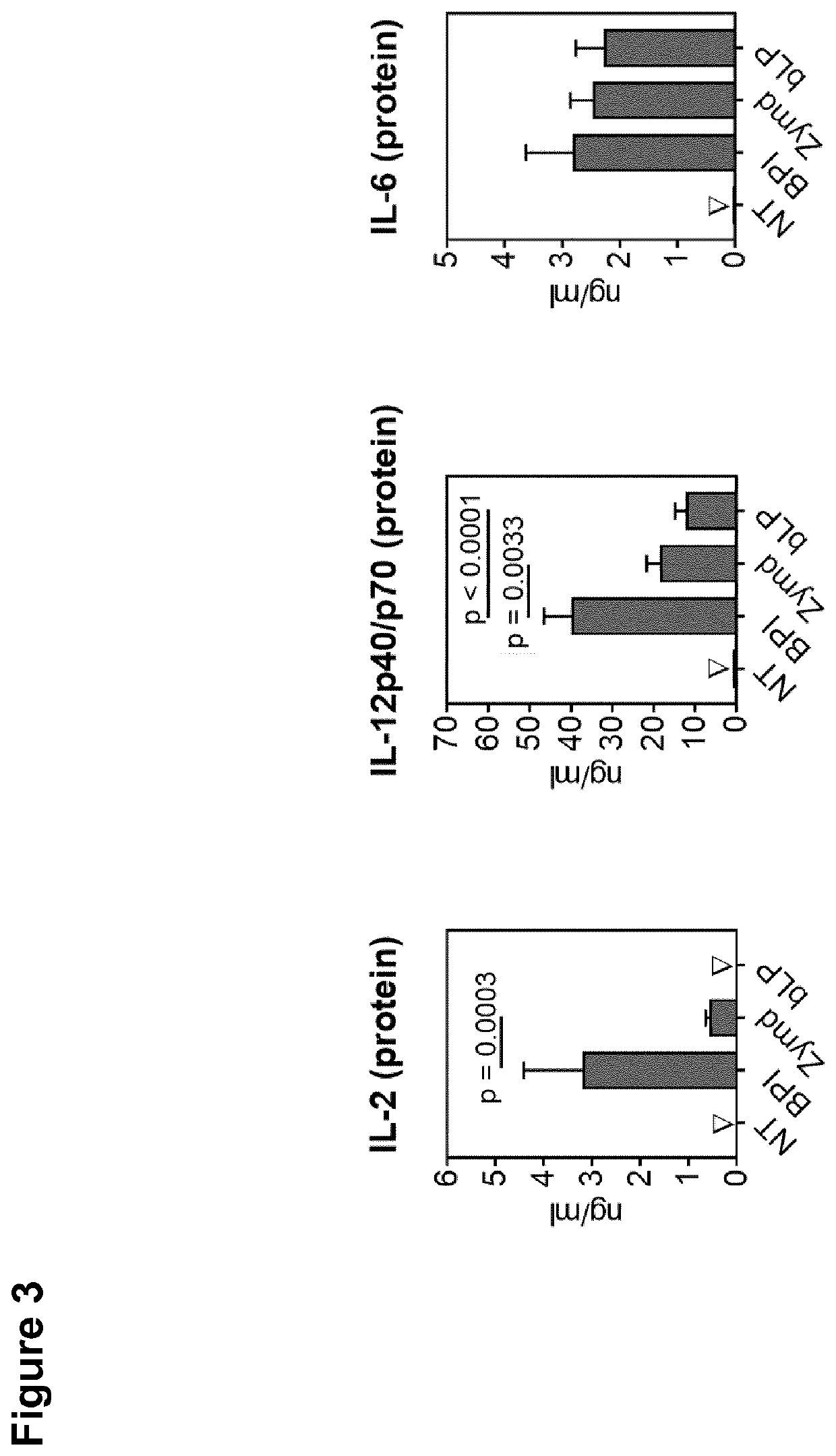
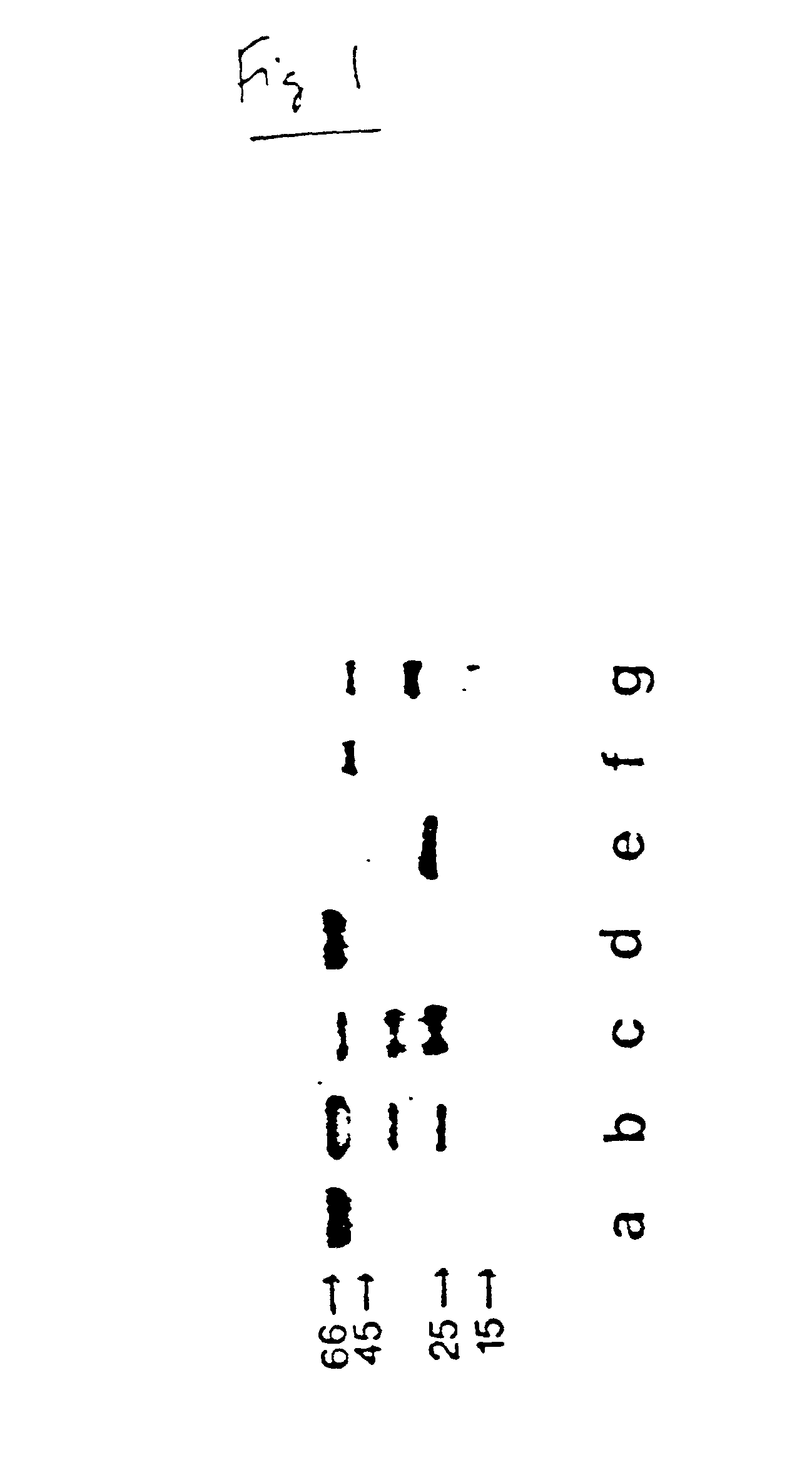
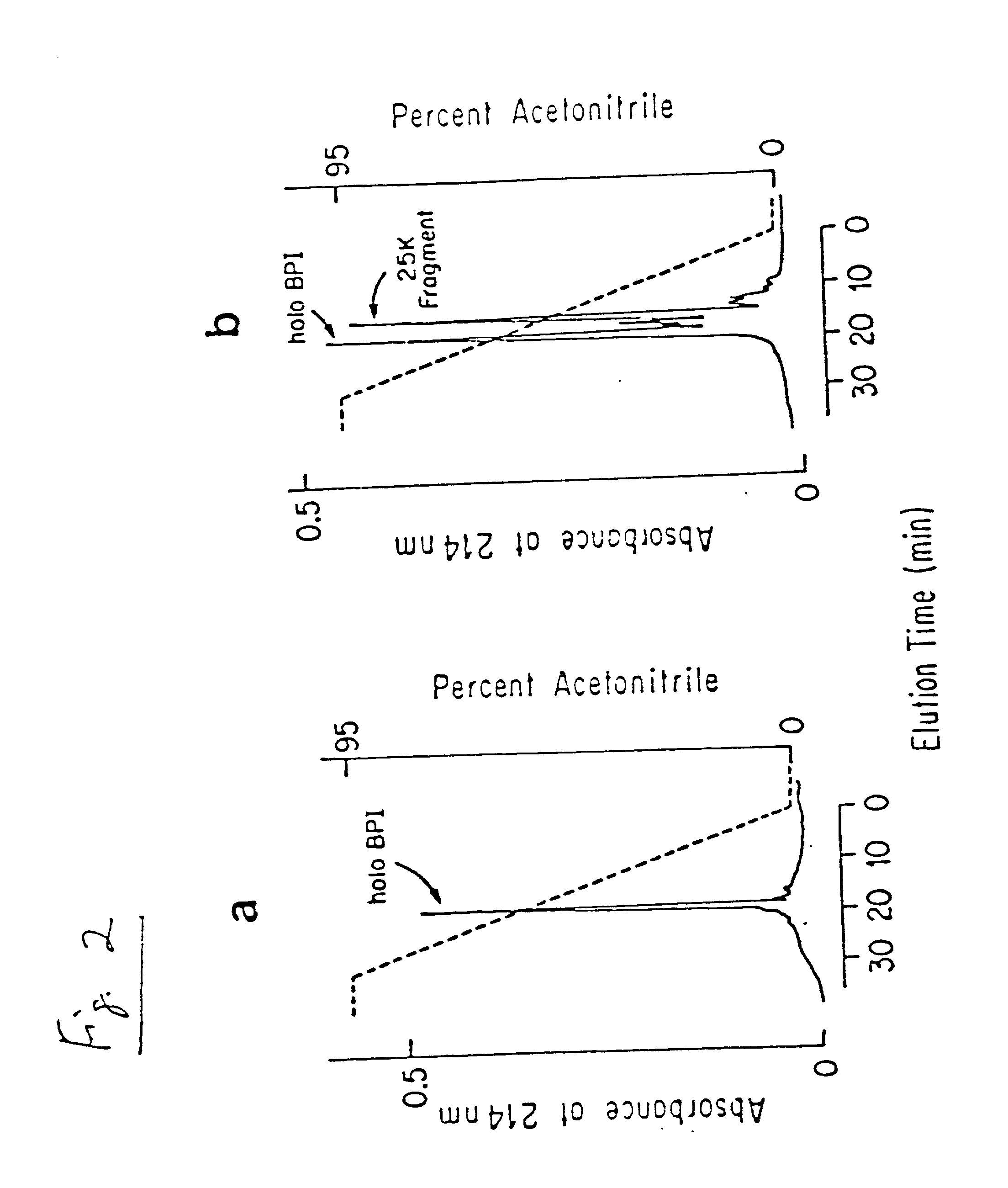
Antimicrobial activity of bovine bactericidal/permeability-increasing protein (BPI)-derived peptides against Gram-negative bacterial mastitis isolates
The antimicrobial activity of bovine bactericidal / permeability-increasing protein (bBPI)-derived synthetic peptides against mastitis-causing Gram-negative bacteria was evaluated. Three peptides were synthesized with sequences corresponding to amino acids 65-99 (bBPI65-99), 142-169 (bBPI142-169), or the combination of amino acids 90-99 and 148-161 (bBPI90-99,148-161) of bBPI. The bBPI90-99,148-161 peptide demonstrated the widest spectrum of antimicrobial activity, with minimum inhibitory (MIC) and bactericidal (MBC) concentration values ranging from 16-64 μg / ml against Escherichia coli, Klebsiella pneumoniae, and Enterobacter spp, and 64-128 μg / ml against Pseudomonas aeruginosa. None of the peptides exhibited any growth inhibitory effect on Serratia marcescens. The antimicrobial activity of bBPI90-99,148-161 was inhibited in milk, but preserved in serum. Finally, both bBPI142-169 and bBPI90-99,148-161 were demonstrated to completely neutralize LPS. The peptide bBPI90-99,148-161 is a potent neutralizer of the highly pro-inflammatory molecule bacterial LPS and has antimicrobial activity against a variety of Gram-negative bacteria.
