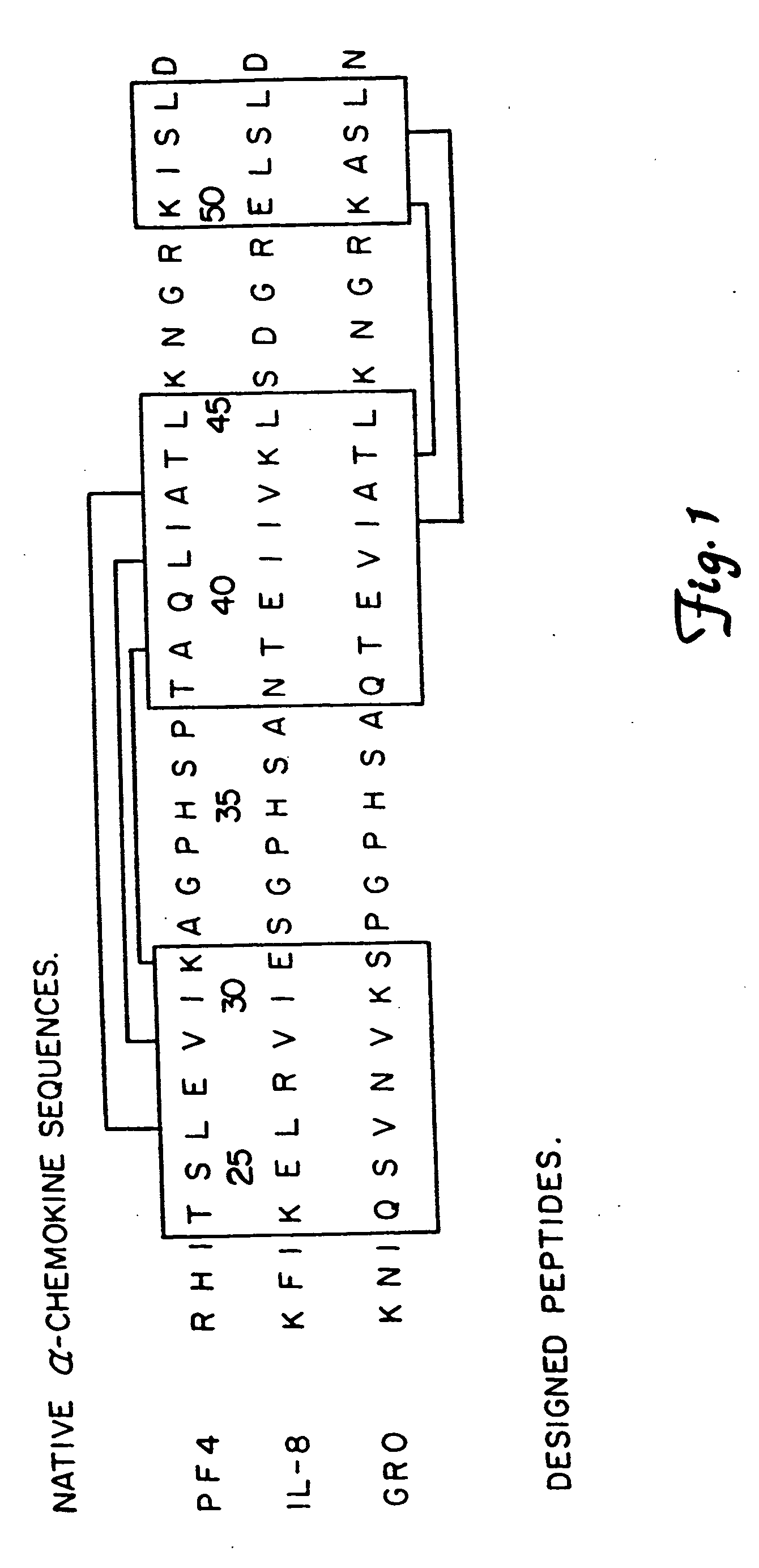
Synthetic approach to designed chemical structures
a chemical structure and synthetic approach technology, applied in the direction of chemokines, peptides, drug compositions, etc., can solve the problems of reducing the usefulness of scales in designing de novo -, peptides in the betabellin and betadoublet series show limited solubility in water, and the design of a structurally stable -polypeptide is dealing with the interaction between -polypeptides,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Design and Synthesis of Water-Soluble β-Sheet Forming Peptides
[0039] Peptides of 33 amino acid residues in length, called βpep-1, βpep-2, βpep-3 and βpep-4, were synthesized on a Milligen Biosearch 9600 automated peptide synthesizer. The procedures used were based on Merrifield solid phase synthesis utilizing Fmoc-BOP chemistry (Stewart et al., 1984. Solid phase peptide synthesis., 2nd ed. Rockford, Ill., Pierce Chemical Co. pp. 135). After the sequence had been obtained, the peptide support and side chain protection groups were acid cleaved (trifluoroacetic acid and scavanger mixture). Crude peptides were analyzed for purity on a Hewlett-Packard 1090M analytical HPLC using a reverse phase C18 VyDac column. Peptides generally were about 90% pure. Further purification was done on a preparative reverse-phase HPLC C-18 column using an elution gradient of 0-60% acetonitrile with 0.1% trifluoroacetic acid in water. Peptides then were analyzed for amino acid composition on a Beckman ...
