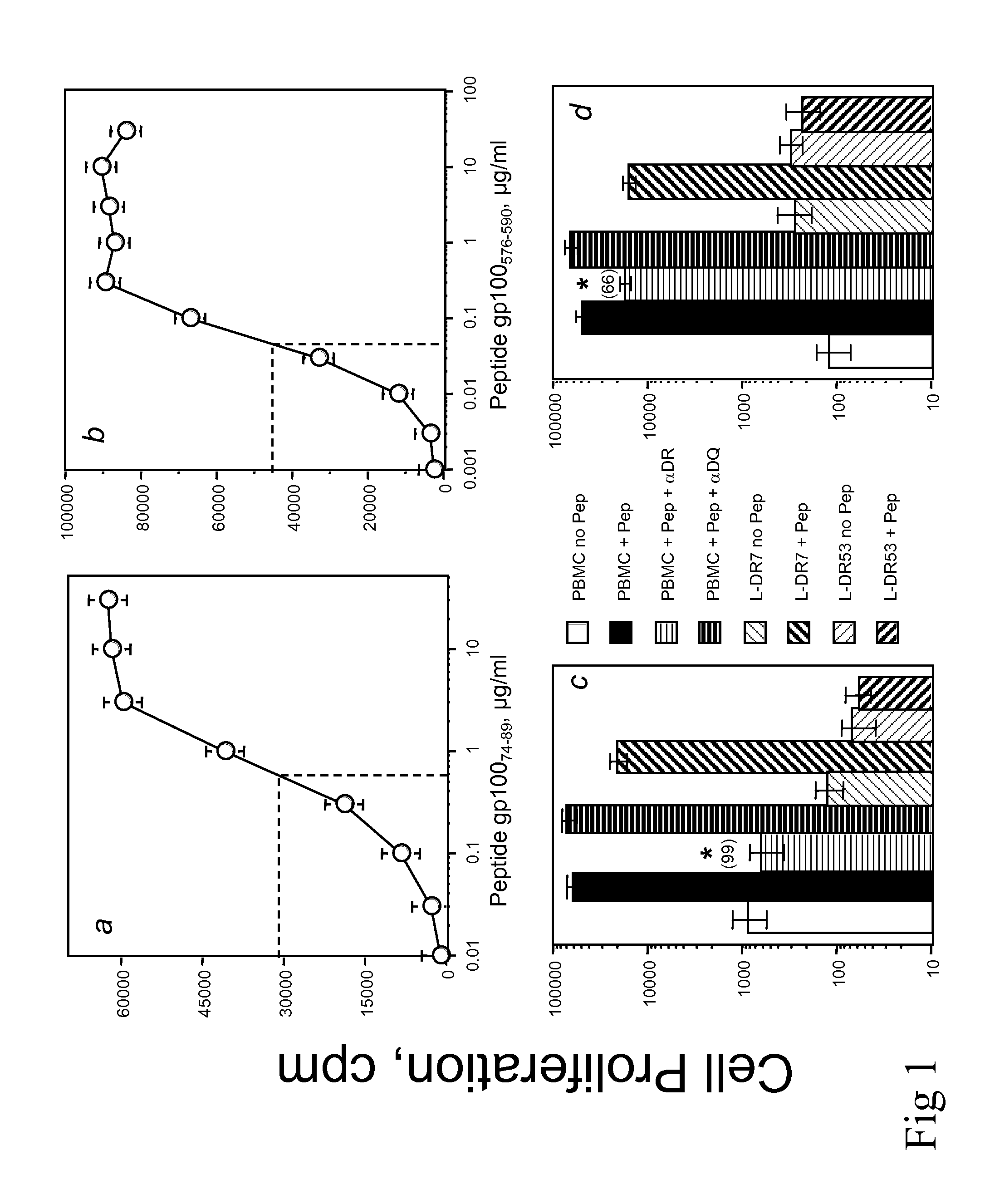
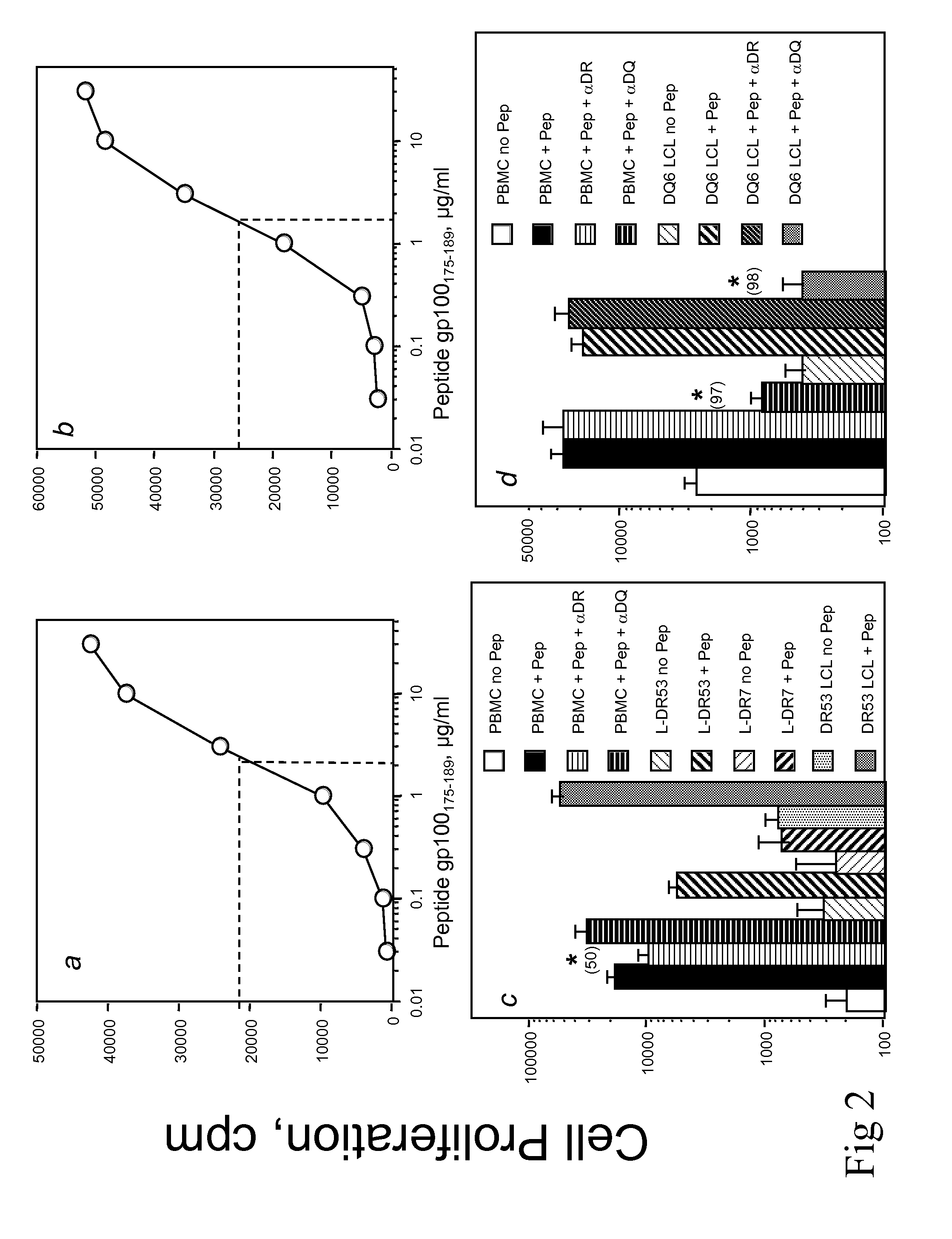
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Melanoma patient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
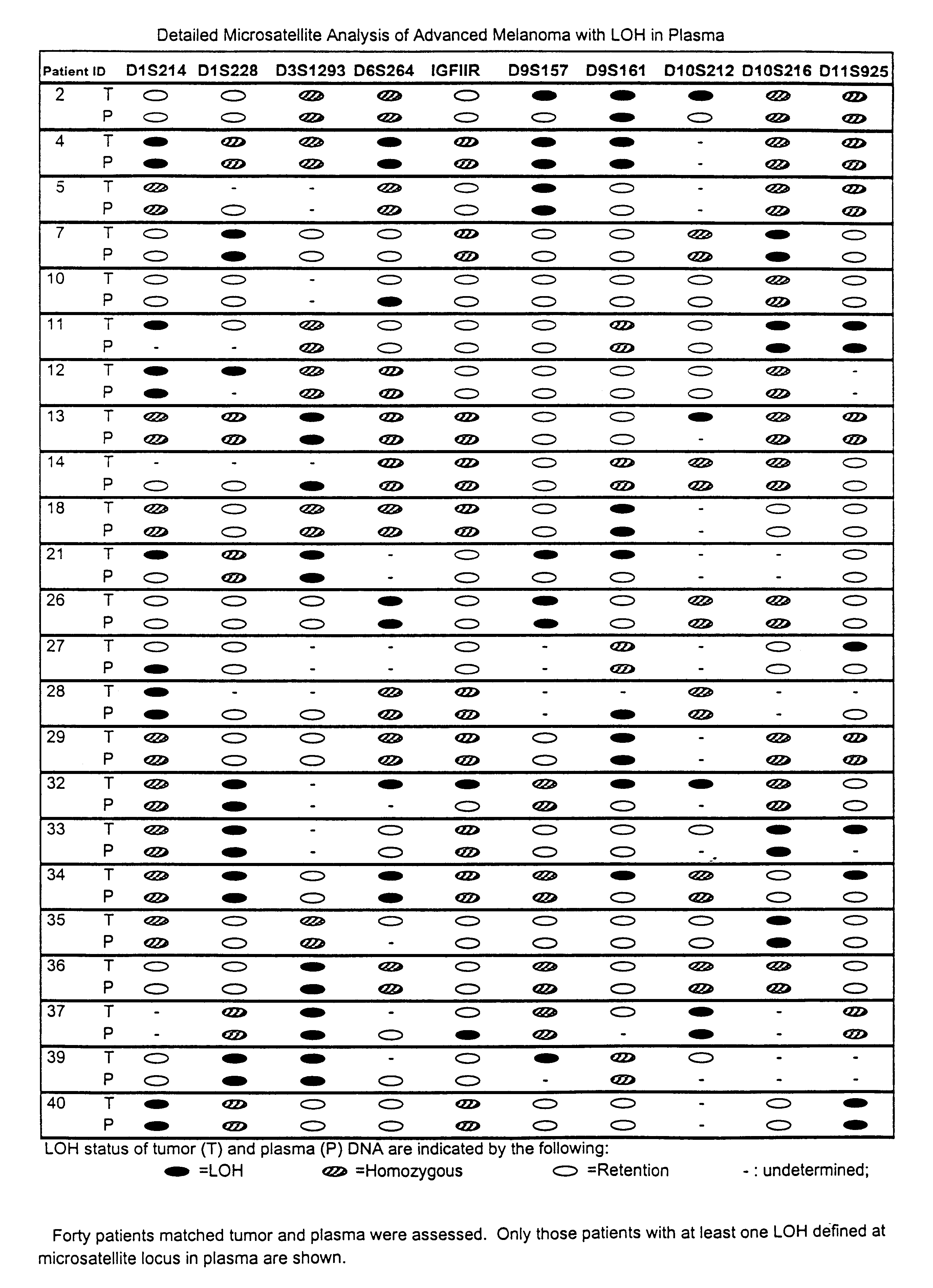
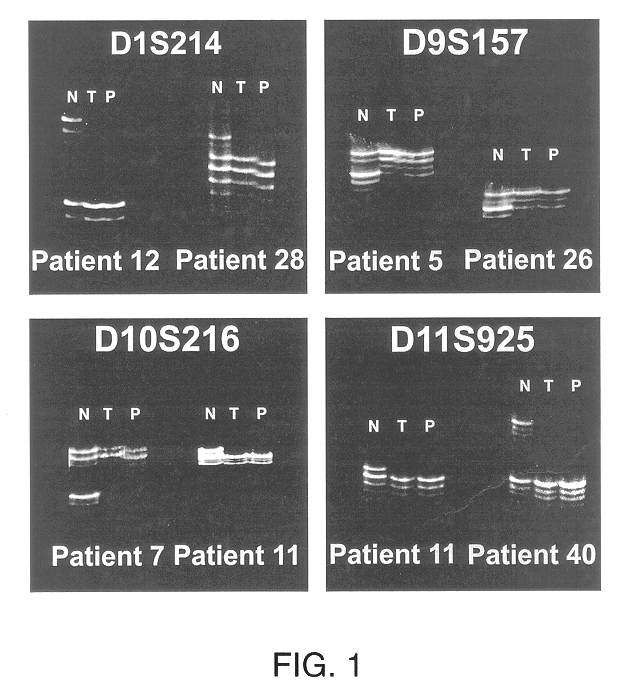
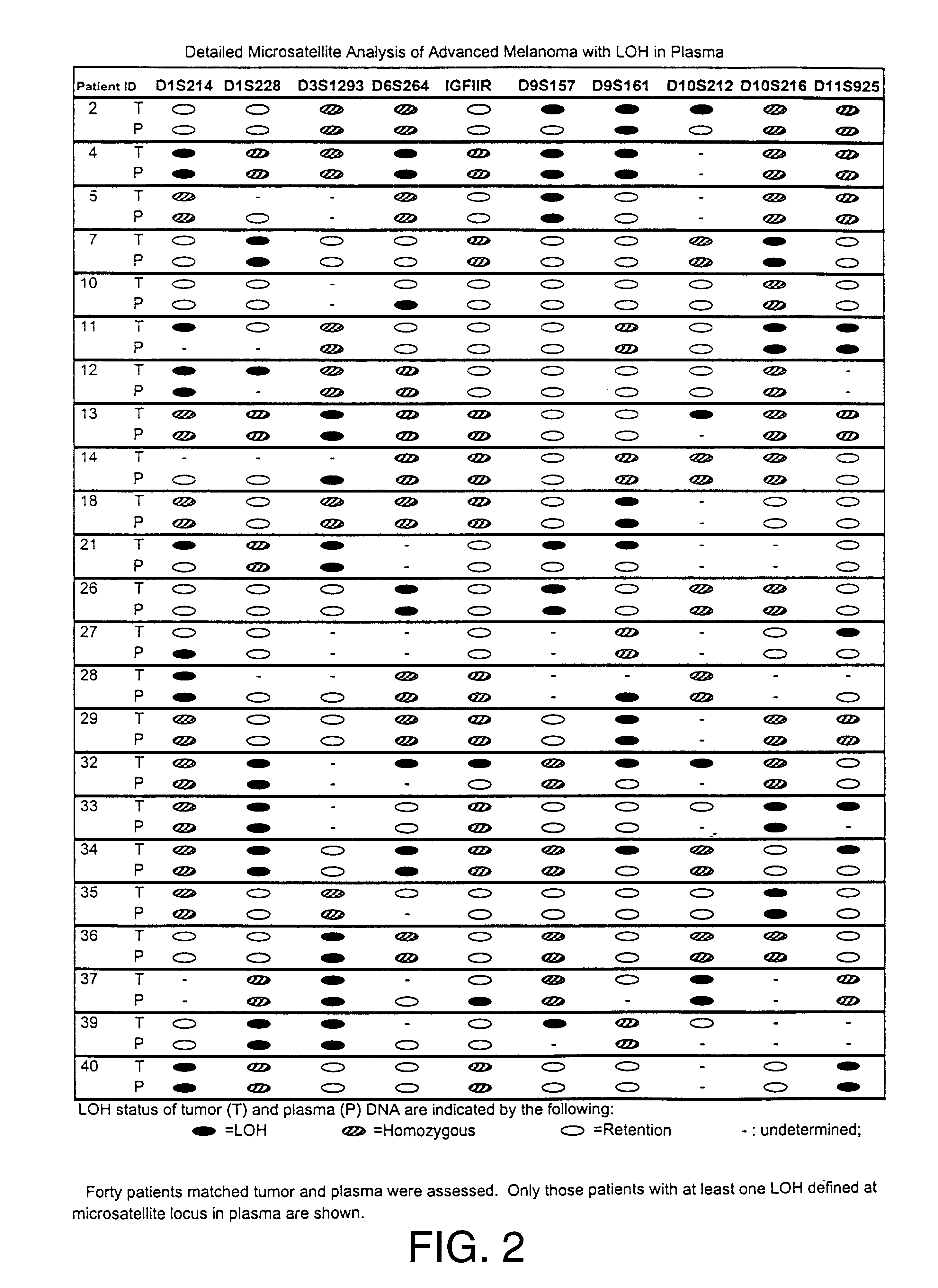
Detection of loss of heterozygosity in tumor and serum of melanoma patients
A method is provided for assessing allelic losses on specific chromosomal regions in melanoma patents. The method relies on the evidence that