Owner:US SEC AGRI
Synthetic peptides with antimicrobial and endotoxin neutralizing properties for management of the sepsis syndrome
A peptide with an amino acid composition such that the peptide is amphipathic, cationic and forms a stable alpha-helix and has the following structure comprising at least 12 amino acidsA=an amino acid selected from the basic amino acids Lys,Arg or HisB=an amino acid selected from the aromatic amino acids Phe, Trp or TyrC=an amino acid selected from the group comprising the hydrophobic amino acids Leu, Ile, Val or Ala, andsaid peptide has either the orientation according to the formula or the retro orientation thereof, wherein at least 0-m of the repetitive sequence motifs (A2-B2-C1-A3) have the retro orientation and the remaining repetitive motifs (A2-B2-C1-A3) have the orientation as presented in the formula and wherein,R1-R2- and R3 are a number of amino acids, and whereinm=1-10, preferably 2-8, more preferably 2-5 andn=1-3, a pharmaceutical composition comprising such a peptide application thereof in treatment or diagnosis related to i.a. parasite infection topical and systemic tumors and septic shock.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT +1
Peptides for the activation of the immune system in humans and animals
The present invention is directed to compositions and methods for the treatment of diseases comprising the administration of compositions comprising one or more peptide(s) having a stimulatory effect on the afflicted host's immune system. Specifically, the invention relates to methods comprising the use of cationic amphipathic peptides having an alpha-helical structure and effecting activation of macrophages when administered in a therapeutically sufficient amount. The methods of the present invention are useful for the treatment of, for example, infectious diseases or cancer.
Owner:MOR AMRAM
Soluble cytoplasmic expression of heterologous proteins in escherichia coli
InactiveUS20130273585A1Specific activityPeptide/protein ingredientsMicrobiological testing/measurementEscherichia coliHeterologous
Soluble variants of recombinant proteins produced in a prokaryotic host cell, where the high expression levels often cause the original proteins to aggregate into insoluble inclusion body aggregates. The variant polypeptides retain biological function while increasing protein solubility with comparable or higher recoverable levels of biologically active protein when expressed in a suitable expression host. Methods of identifying critical residues and substituting them are provided to produce the variants.
Owner:GANGAGEN
Genetic markers for improved disease resistance in animals (BPI)
A method for determining improved disease resistance in animals is disclosed. The method assays for a novel genetic alleles of the BPI gene of the animal. The alleles are correlated with superior disease resistance. Novel nucleotide sequences, assays and primers are disclosed for the methods of the invention.
Owner:PIG IMPROVEMENT UK +2
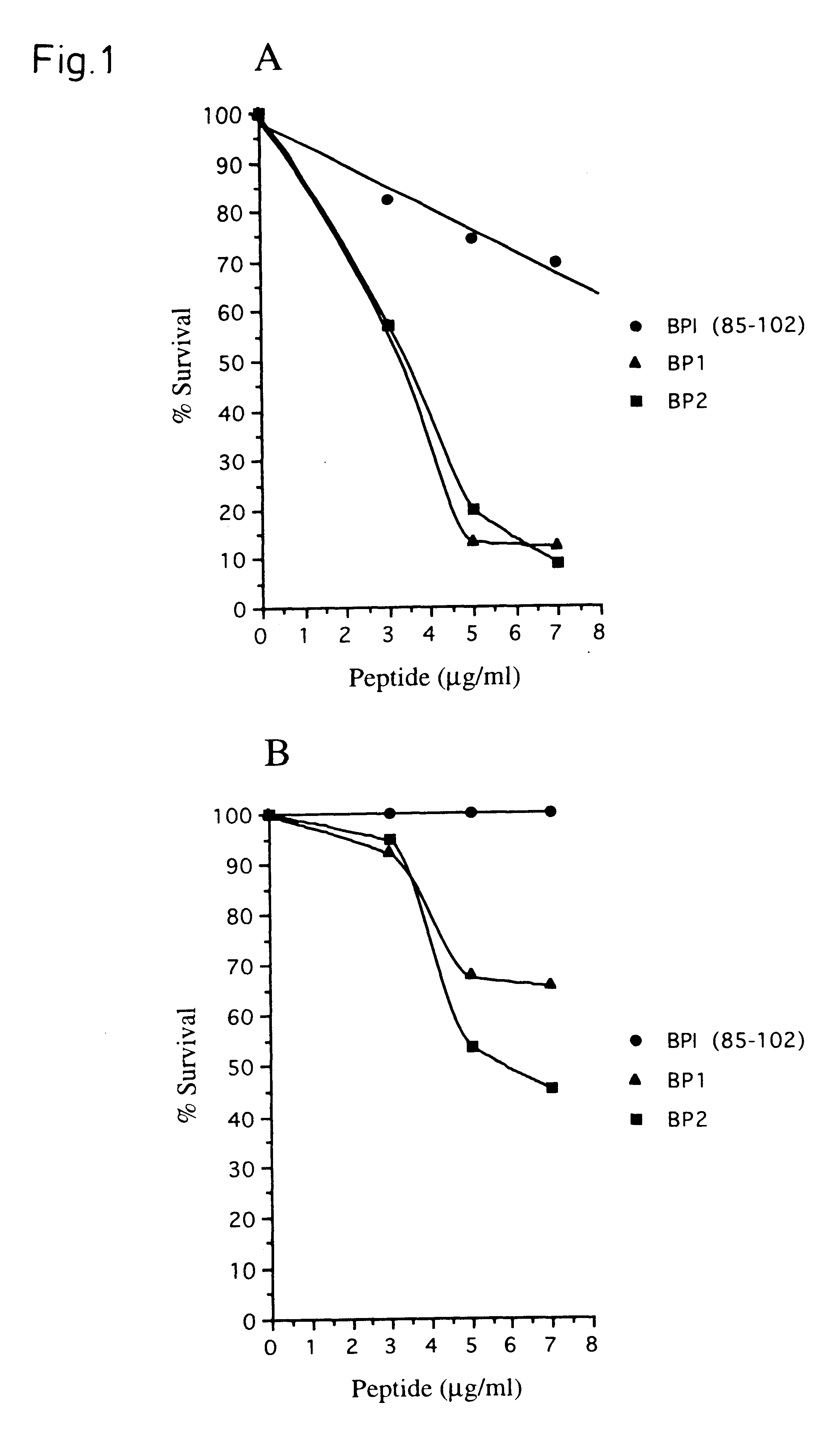
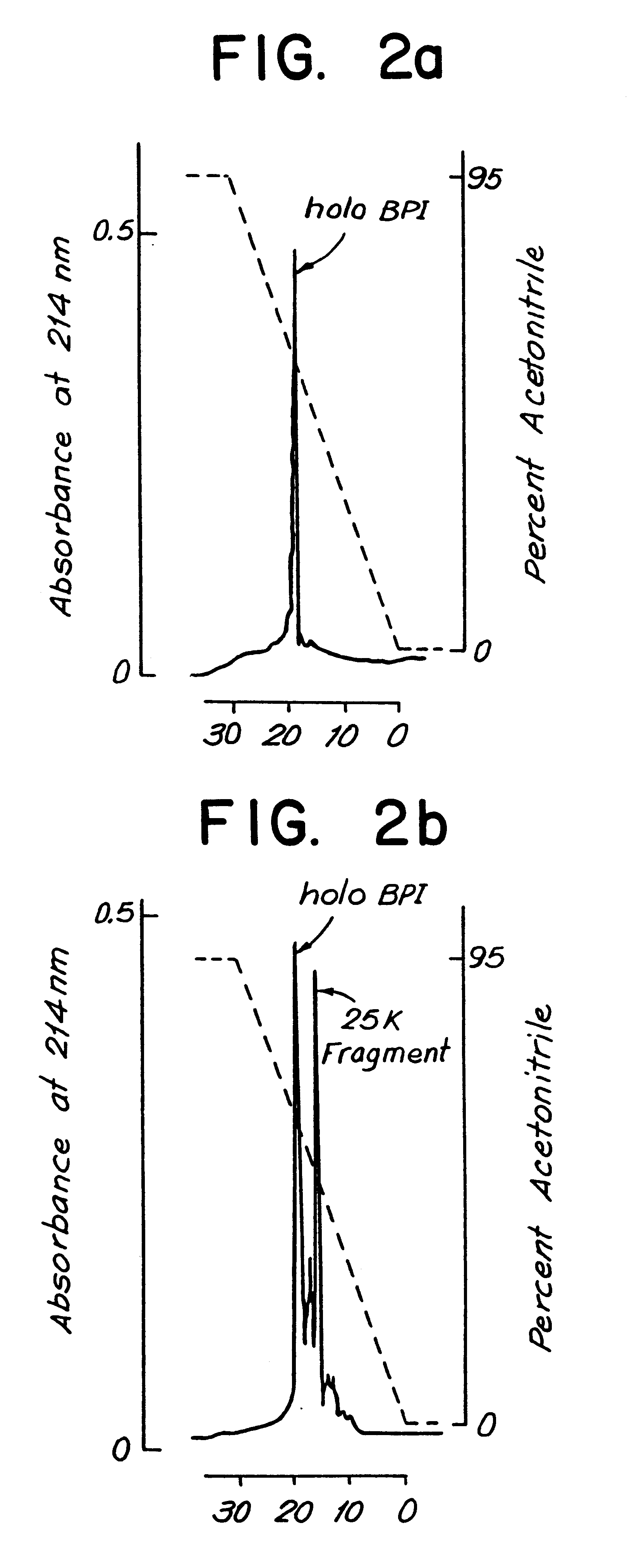
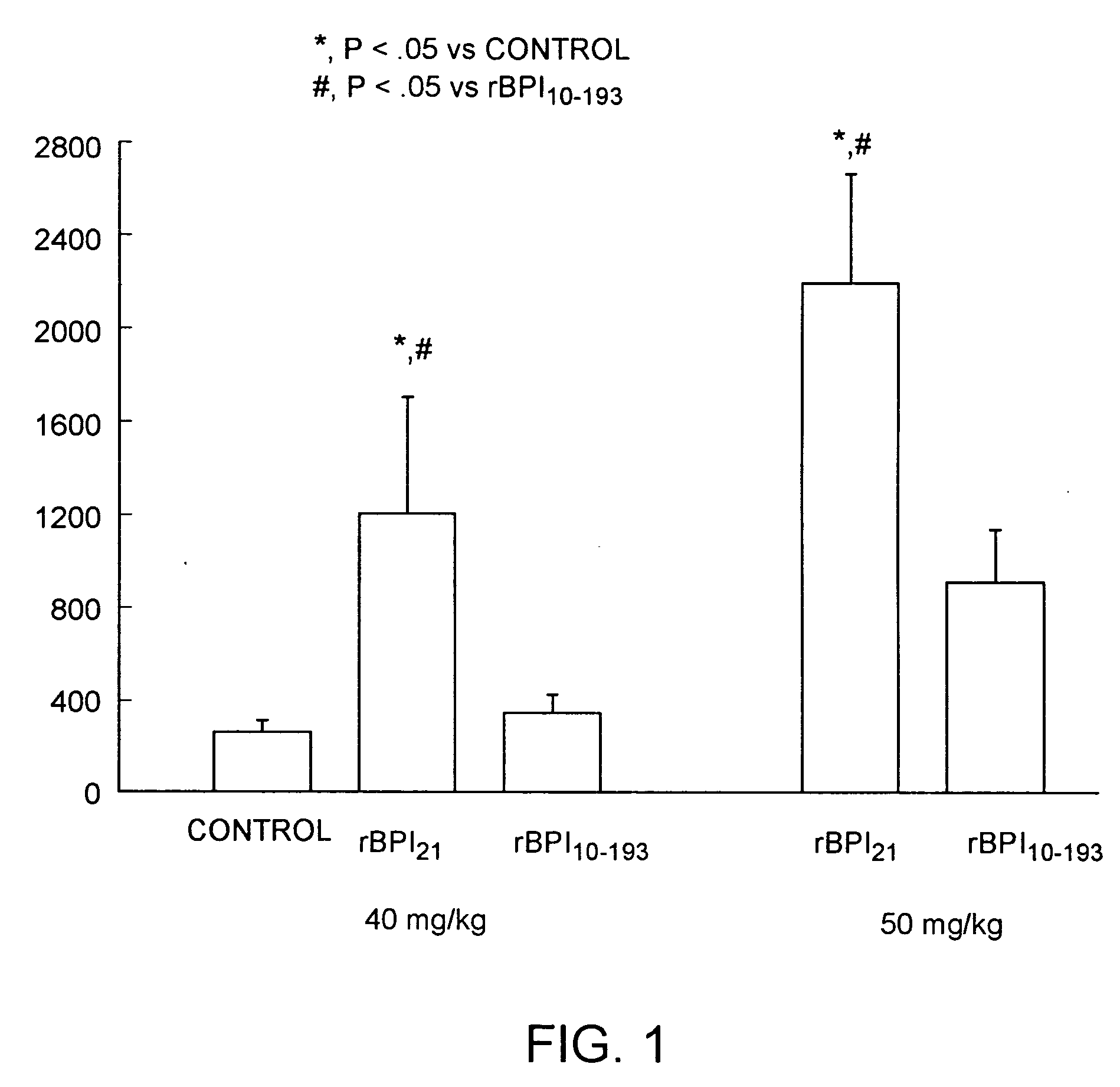
Therapeutic uses of biologically active bactericidal/permeability-increasing protein fragments
InactiveUS6132775AImprove efficiencyImprove breathabilityAntibacterial agentsBiocideBactericidal/permeability-increasing proteinProtein Fragment
Therapeutic methods using a purified isolated human bactericidal / permeability-increasing protein and biologically-active fragments thereof is provided.