example 2
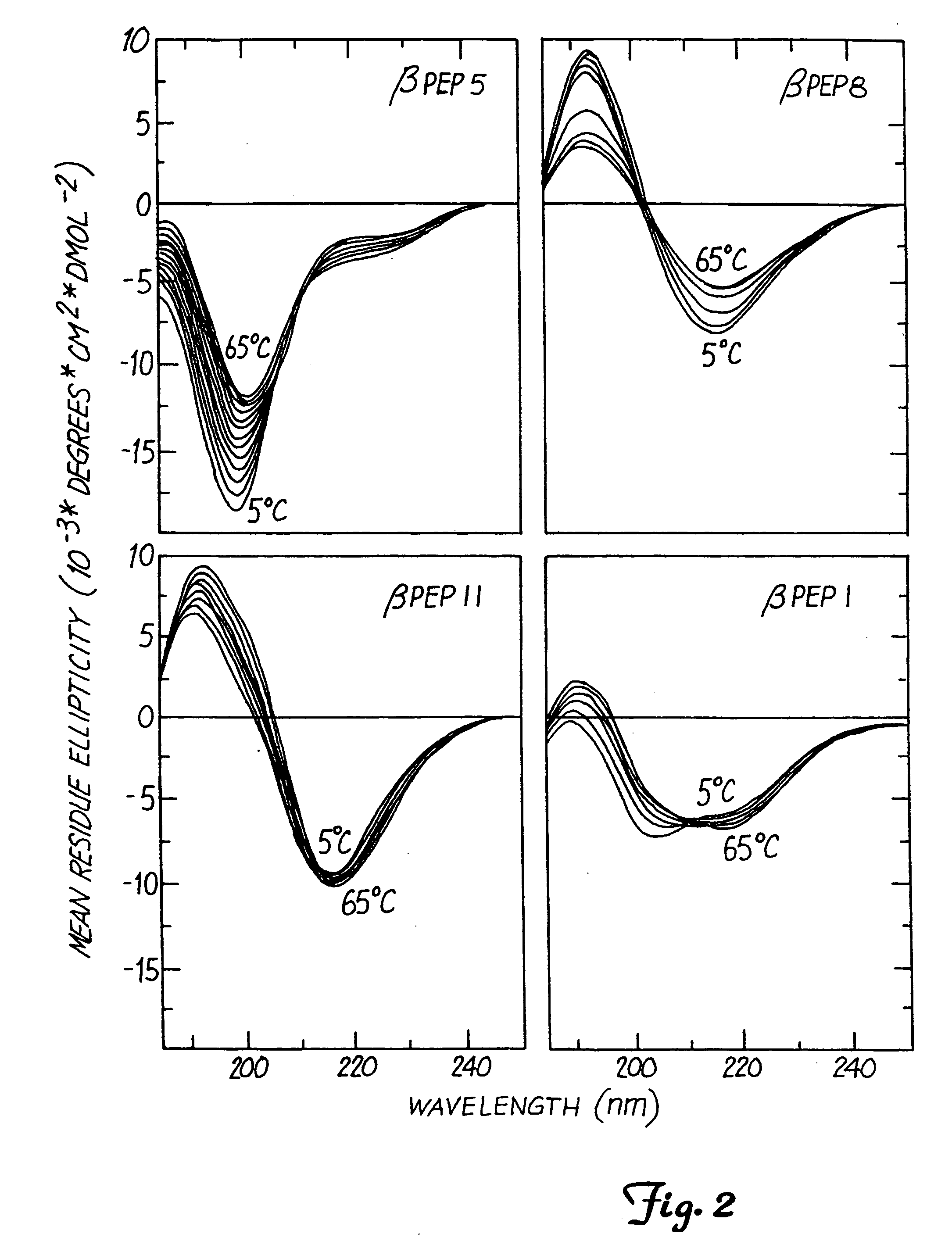
Circular Dichroism (CD) of the β-Peptide Series
[0044] Circular dichroism (CD) is one way to measure formation of a β-sheet structure. CD spectra were measured on a JASCO JA-710 (Jasco, Eastern, Md.) automatic recording spectropolarimeter coupled with a data processor. Curves were recorded digitally and fed through the data processor for signal averaging and baseline subtraction. Spectra were recorded from 5° C. to 65° C. in the presence of 10 mM potassium phosphate, over a 185 nm to 250 nm range using a 0.5 mm path-length, thermally-jacketed quartz cuvette. Temperature was controlled by using a NesLab water bath. Peptide concentration was varied from 0.014 to 0.14 mM. The scan speed was 20 nm / min. Spectra were signal-averaged 8 times, and an equally signal-averaged solvent baseline was subtracted. These experiments are well known in the art.