free DNA may be released in the plasma / serum of cancer patients allowing the detection of DNA with LOH in the plasma / serum of cancer patients by analysis for microsatellite markers. The amount of and specific allelic loss allows a prognosis to be made regarding tumor diagnosis and progression, tumor metastasis, tumor recurrence, and mortality.
Owner:JOHN WAYNE CANCER INST
Method to treat melanoma in braf inhibitor-resistant subjects
InactiveUS20120053185A1Better respondEffective treatmentCompound screeningBiocideBRAF inhibitorTyrosine
Methods to treat cancer patients, especially melanoma patients, who have BRAF mutations and have become resistant to BRAF mutant kinase inhibitors employ inhibitors of multiple receptor tyrosine kinases. In addition, methods are described for identifying pharmaceutical compositions and drugs that will be successful in treating these patients.
Owner:GENEKEY CORP
Methods for predicting survival in metastatic melanoma patients
InactiveUS20110275089A1Prolong survival timeStimulate immune responseMicrobiological testing/measurementDisease diagnosisDifferential survivalMetastatic melanoma
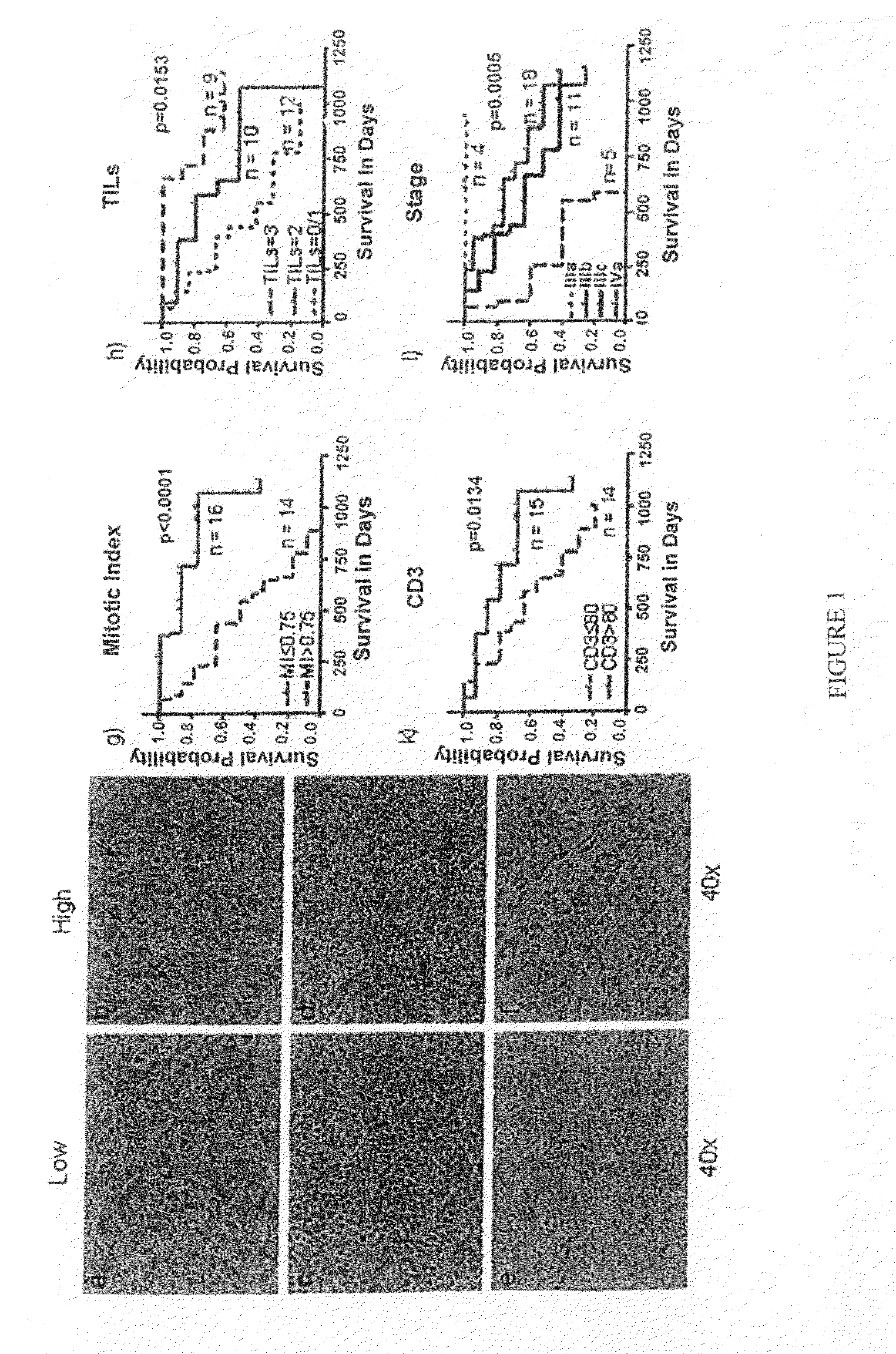
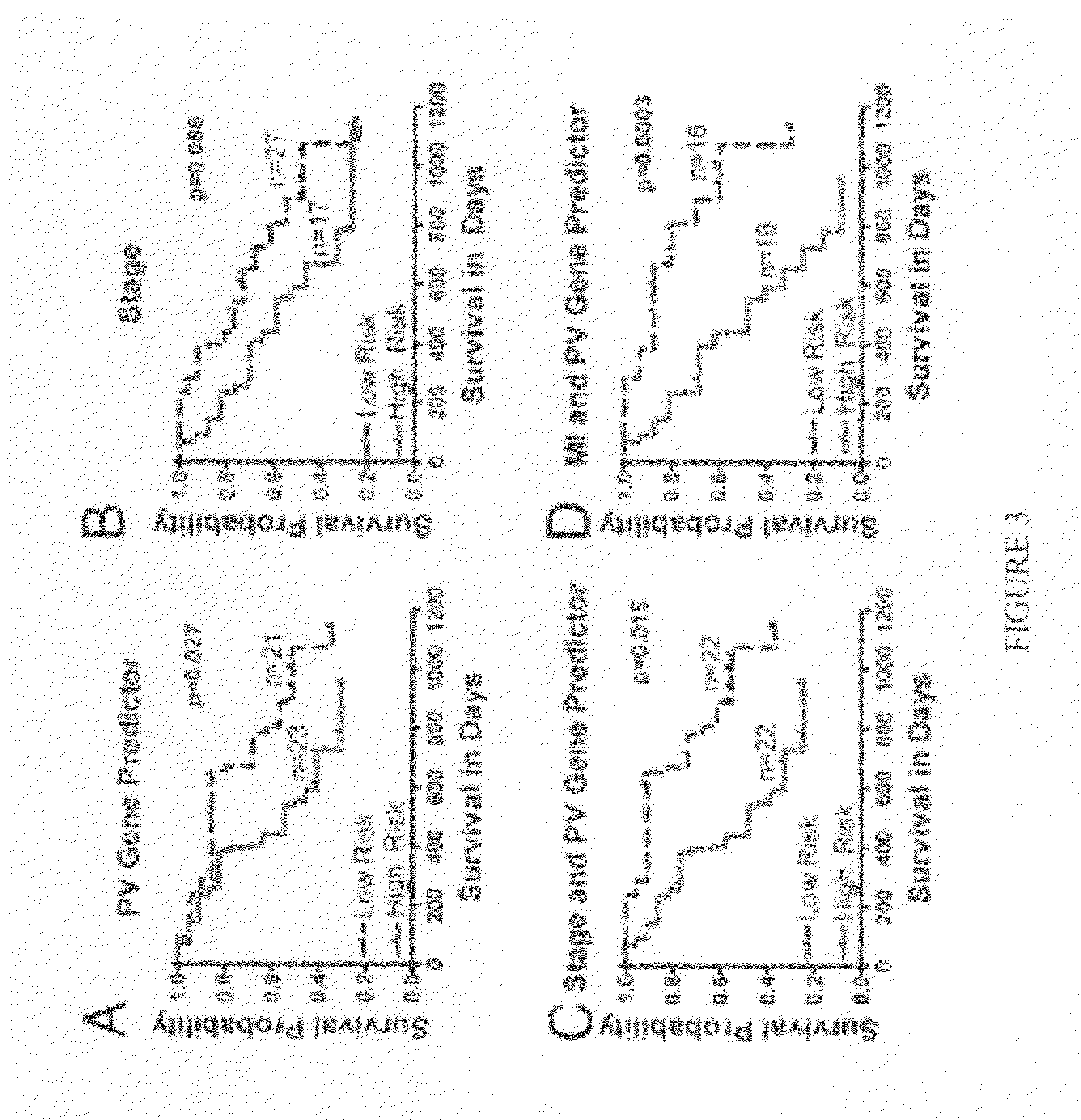
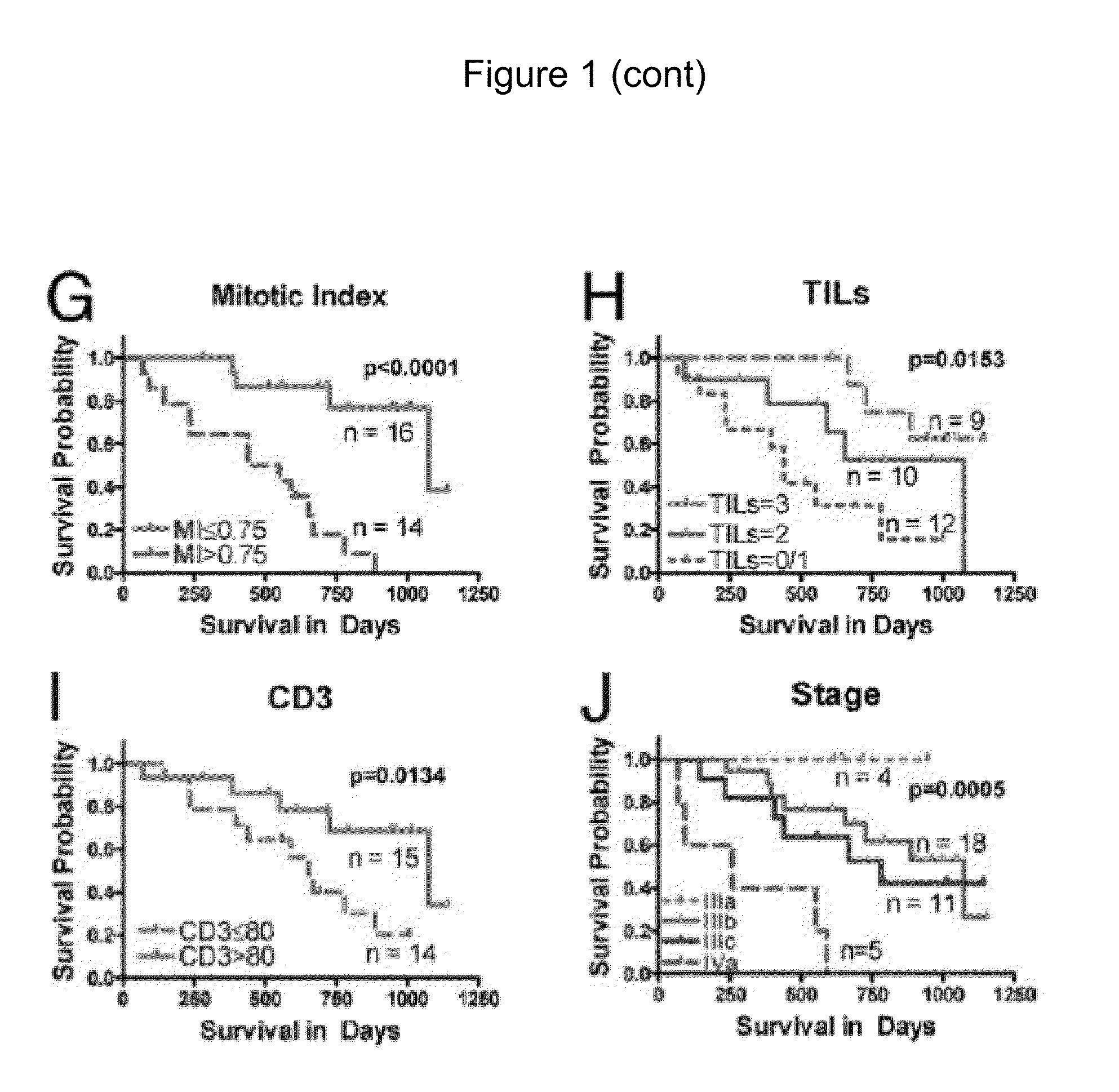
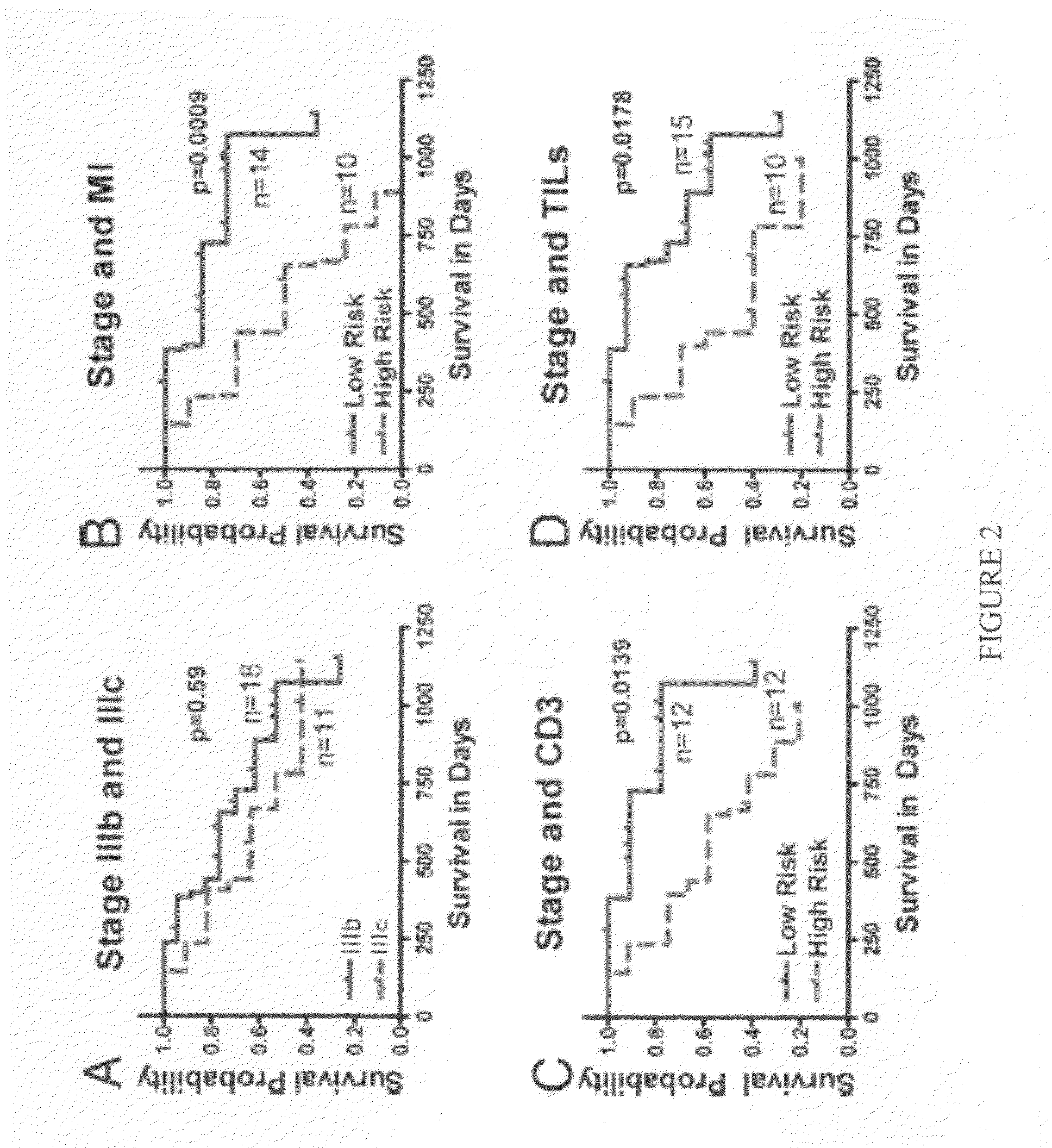
Cellular and genetic signatures and methods of using same for subcategorizing stage III melanoma tumors are described herein. The signatures and methods are particularly useful with regard to establishing more distinct criteria on which basis to differentiate stage IIIB and IIIC melanoma patients. Assessment of the cellular and genetic signatures of a melanoma sample using methods described herein yields information on which basis differential survival duration and sensitivity to various cancer therapies can be predicted for a Stage IIIB or Stage IIIC melanoma patients. As described herein, gene expression profiling, determination of mitotic index (MI), and quantification of tumor infiltrating leukocytes (TILs) and CD3+ cells in metastatic lesions may be utilized to predict or assess drug response, drug sensitivity, and clinical outcome in metastatic melanoma patients.