Owner:NEW YORK UNIV
Synthetic approach to designed chemical structures
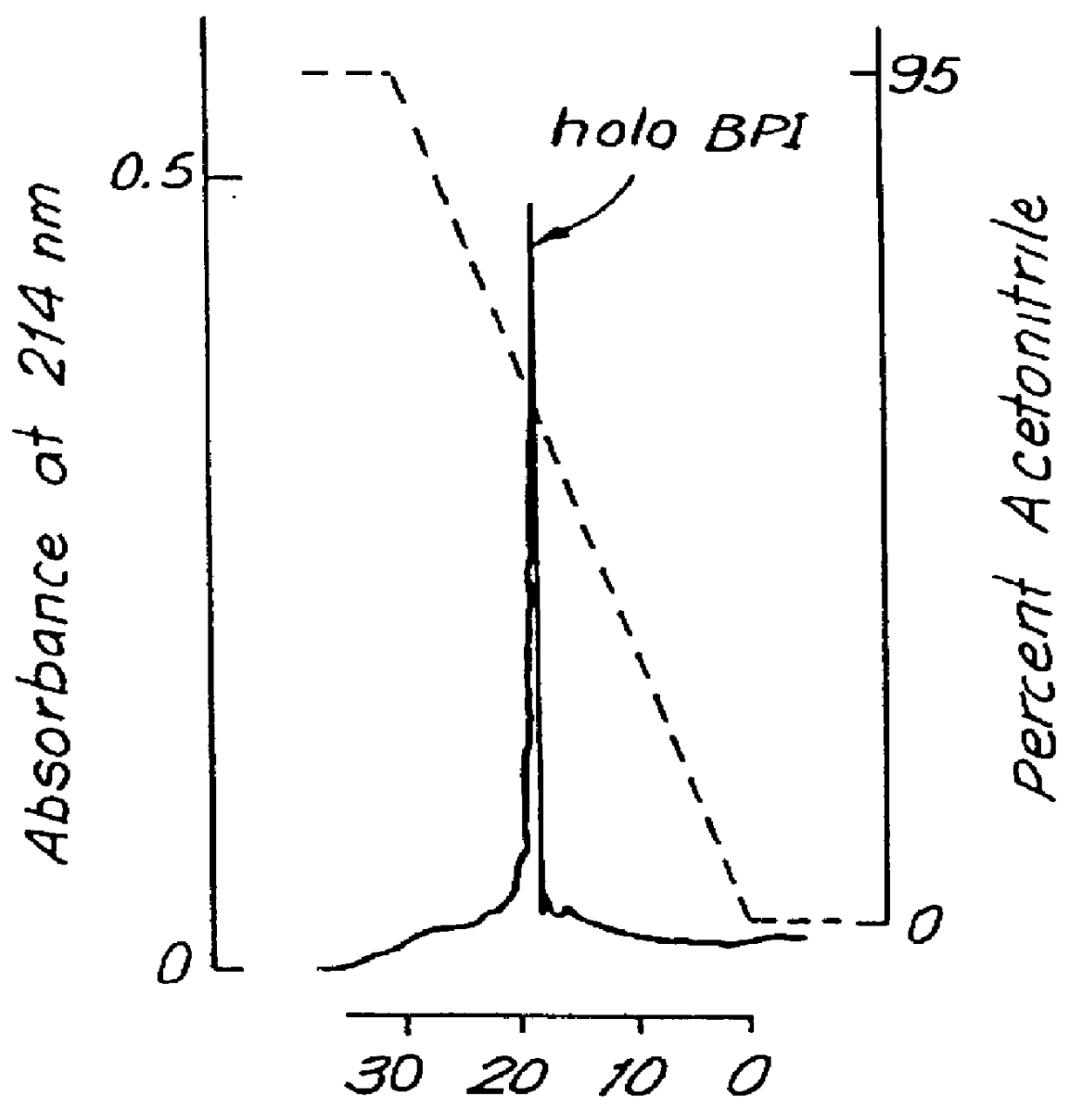
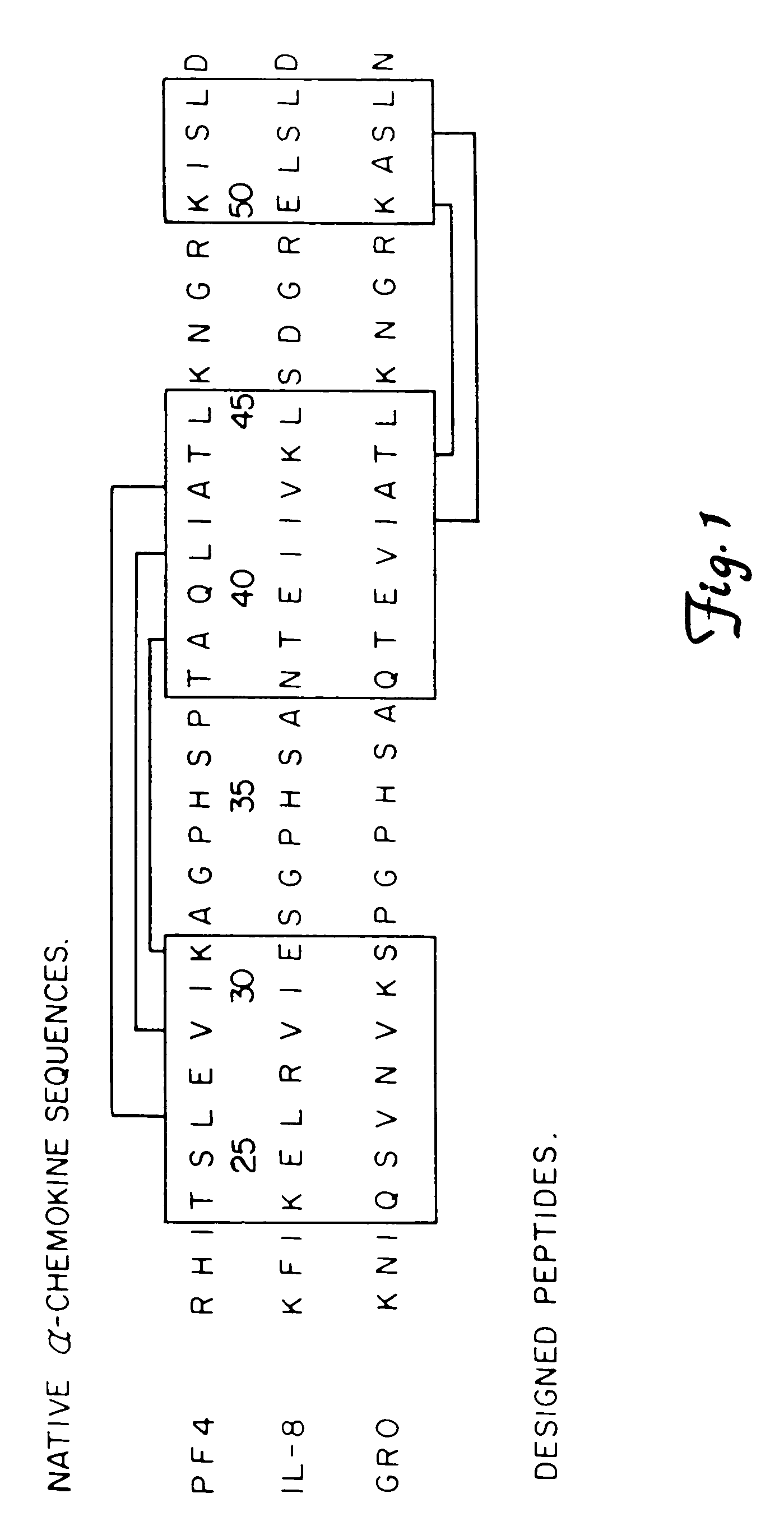
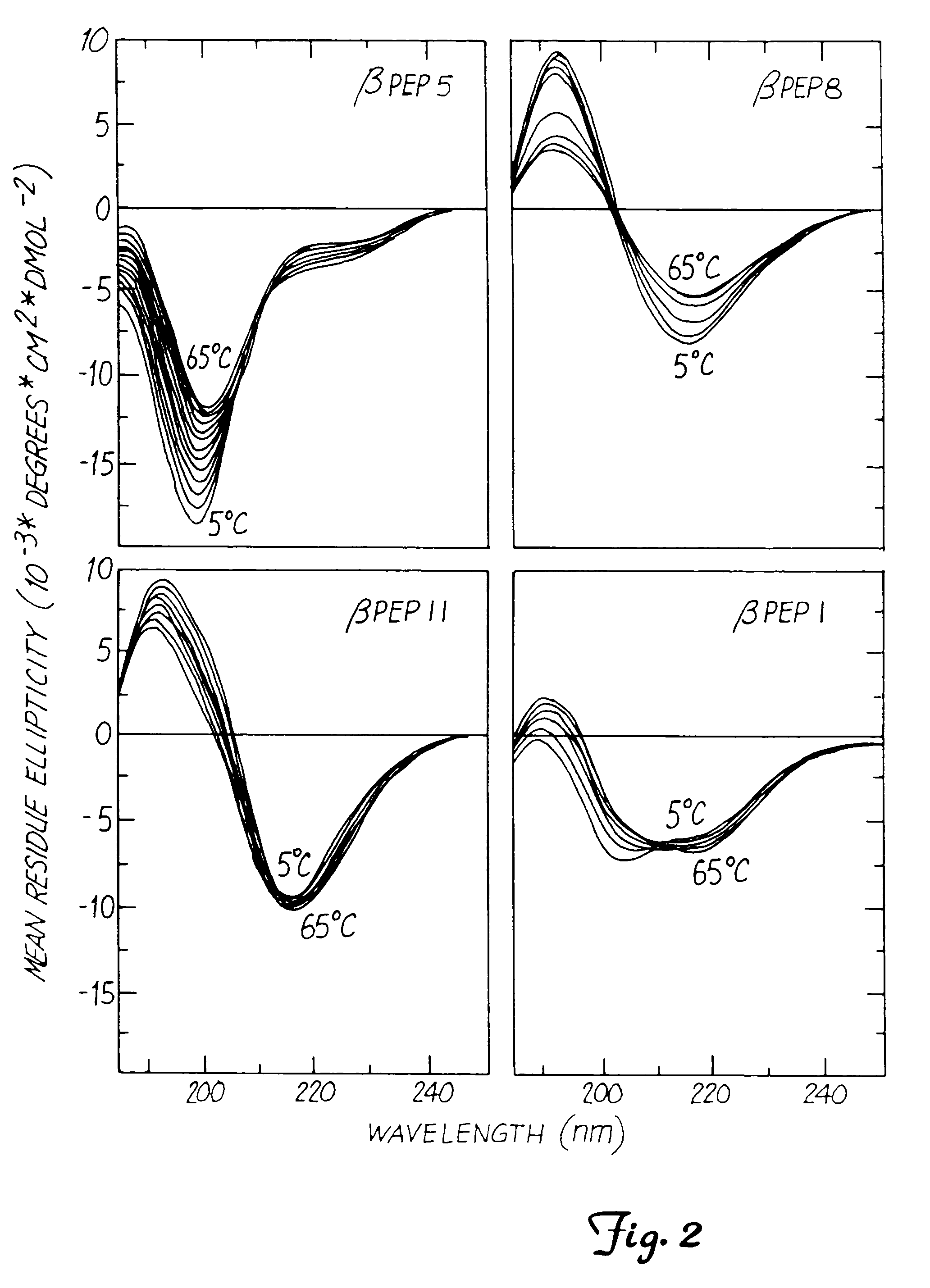
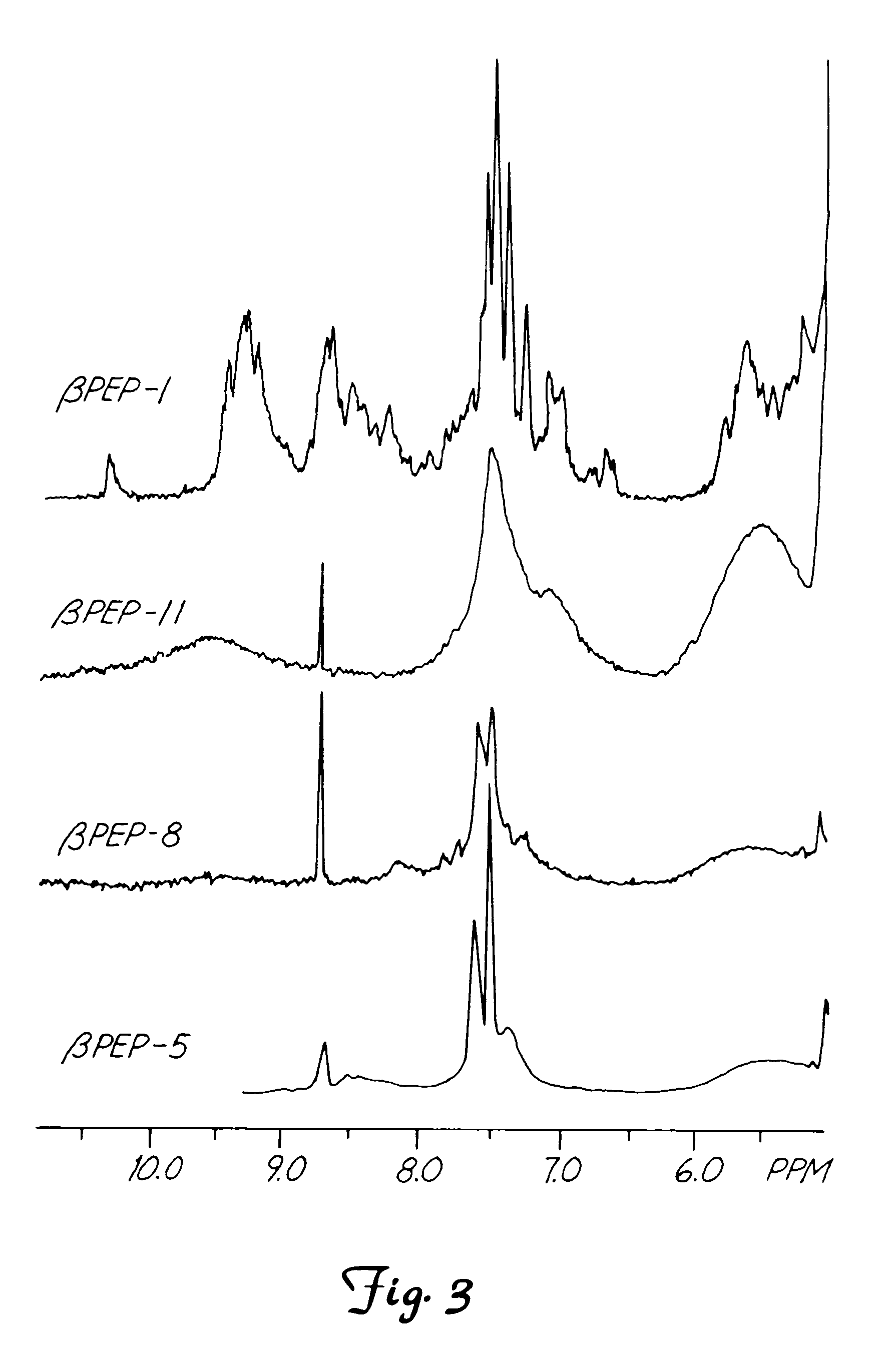
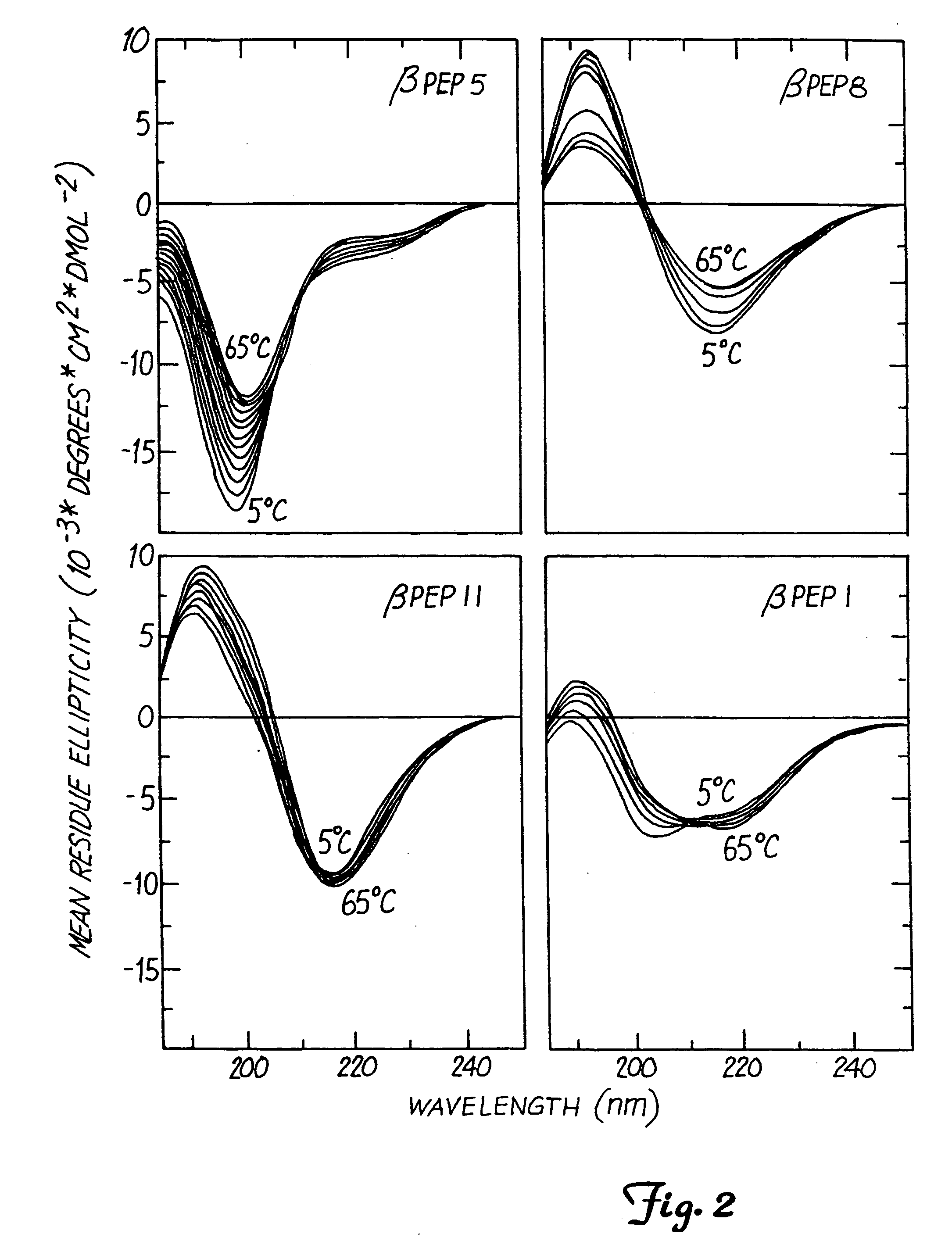
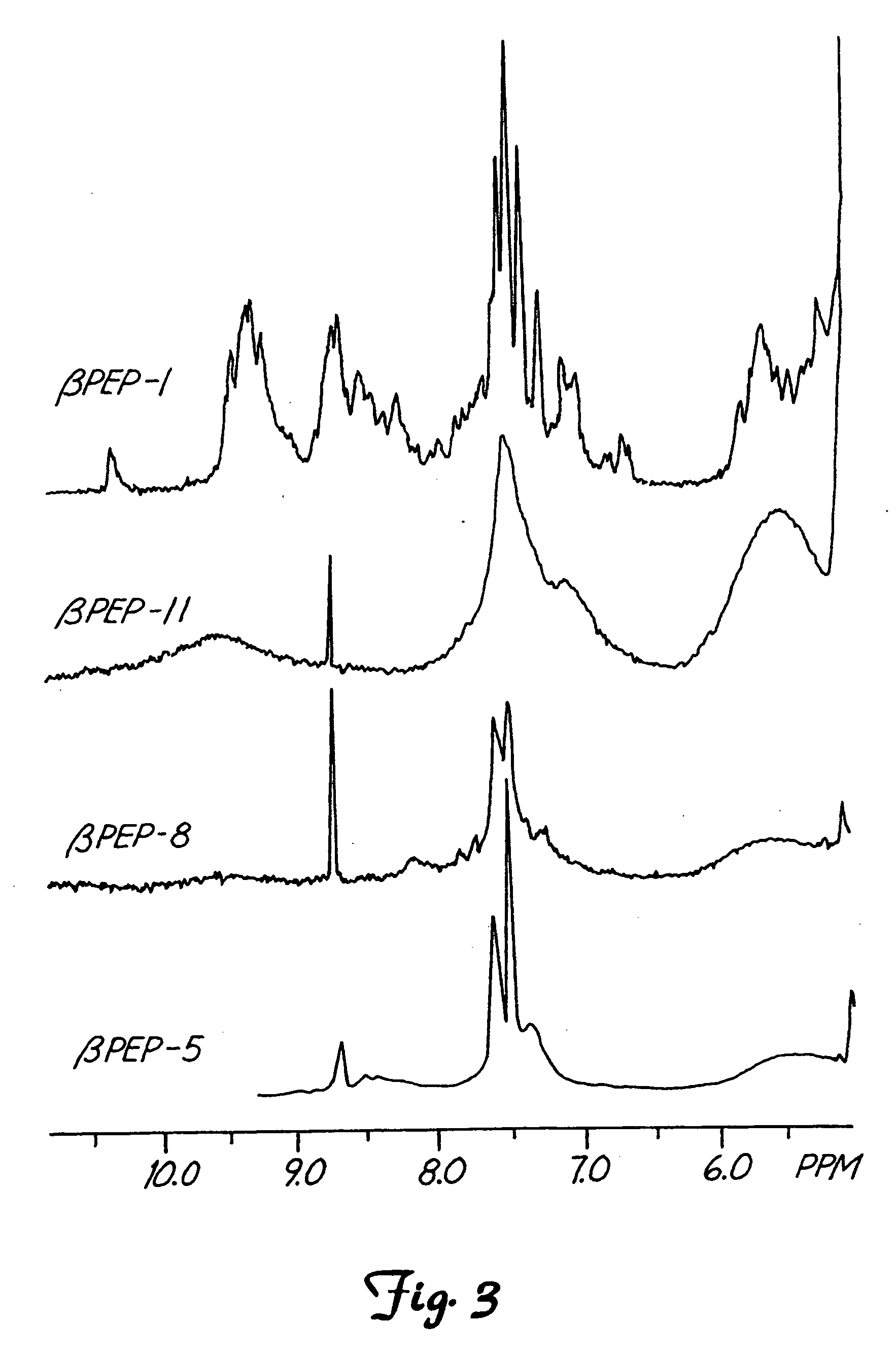
This invention relates to the chemical design and production of peptides, peptide structure and three dimensional conformation was assessed using NMR, circular dichroisin and pulsed field gradient NMR. In addition, this invention relates to peptides produced by these methods and to methods for using the peptides.
Owner:RGT UNIV OF MINNESOTA
Peptides for the activation of the immune system in humans and animals
The present invention is directed to compositions and methods for the treatment of diseases comprising the administration of compositions comprising one or more peptide(s) having a stimulatory effect on the afflicted host's immune system. Specifically, the invention relates to methods comprising the use of cationic amphipathic peptides having an alpha-helical structure and effecting activation of macrophages when administered in a therapeutically sufficient amount. The methods of the present invention are useful for the treatment of, for example, infectious or cancer.
Owner:CENT NAT DE LA RECHERCHE SCI
Novel synthetic peptides with antimicrobial and endotoxin neutralizing properties for management of the sepsis syndrome
InactiveUS20040049011A1Prevent septic shockPeptide/protein ingredientsPeptide preparation methodsAlpha helixSaxitoxin
A peptide with an amino acid composition such that the peptide is amphipathic, cationic and forms a stable alpha-helix and has the following structure comprising at least 12 amino acids R1-R2-A1-B1-(A2-B2-C1-A3)m-(C2)n-R3, wherein A=an amino acid selected from the basic amino acids Lys,Arg or His B=an amino