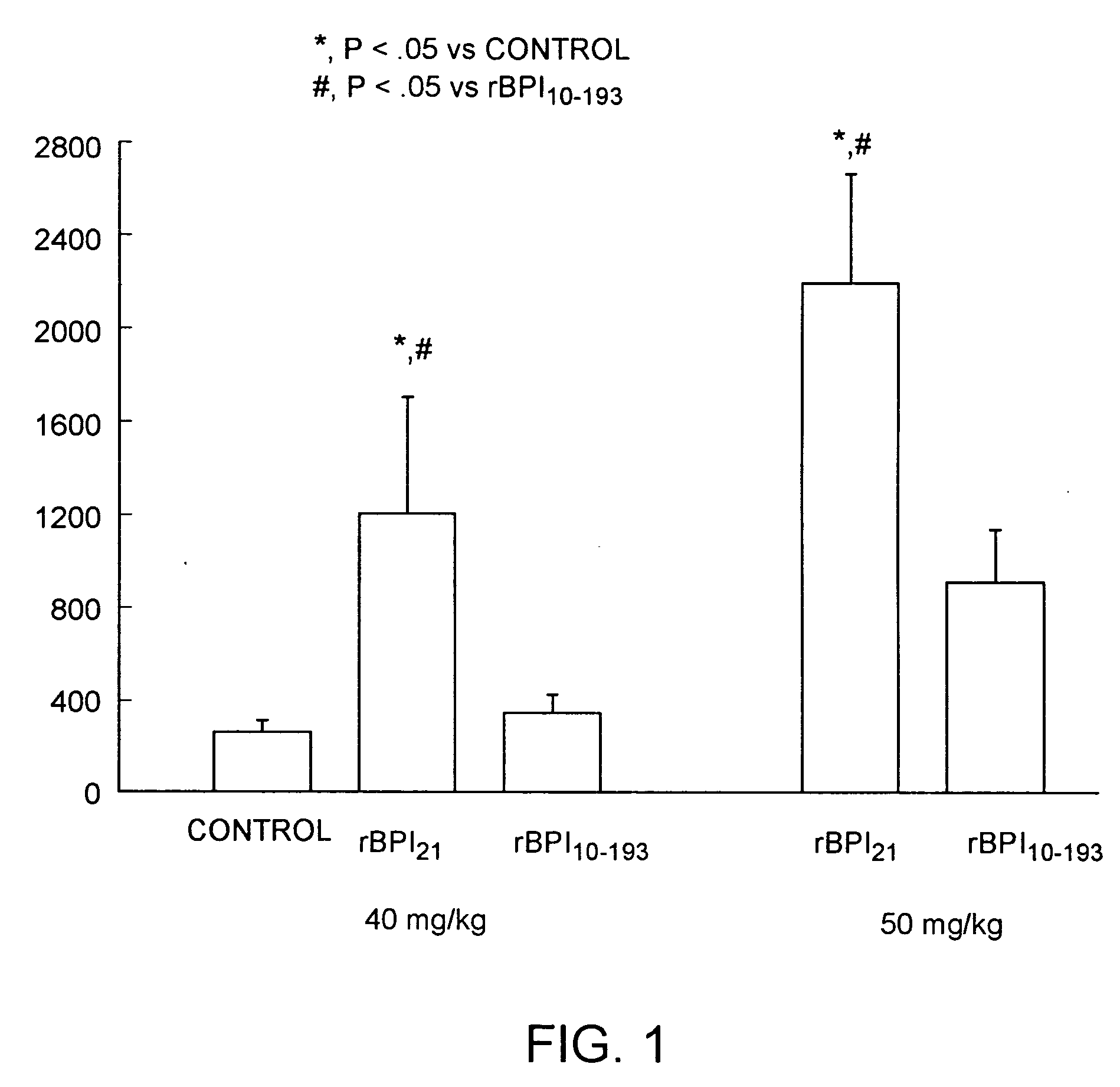
Bacterical/permability-increasing protein(BPI) deletion analogs
a technology of permability and protein, applied in the field of bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-bpi-b
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Expression Vector pING1742
[0034] The rBPI(10-193)C132A expression vector, pING1742, was constructed as follows. The expression vector pING4155 was first constructed by ligating a BamHI-BsaI fragment containing the neo gene from pING3174 with a BsaI-XhoI fragment containing the CMV promoter and rBPI21 gene from pING4144 and an XhoI-BamHI fragment containing the mouse (kappa) light chain 3′ untranslated region from pING4537 (pING3174, pING4144 and pING4537 are described in U.S. Pat. No. 5,420,019, incorporated by reference). The resulting pING4155 vector contains the gene encoding rBPI21 fused to the human IgG enhancer, the human CMV promoter and the mouse (kappa) light chain 3′ untranslated region. It also contains the neo gene encoding neomycin phosphotransferase, for selection of transfectants resistant to the antibiotic Geneticin® (G418).
[0035] The vector pING1732 was produced by deleting the 0.7 kbp HindIII-HindIII fragment of pING4155 containing the human Ig en...
example 2
Transformation of CHO Cells with pING1742
[0037] CHO-K1 cells (American Type Culture Collection (ATCC) Accession No. CCL61) were adapted to growth in serum-free Ex-Cell 301 medium as follows. CHO-K1 cells grown in Ham's F12 medium were trypsinized, centrifuged and resuspended in Ex-Cell 301 medium. Cells were grown in a 125-ml flask at 100 rpm and passaged every two to three days in either a 125-ml or 250-ml flask.
[0038] These Ex-Cell 301-adapted CHO cells were transfected by electroporation with pING1742. Prior to transfection, pING1742 was digested with NotI, which linearizes the plasmid. Following a 48-hour recovery, cells were plated at approximately 104 cells / well into 96-well plates containing Ex-Cell 301 medium supplemented with 0.6 mg / mL G418 (Life Technologies, Gaithersburg, Md.). At approximately 2 weeks, supernatants from approximately 250 wells containing single colonies were screened by ELISA for the presence of BPI-reactive protein using an anti-BPI monoclonal antibod...
example 3
Production and Purification of rBPI(10-193)C132A
[0041] Large quantities of rBPI(10-193)C132A were produced for characterization by growing Clone 139 cells in 2-liter research fermenters (Biolafitte, St. Germain en Laye, France) and then in a 500 liter ABEC fermenter (ABEC, Allentown, Pa.). Protein product obtained from the 2-liter fermenters was used for the in vitro studies described below, while product obtained from the 500 liter fermenter was used for animal toxicology and efficacy studies.
[0042] A. Growth in Two-Liter Fermenters
[0043] Clone 139 cells were passaged in spinner flasks of increasing volumes containing Ex-Cell medium supplemented with 1% FBS until sufficient volume and cell density was achieved to inoculate the 2 liter bioreactors at approximately 2×105 cells / mL. Cells were grown in three 2-liter fermenters in Ex-Cell medium supplemented with 1% FBS, at 37° C., pH 7.2, 150 rpm with dissolved oxygen maintained at 5-10%. Large sterile SP-Sepharose beads (Pharmacia ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com