[0045] For βpep-1, CD data resembled those observed for β-sheet-forming PF4 peptide. Based on CD data alone, βpep-1 appeared not to form any β...
example 3
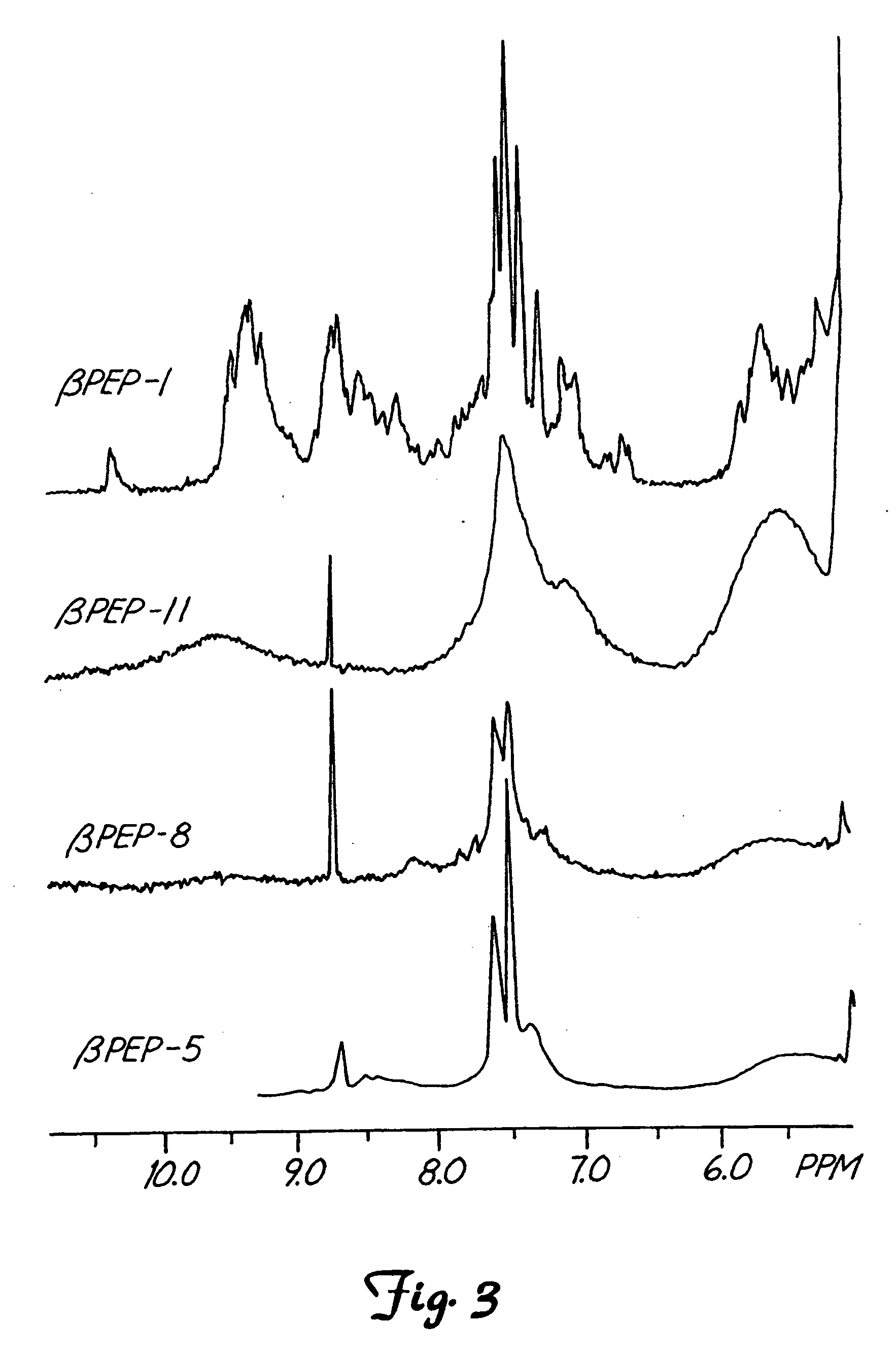
Nuclear Magnetic Resonance (NMR) of the β-Peptide Series
[0047] Since CD data indicate maximal β-sheet formation at about 40° C., NMR spectra (FIG. 3) were accumulated for all four peptides at pH 6.3, 20 mM NaCl and 40° C. For NMR measurements, freeze-dried peptide was dissolved either in D2O or in H2O / D2O (9:1). Polypeptide concentration normally was in the range of 1 to 5 mM. pH was adjusted by adding μL quantities of NaOD or DCl to the peptide sample. NMR spectra were acquired on a Bruker AMX-600 (Bruker Instrument, Inc., Bruker, Mass.) or AMX-500 NMR spectrometer. For resonance assignments, double quantum filtered COSY (Piantini et al. J. Am. Chem. Soc. 104:6800-6801, 1982) and 2D-homonuclear magnetization transfer (HOHAHA) spectra, obtained by spin-locking with a MLEV-17 sequence with a mixing time of 60 ms, were used to identify spin systems (See Bax, et al. J. Magn. Reson. 65: 355-360, 1985). NOESY experiments (see Jeener et al., J. Chem. Phys. 71:4546-4550, 1979 and Wider et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com