acid selected from the aromatic amino acids Phe, Trp or Tyr C=an amino acid selected from the group comprising the hydrophobic amino acids Leu, Ile, Val or Ala, and said peptide has either the orientation according to the formula or the retro orientation thereof, wherein at least 0-n of the repetitive sequence motifs (A2-B2-C1-A3) have the retro orientation and the remaining repetitive motifs (A2-B2-C1-A3) have the orientation as presented in the formula and wherein, R1-R2- and R3 are a number of amino acids, and wherein m=1-10, preferably 2-8, more preferably 2-5 and n=1-3, a pharmaceutical composition comprising such a peptide application thereof in treatment or diagnosis related to i.a. parasite infection topical and systemic tumors and septic shock.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Chimeric antibacterial polypeptides
ActiveUS20140050713A1Inhibiting bacterial cell growthReduce in quantityAntibacterial agentsPolypeptide with localisation/targeting motifChemistry
Owner:BACTOCLEAR HLDG PTE LTD
Synthetic approach to designed chemical structures
InactiveUS20060183191A1Antibacterial agentsOrganic active ingredientsChemical structureCompound (substance)
This invention relates to the chemical design and production of peptides, peptide structure and three dimensional conformation was assessed using NMR, circular dichroisin and pulsed field gradient NMR.
Owner:RGT UNIV OF MINNESOTA
Therapeutic peptide-based constructs
InactiveUS6906037B2Inhibiting endothelial cell proliferationInhibit angiogenesisBiocidePeptide/protein ingredientsBactericidal/permeability-increasing proteinSmall peptide
The present invention relates generally to small peptide-based constructs and their therapeutic uses. The sequences of these peptide-based constructs are based on a reverse subsequence derived from Domain II of bactericidal / permeability-increasing protein (BPI).