Owner:NEW YORK UNIV
Method to treat melanoma in BRAF inhibitor-resistant subjects
Owner:GENEKEY CORP
Low dose haptenized tumor cell and tumor cell extract immunotherapy
InactiveUS7297330B2Effective treatmentBiocidePhosphorous compound active ingredientsAdjuvantDinitrophenyl
This invention relates to compositions comprising haptenized tumor cells and extracts thereof, methods for preparing the compositions, vaccines comprising such haptenized tumor cells, and methods for treating cancer with such vaccines. In a specific embodiment, melanoma cells are haptenized with a dinitrophenyl group, and used for treatment of melanoma patients having metastatic disease. Preferably, patients are given a first vaccine dose containing haptenized cells to “prime” the immune system. Subsequently, patients are injected with an immunomodulatory compound such as cyclophosphamide. In a preferred embodiment, an appropriate time period after the “priming” vaccine dose, additional vaccine doses containing a mixture of haptenized cells and an adjuvant are administered. The described treatment plan is more effective for eliciting favorable anti-tumor immune responses.
Owner:THOMAS JEFFERSON UNIV
Long non-coding RNA related to melanoma and application thereof
InactiveCN110964831AAchieve early diagnosisImprove survival rateMicrobiological testing/measurementDNA/RNA fragmentationPharmaceutical drugOncology
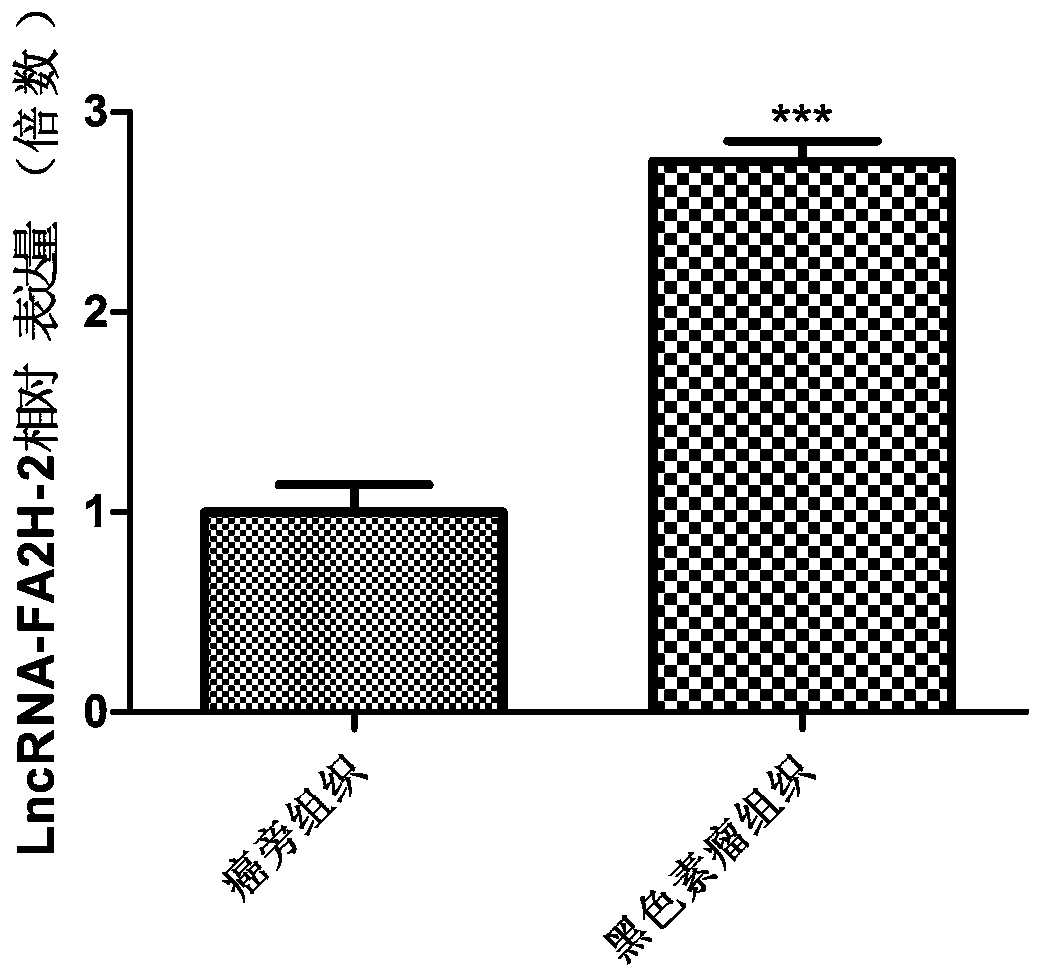
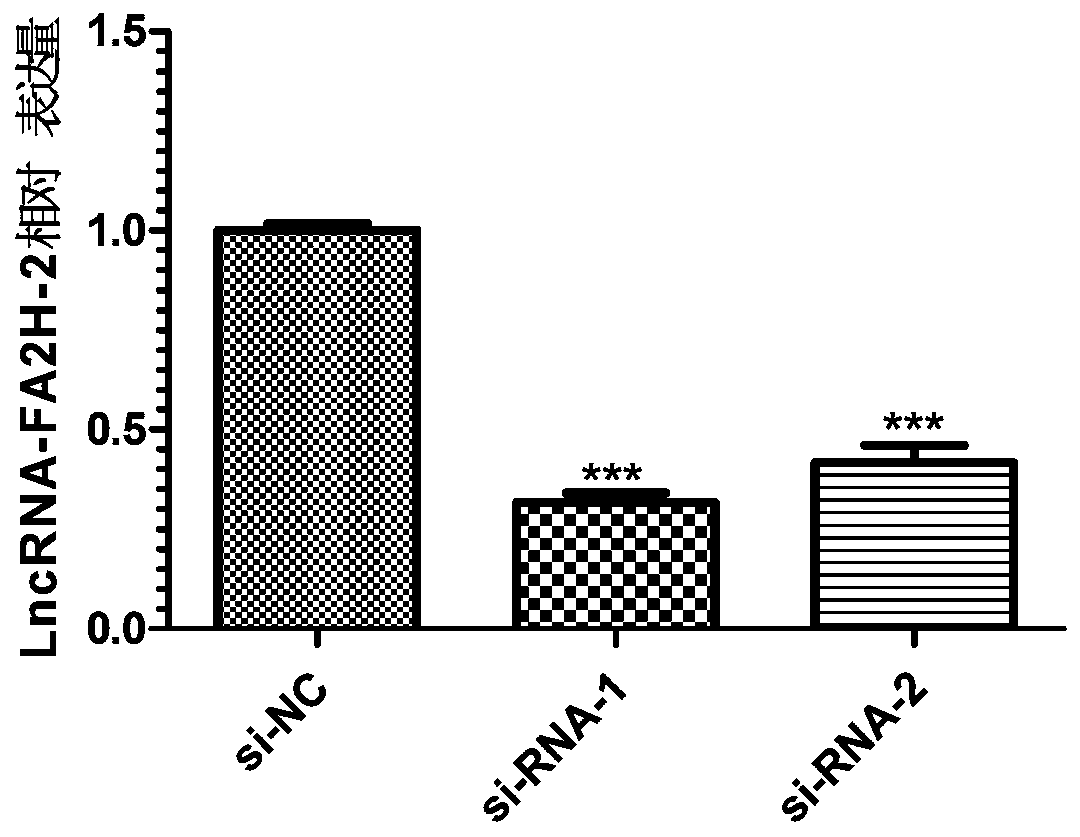
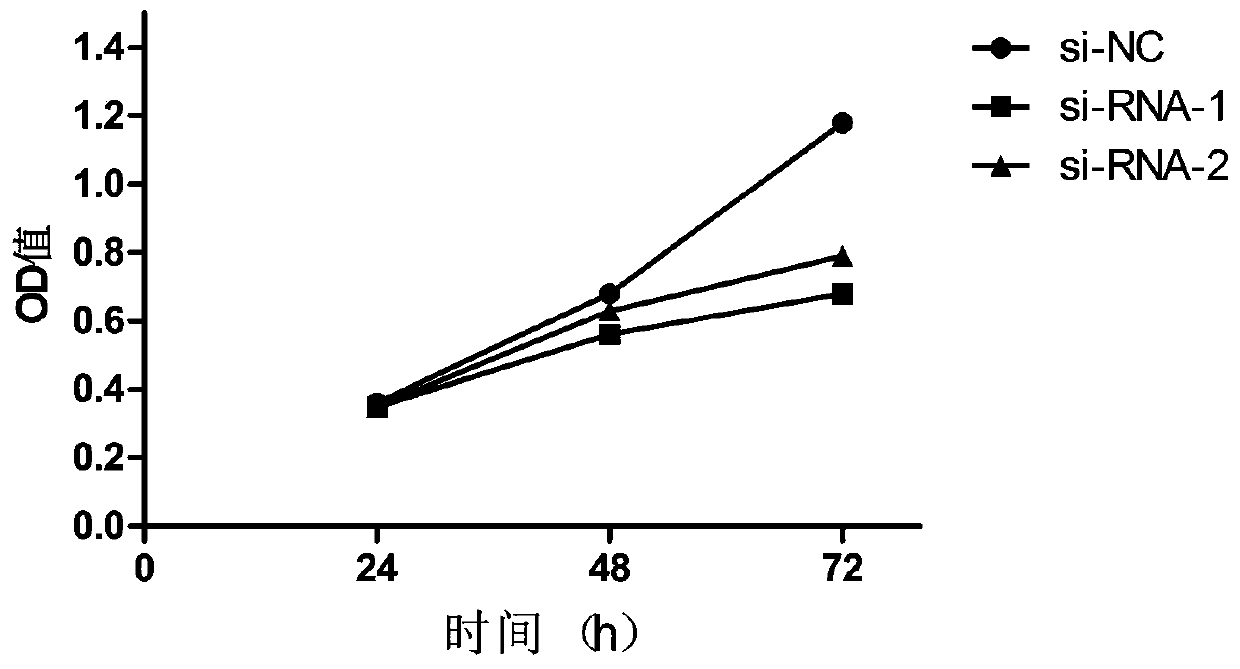
The invention discloses a long non-coding RNA related to melanoma and application thereof. The long non-coding RNA is LncRNA-FA2H-2. Researches prove that the expression of the LncRNA-FA2H-2 in tissues of a melanoma patient is up-regulated; the proliferation capacity of melanoma cells A357 can be remarkably reduced by knocking down the LncRNA-FA2H-2 through the si-RNA; and thus the result indicates that the LncRNA-FA2H-2 can be used as a molecular marker and a target point of melanoma for diagnosis and treatment of the melanoma. The long non-coding RNA related to melanoma has the beneficial effects that the biomarker LncRNA-FA2H-2 is provided for the diagnosis of melanoma, and the early diagnosis of melanoma can be realized by detecting the expression of the gene; the invention also provides application of siRNA of LncRNA-FA2H-2 in preparation of a melanoma pharmaceutical composition; and the long non-coding RNA has important clinical significance and broad application prospects.