Owner:XOMA TECH LTD
Anti-fungal peptides
The present invention relates generally to anti-fungal peptides derived from or based on Domain III (amino acids 142-169) of bactericidal / permeability-increasing protein (BPI) and therapeutic uses of such peptides.
Owner:XOMA CORP
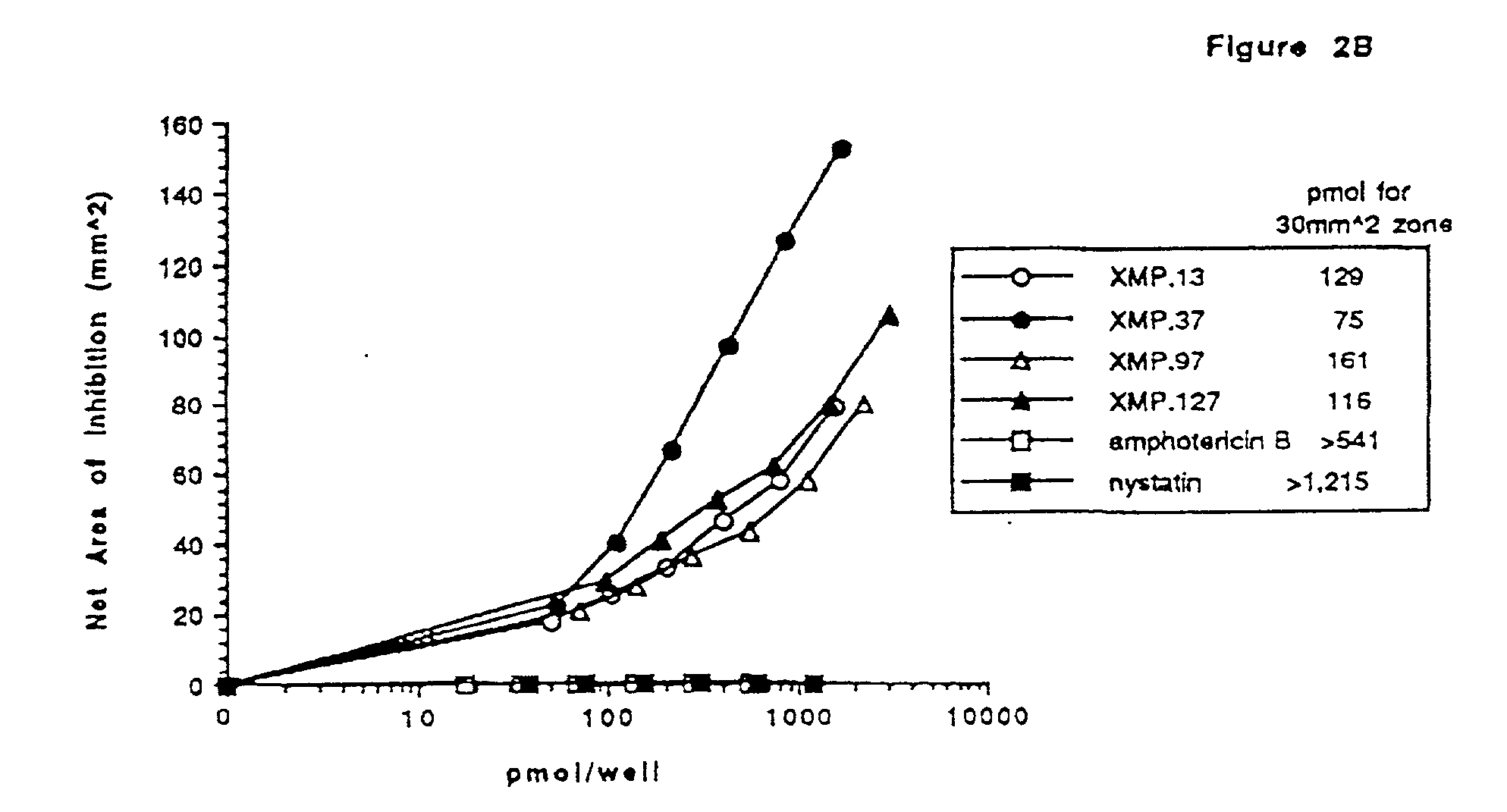
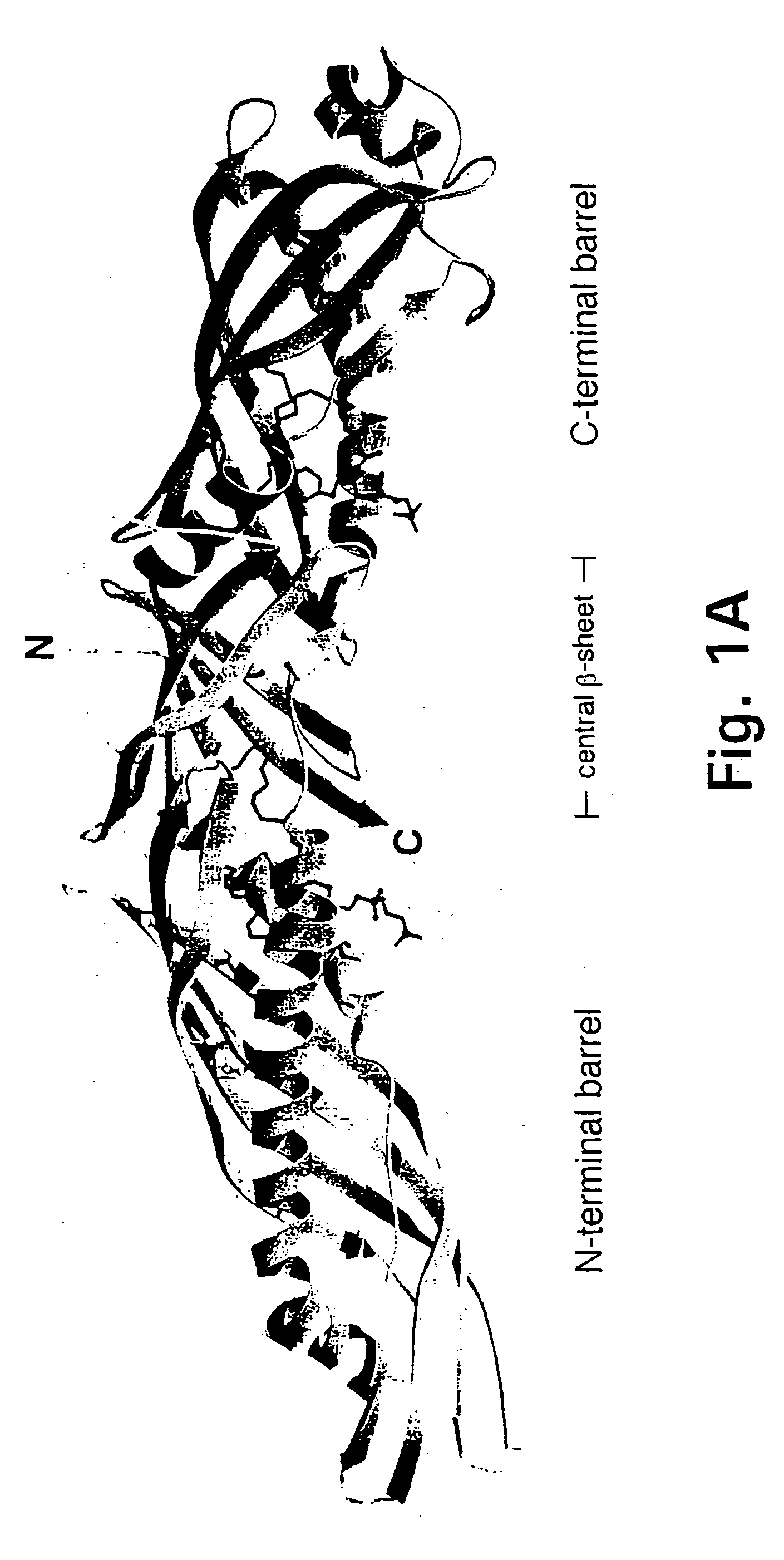
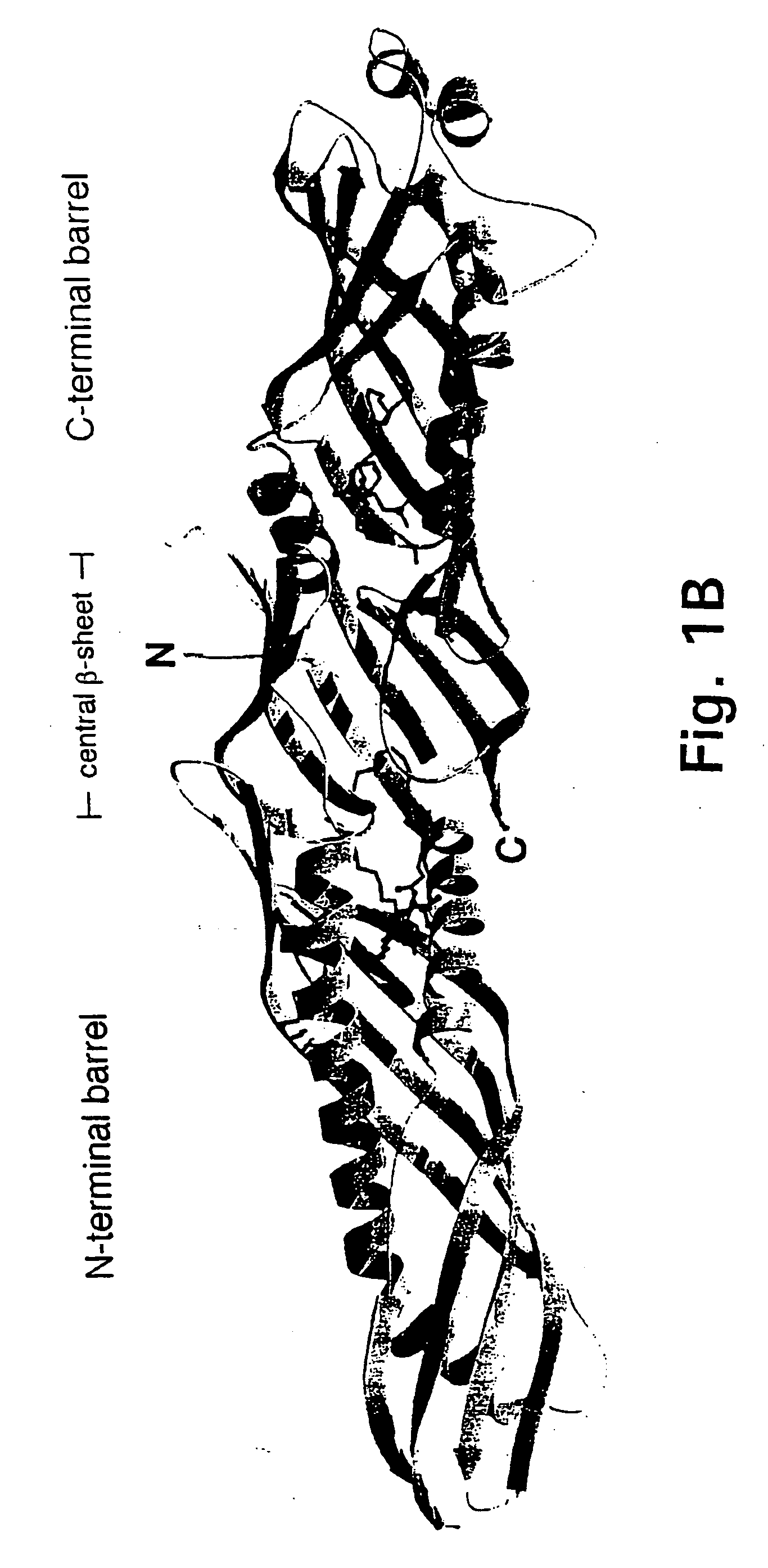
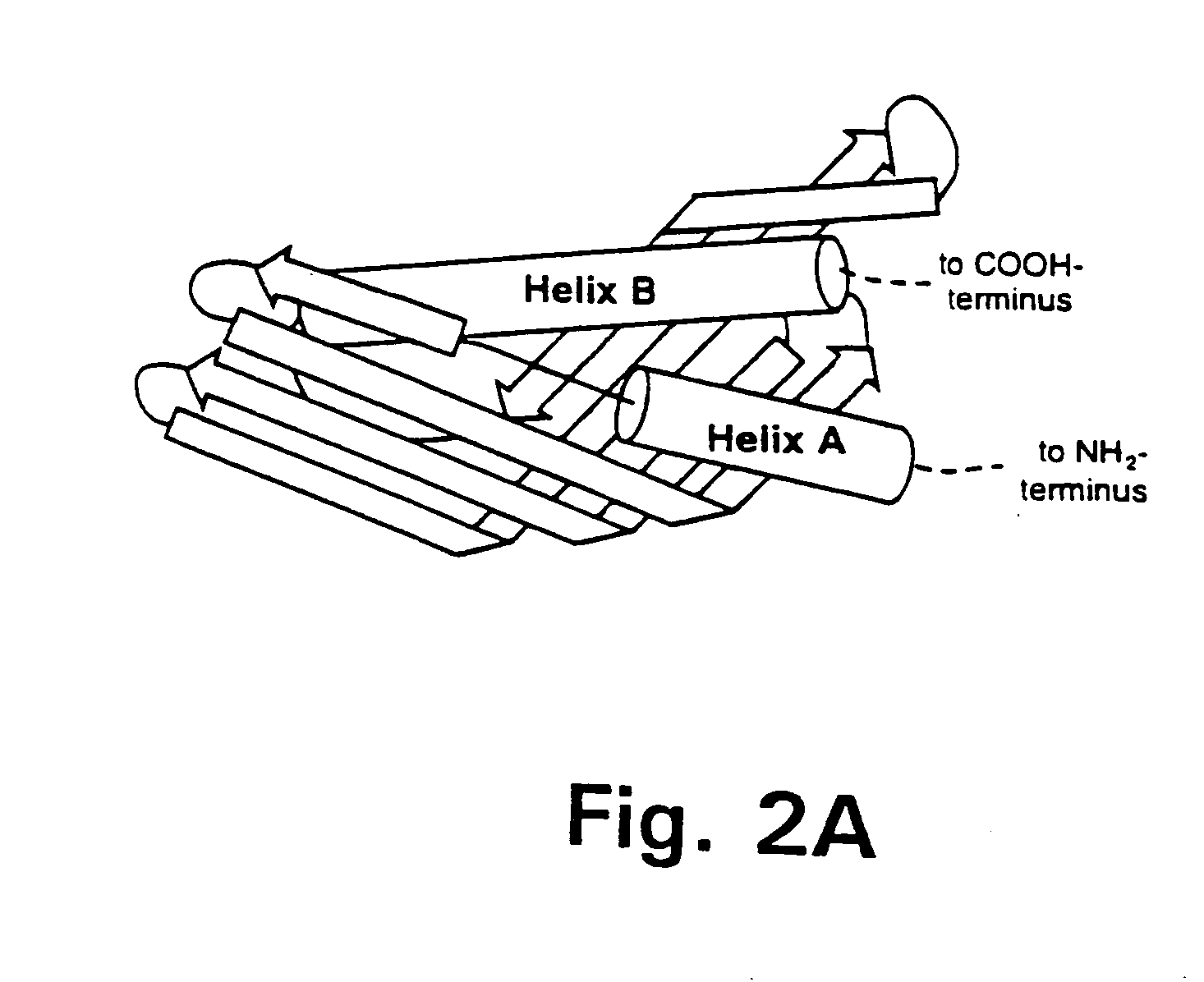
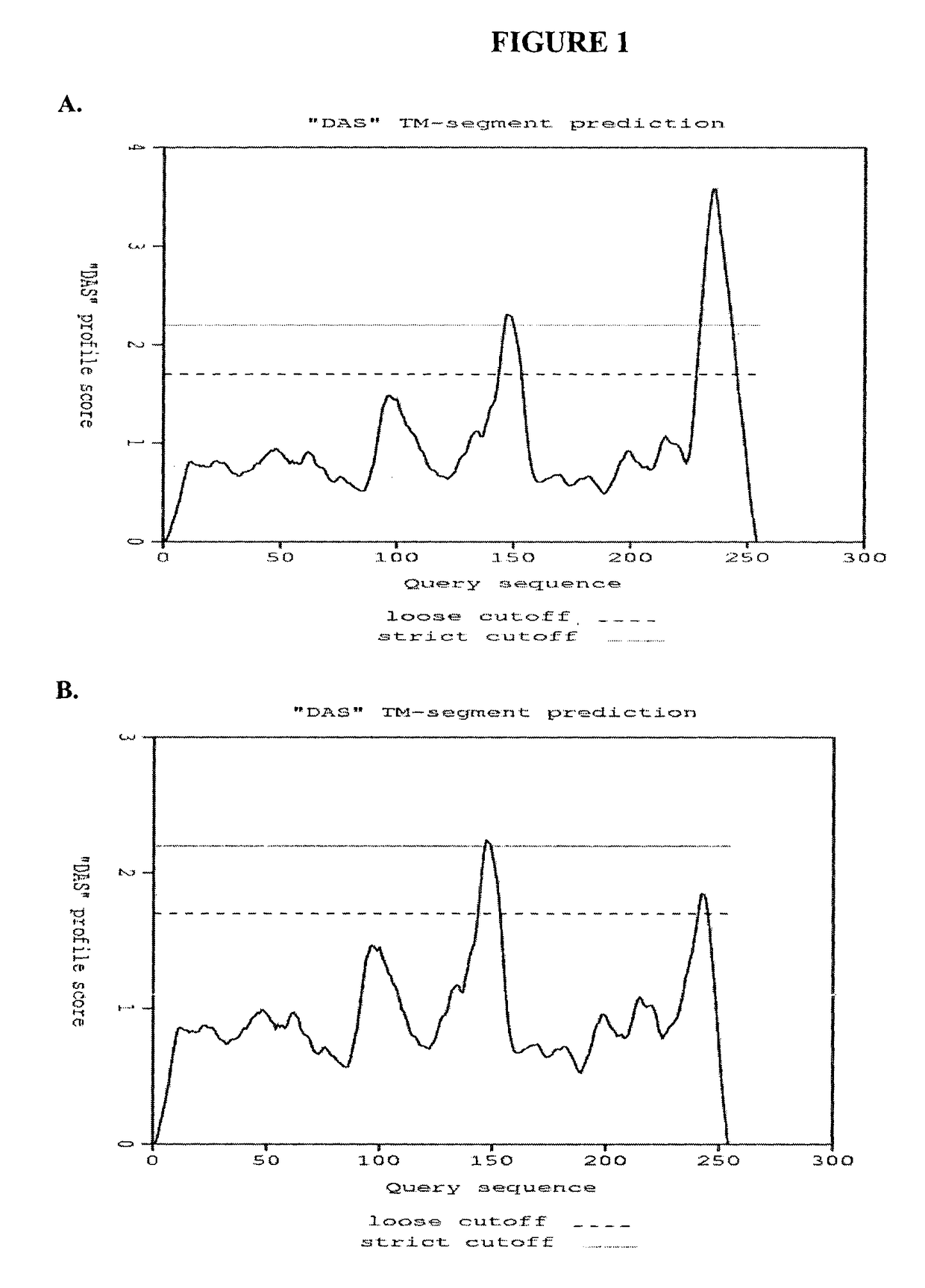
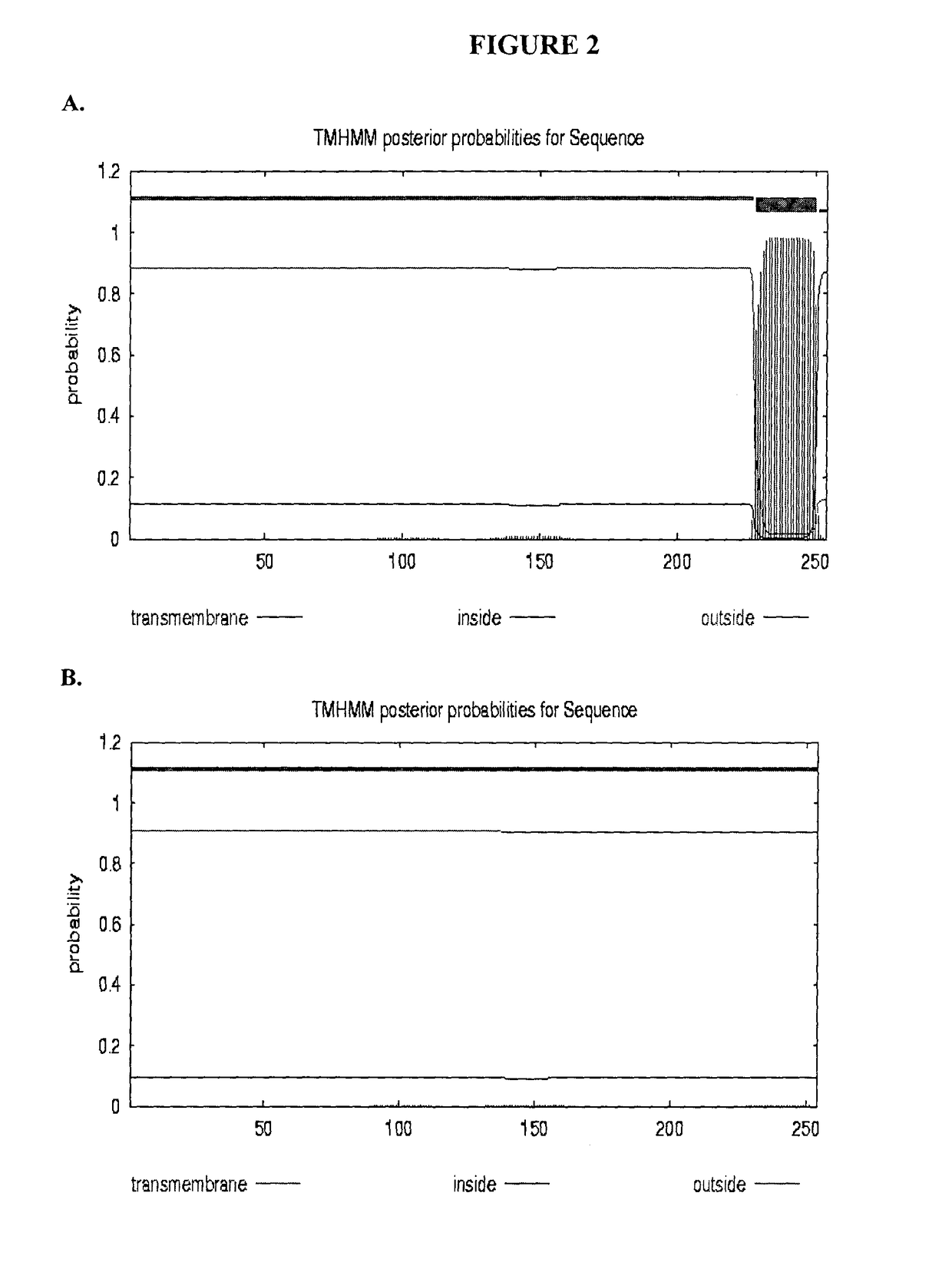
Bactericidal/permeability-increasing protein: crystallization, x-ray diffraction, three-dimensional structure determination, rational drug design and molecular modeling or related proteins
InactiveUS20050209837A1Change surface chargeAltered lipid binding pocketsAnalogue computers for chemical processesBactericidal/permeability-increasing proteinLipid formationLipid Transport
The present invention solves the three-dimensional structure of BPI and thereby provides atomic coordinates of BPI from the analysis of x-ray diffraction patterns of sufficiently high resolution for three-dimensional structure determination of the protein, as well as methods for rational drug design, based on using amino acid sequence data and / or x-ray diffraction data provided on computer readable media, as analyzed on a computer system having suitable computer algorithms; and atomic coordinates are provided yielding structural information on related proteins, including the lipid binding and lipid transport protein family that includes BPI, LBP, CETP and PLTP.
Owner:BEAMER LESA +2
Bactericidal/permeability-increasing protein (BPI) compositions
InactiveUS6057293ASurface tension of solutionSurface tension is alteredPeptide/protein ingredientsPeptide preparation methodsBactericidal/permeability-increasing proteinPhospholipid
Owner:XOMA CORP
Therapeutic peptide-based constructs
InactiveUS20060148714A1Peptide/protein ingredientsDepsipeptidesBactericidal/permeability-increasing proteinSmall peptide
Owner:LITTLE ROGER +2
Variant of BPIFB4 protein
ActiveCN104955836ASenses disorderNervous disorderBactericidal/permeability-increasing proteinCell biology
The present invention relates to an variant of BPIFB4 (Bactericidal / Permeability Increasing protein family B, member 4) protein and its use for the treatment of pathologies involving impairment of nitric oxide signalling.
Owner:LGV1 SRL
Bactericidal/permeability-increasing protein delation analogs
InactiveCN1312858AAntibacterial agentsBiocideBactericidal/permeability-increasing proteinPolynucleotide
Owner:XOMA TECH LTD
Variant of bpifb4 protein
ActiveCN104955836BSenses disorderNervous disorderBactericidal/permeability-increasing proteinCell biology
The present invention relates to variants of the BPIFB4 (bactericidal / permeability-increasing protein family B, member 4) protein and their use for the treatment of conditions involving impaired nitric oxide signaling.
Owner:LGV1 SRL
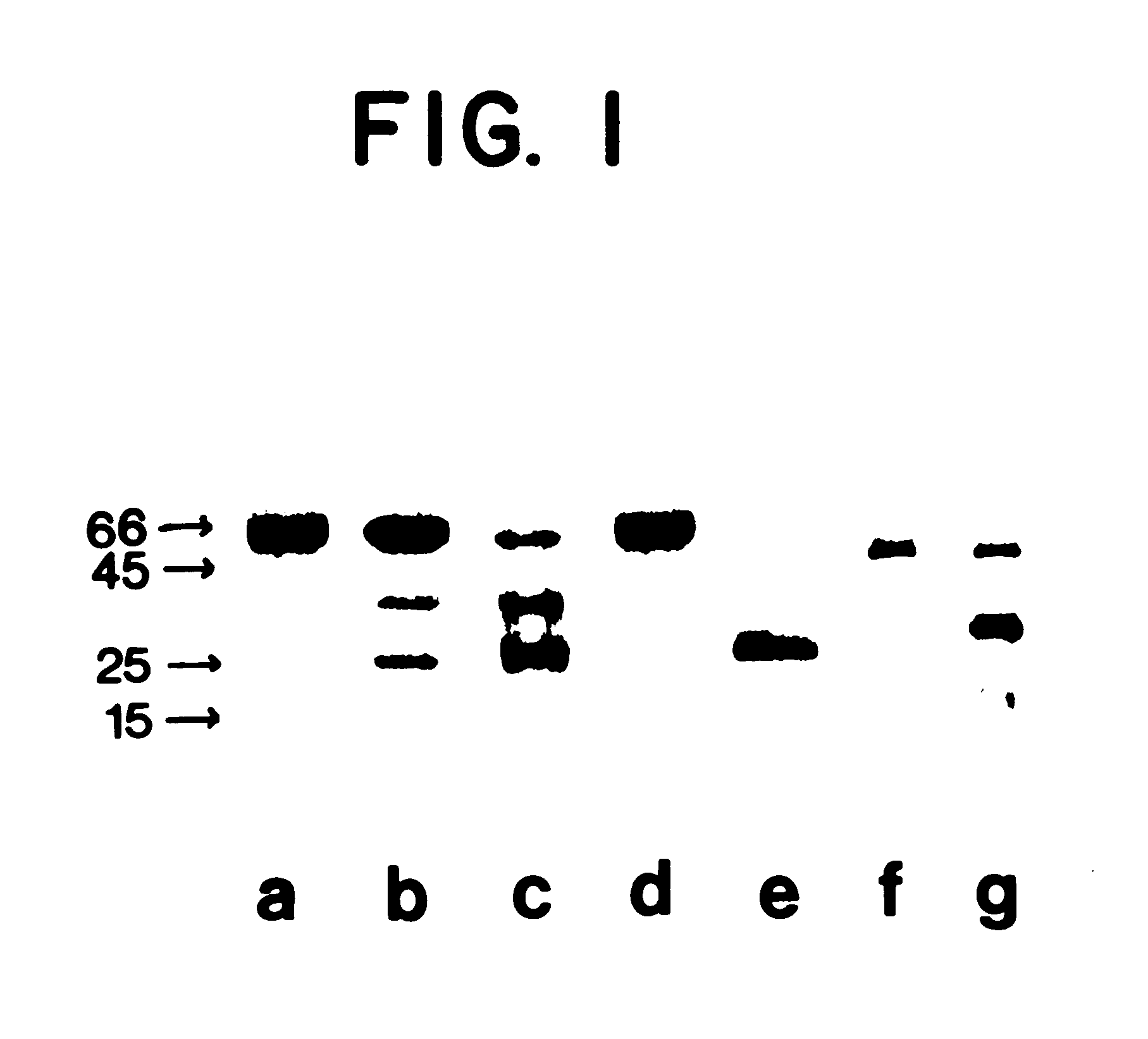
Biologically active bactericidal/permeability-increasing protein fragments
InactiveUS6287811B1Improve efficiencyImprove breathabilityAntibacterial agentsBacteriaBactericidal/permeability-increasing proteinProtein Fragment
Owner:NEW YORK UNIV
Bactericidal/permeability increasing protein for use in a method of immunization, preferably as an adjuvant in a method of vaccination
PendingUS20210299252A1Enhance immune stimulationHigh activityBacterial antigen ingredientsImmunological disordersBactericidal/permeability-increasing proteinAdjuvant
The present invention relates to bactericidal / permeability increasing protein (BPI) for use in a method of immunization of a patient, preferably as an adjuvant in a method of vaccination. The present invention also relates to a preparation comprising BPI for use in a method of immunization of a patient, and optionally an immunomodulatory agent. The present invention further relates to a process of producing a preparation including BPI for use in a method of immunization of a patient.
Owner:UNIV REGENSBURG
Diastereo analog of peptide SPFK-amide with selective anti-microbial activity and a method thereof
InactiveUS20030186888A1Reduced and no hemolytic activityAntibacterial agentsBiocideMicroorganismAmino acid
The present invention relates to a novel Diastereo analog Dsam of peptide SPFK-amide of amino acid sequence PKLLKTFLSKWIG with D-Leu residues at positions 4 and 8 of analog, having selective anti-microbial activity and no hemolytic activity of said SPFK, and a method of producing said Diastereo analog.
Owner:COUNCIL OF SCI & IND RES
Biologically active bactericidal/permeability-increasing protein fragments
InactiveUS20020137050A1Improve antibacterial propertiesReduce resistanceAntibacterial agentsBacteriaBactericidal/permeability-increasing proteinProtein Fragment
Owner:NEW YORK UNIV
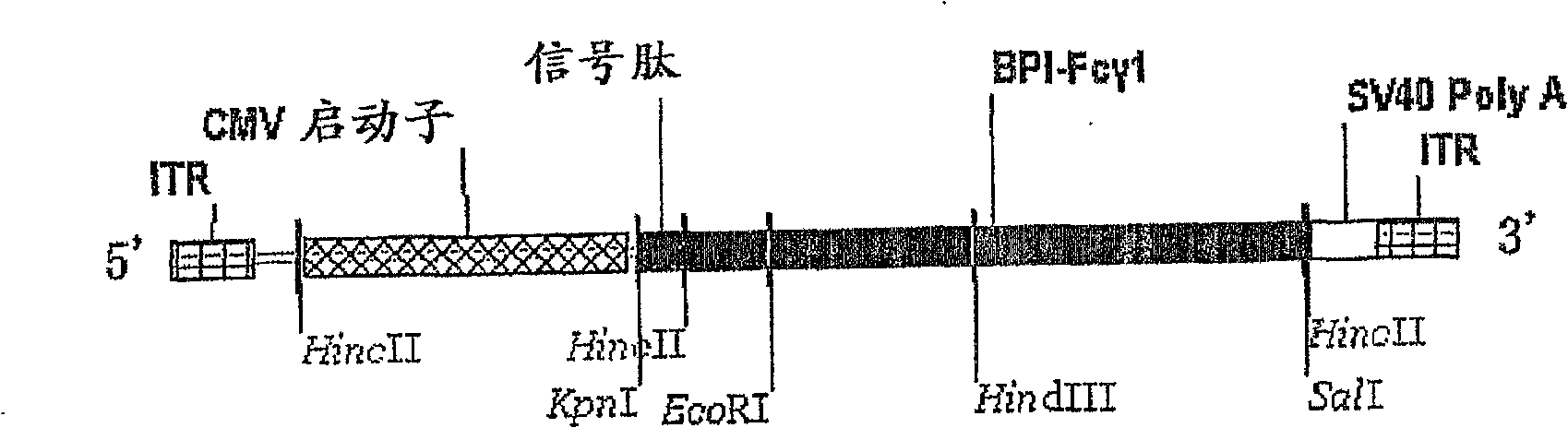
A recombinant virus comprising BPI gene and a pharmaceutical composition containing the same and uses thereof
The present invention discloses a recombinant virus, which comprises a viral vector, and a gene construct selected from the following: 1) a human BPI gene or a functional fragment gene thereof, or a degenerate Sequence thereof, and 2) a chimeric gene comprising a human BPI gene or a functional fragment gene thereof, or a degenerate Sequence thereof, wherein the chimeric gene further comprises a Fc constant region gene or its allelic gene of a heavy chain of a human immunoglobulin in the 3' end of the human BPI gene or the functional fragment gene thereof. This invention also discloses the use of the gene construct comprising the human BPI gene or functional fragment gene thereof in the preparation of the pharmaceutical composition of gene therapy of the GNB and / or GNB like pathogen infection in Mammal. The present invention further provides a gene therapy method of the GNB and / or GNB like pathogen infection using the recombinant virus or the pharmaceutical composition.
Owner:XIAMEN LIANHEANJIN BIOTECH CO LTD +1
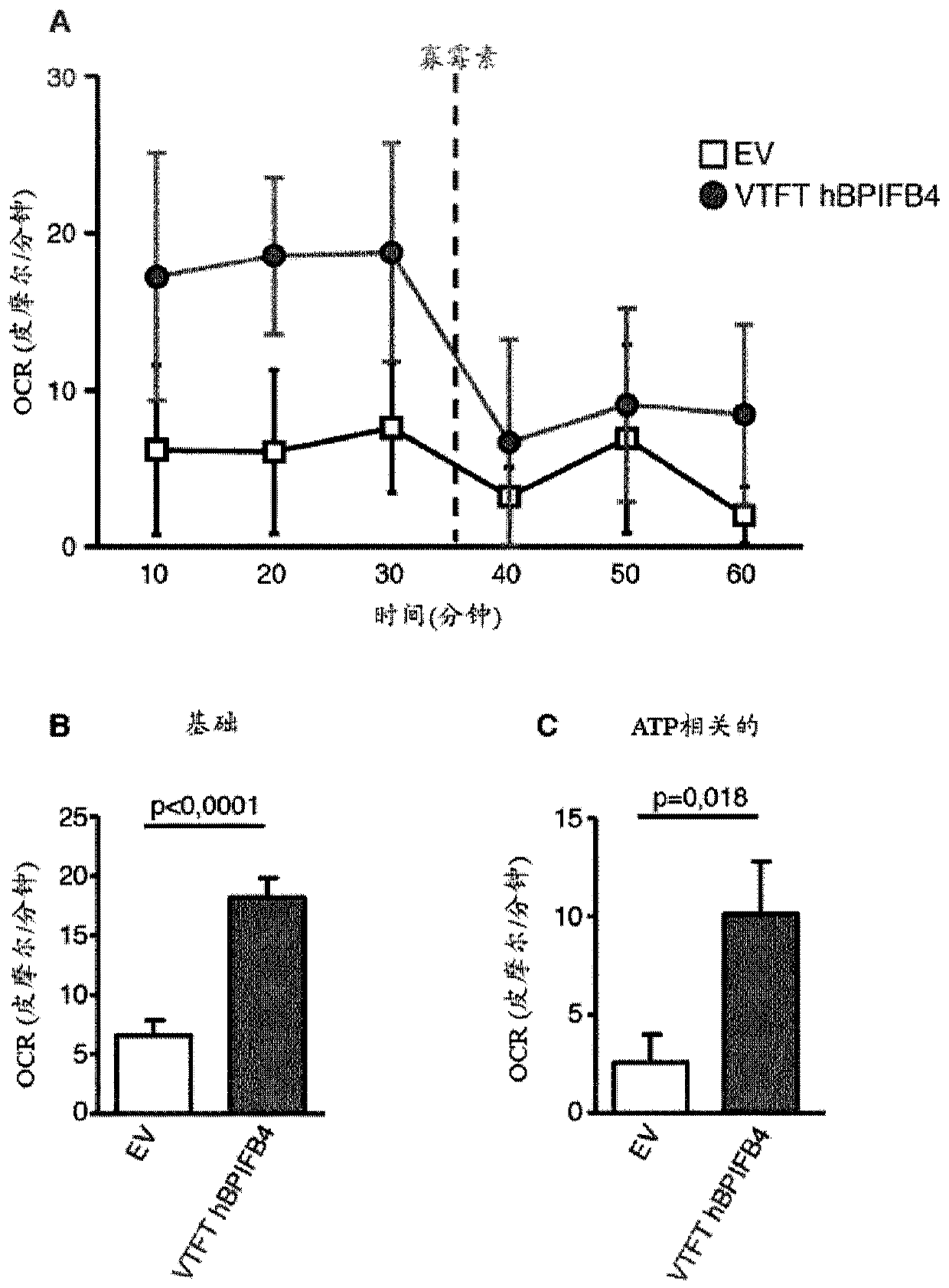
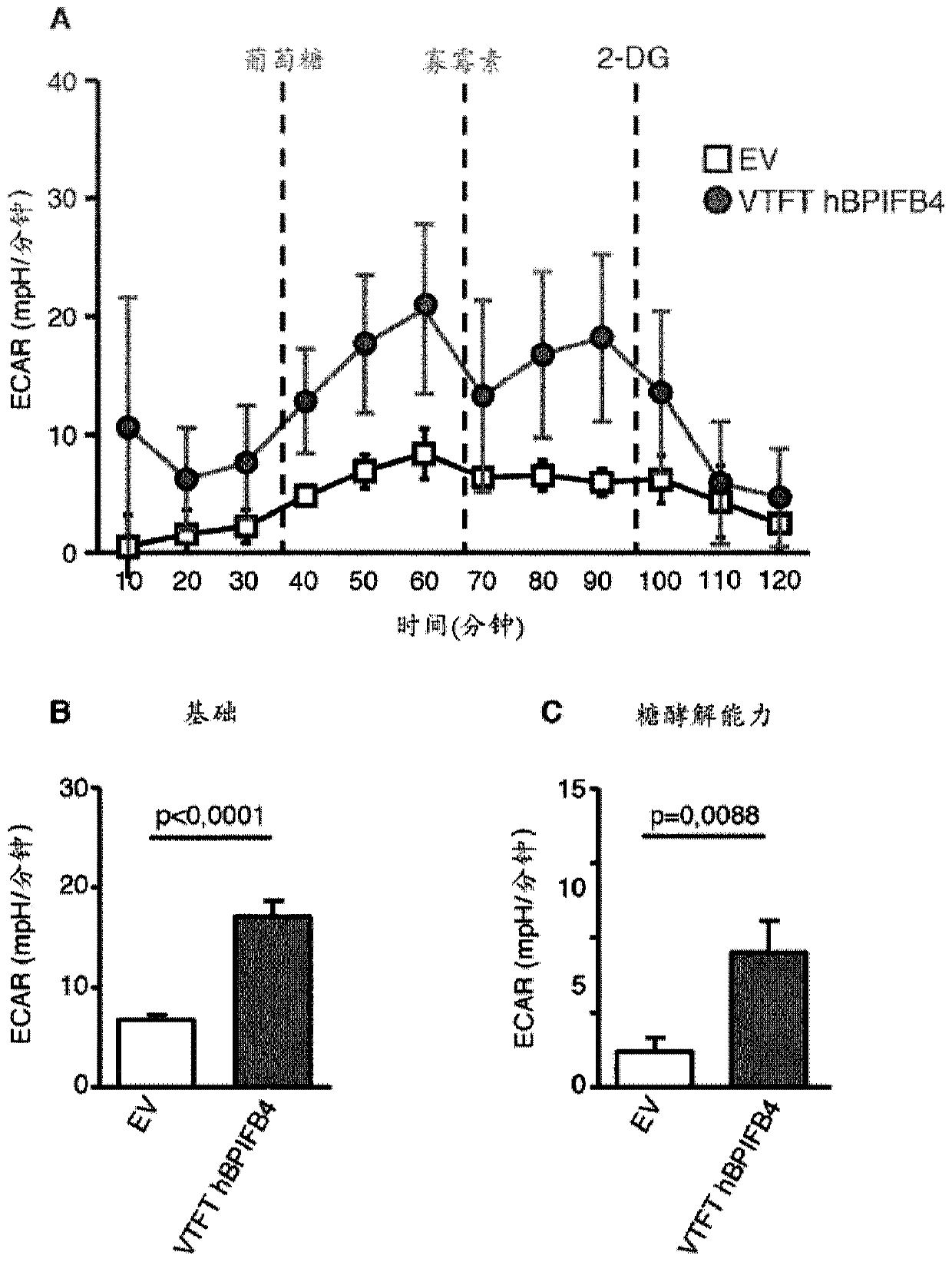
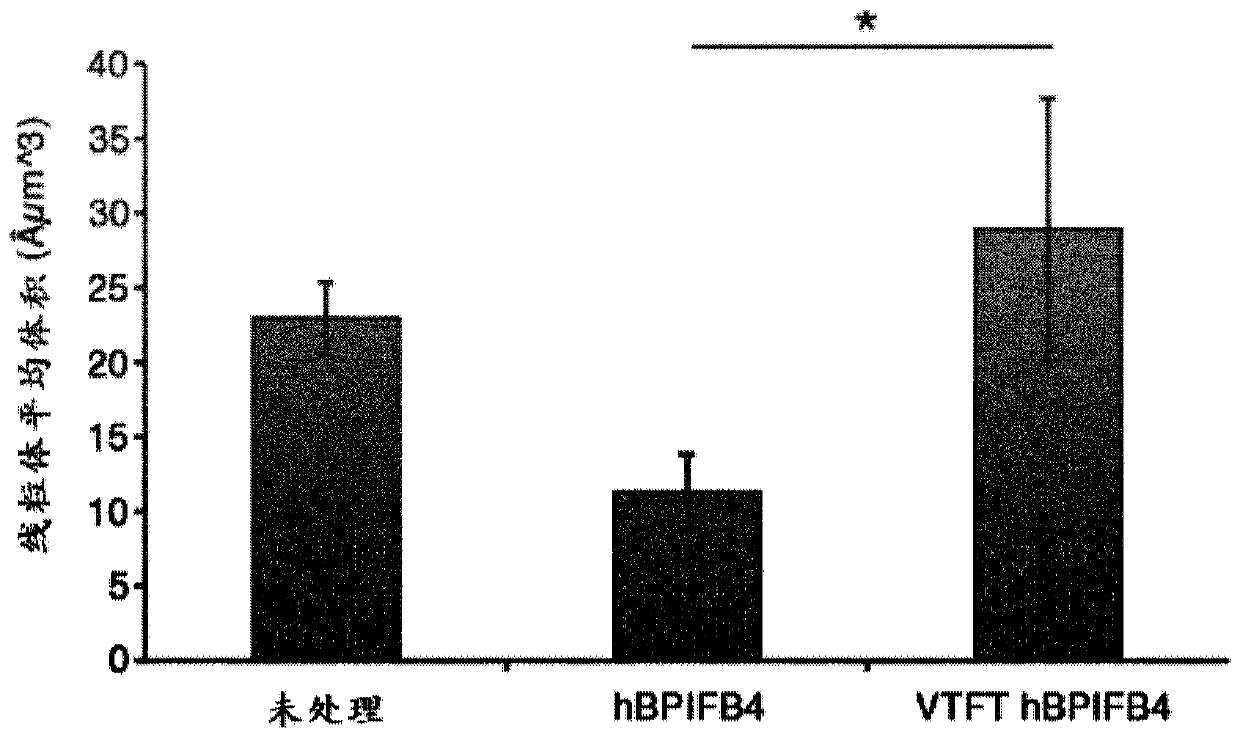
Vtft isoform of a bpifb4 protein for use in neuronal diseases and injuries
The invention relates inter alia to a protein which is a VTFT isoform of a BPIFB4 protein or a functional fragment thereof for use in the treatment or prophylaxis of a condition selected from neuronaldiseases and injuries, said diseases and injuries being associated with mitochondrial dysfunction and / or protein aggregation and / or ameliorated by CXCR4 activation.
Owner:LGV1 SRL
Bacterical/permability-increasing protein(BPI) deletion analogs
InactiveUS20050130889A1Improve homogeneityImprove stabilityAntibacterial agentsBiocideCysteine thiolateNucleotide
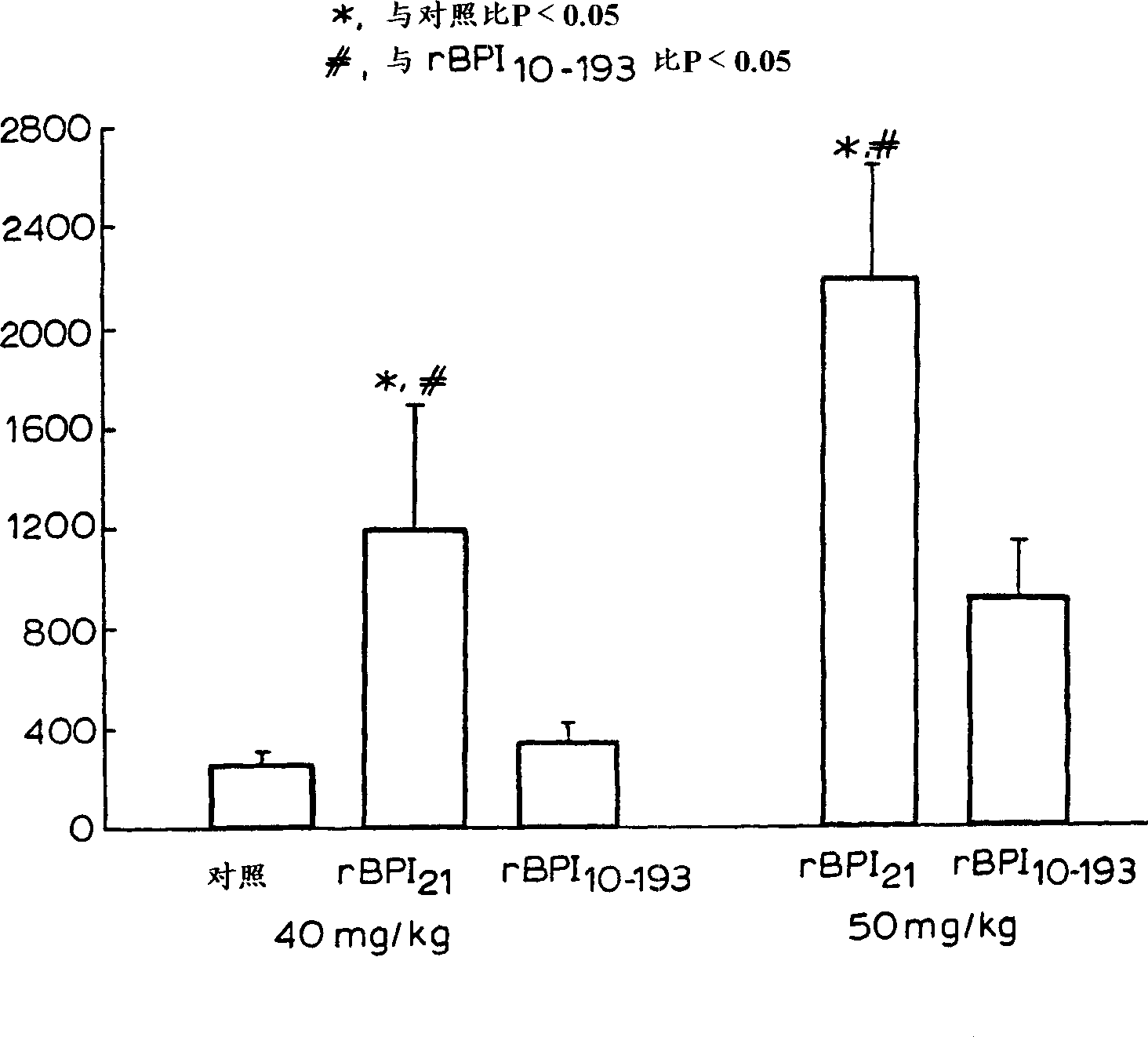
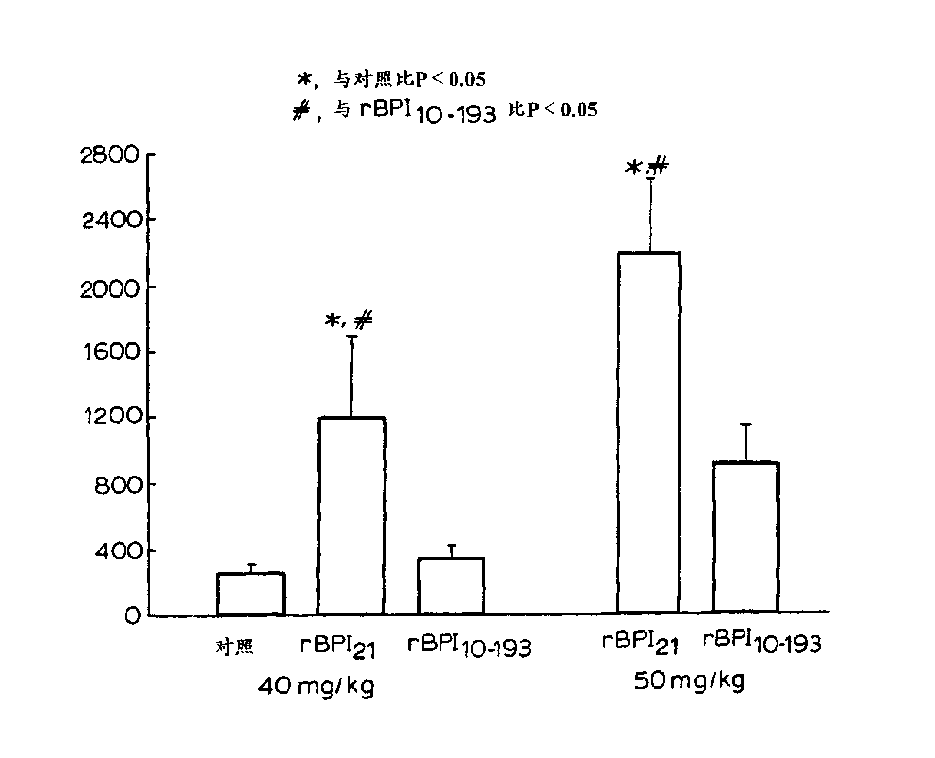
Novel BPI deletion analogs are provided that consist of amino acid residues 10 through 193 of mature human BPI wherein the cysteine residue at BPI amino acid position 132 is replaced by another amino acid. Fusion proteins comprising these analogs are also provided, as are polynucleotides encoding these products, materials and methods for their recombinant production, compositions and medicaments of these products, and therapeutic uses for these products.
Owner:XOMA CORP
Methods for Recombinant Peptide Production
InactiveUS20090246831A1Efficiently and economically producedSimple methodBacteriaAntibody mimetics/scaffoldsBiotechnologyRecombinant peptide
Owner:XOMA TECH LTD
Chimeric antibacterial polypeptides
ActiveUS9605250B2Decreased cfuReduce in quantityAntibacterial agentsPolypeptide with localisation/targeting motifChemistry
Owner:BACTOCLEAR HLDG PTE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com