Owner:刘秀玲
Long-chain non-coding RNA marker for early diagnosis of malignant melanoma and application thereof
PendingCN111088361AImprove early detection rateHigh potential for clinical applicationMicrobiological testing/measurementDNA/RNA fragmentationAbnormal expressionMalignancy
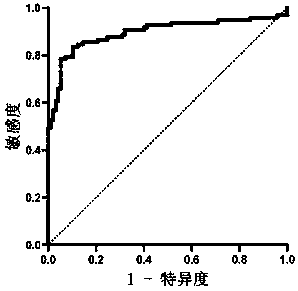
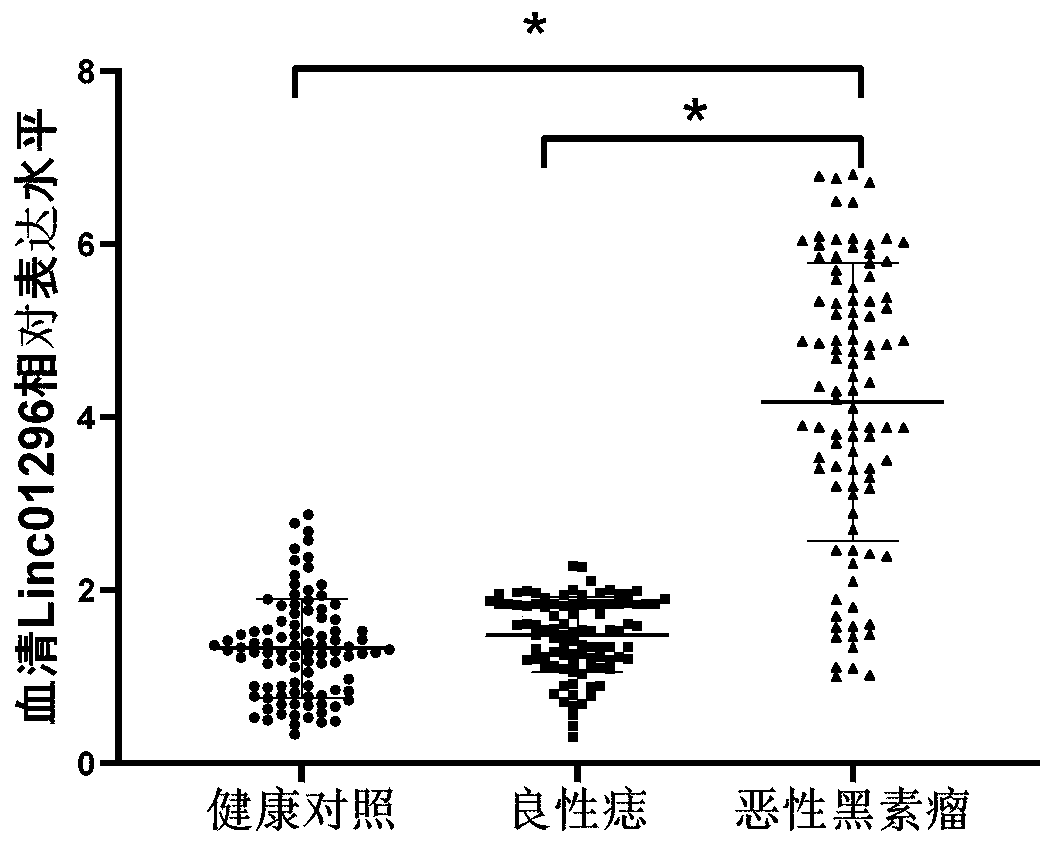
The invention relates to a long-chain non-coding RNA marker for early diagnosis of malignant melanoma and application thereof. The long-chain non-coding RNA marker is Linc01296 with a corresponding DNA sequence as shown in SEQ. ID. NO. 1. The application is an application of the long-chain non-coding RNA marker Linc01296 in the preparation of an early diagnosis reagent for malignant melanoma as atarget RNA. According to the invention, lcnRNA, Linc0126, with abnormal expression in serum of malignant melanoma is successfully screened, and expression of Linc01296 is significantly high in the serum of malignant melanoma patients. In addition, Linc01296 can effectively distinguish malignant melanoma patients from healthy people and patients with benign skin moles. The marker has high sensitivity and specificity, wherein the sensitivity can reach 84.69%, the specificity can reach 88.77%, and the marker has high diagnostic characteristics.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
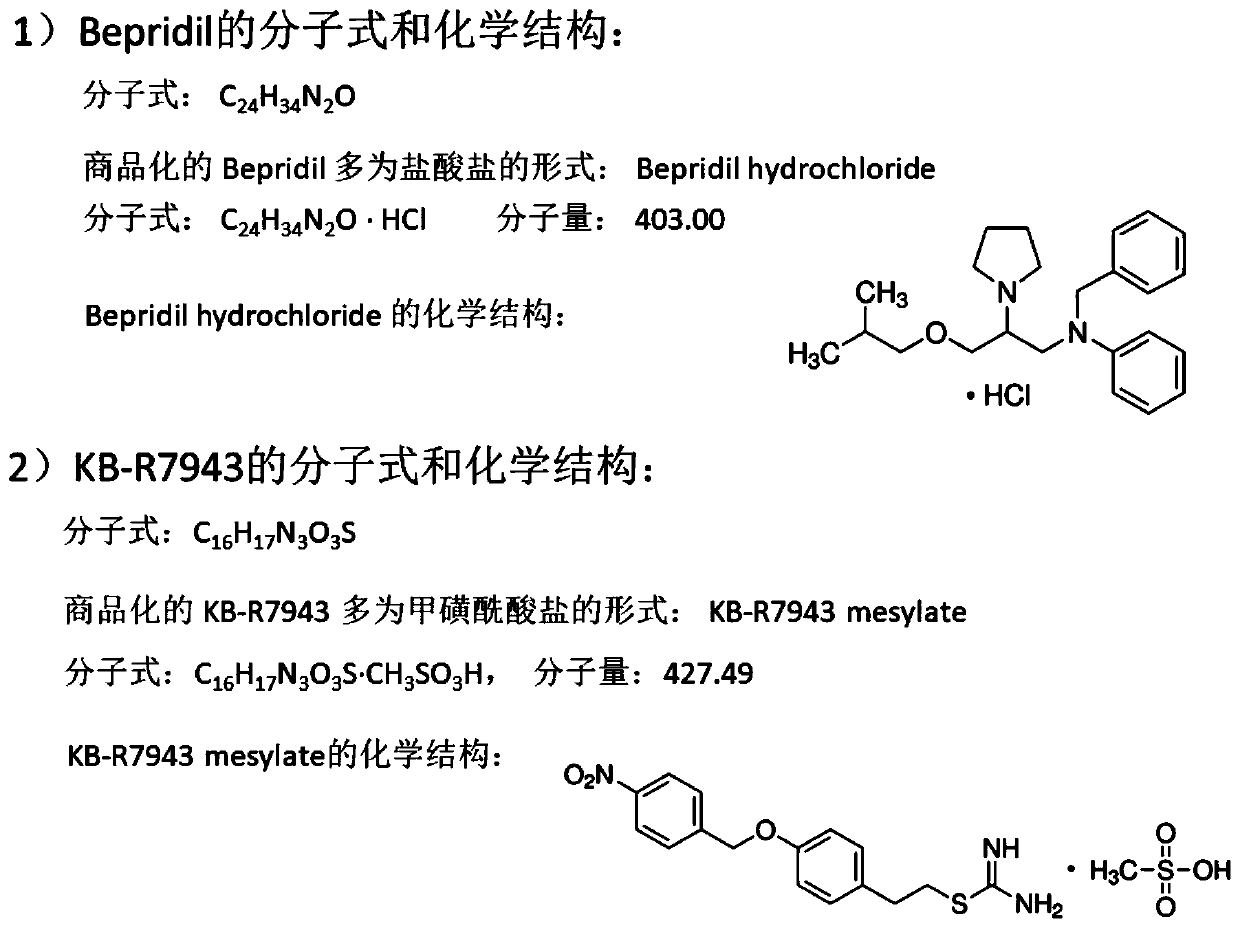
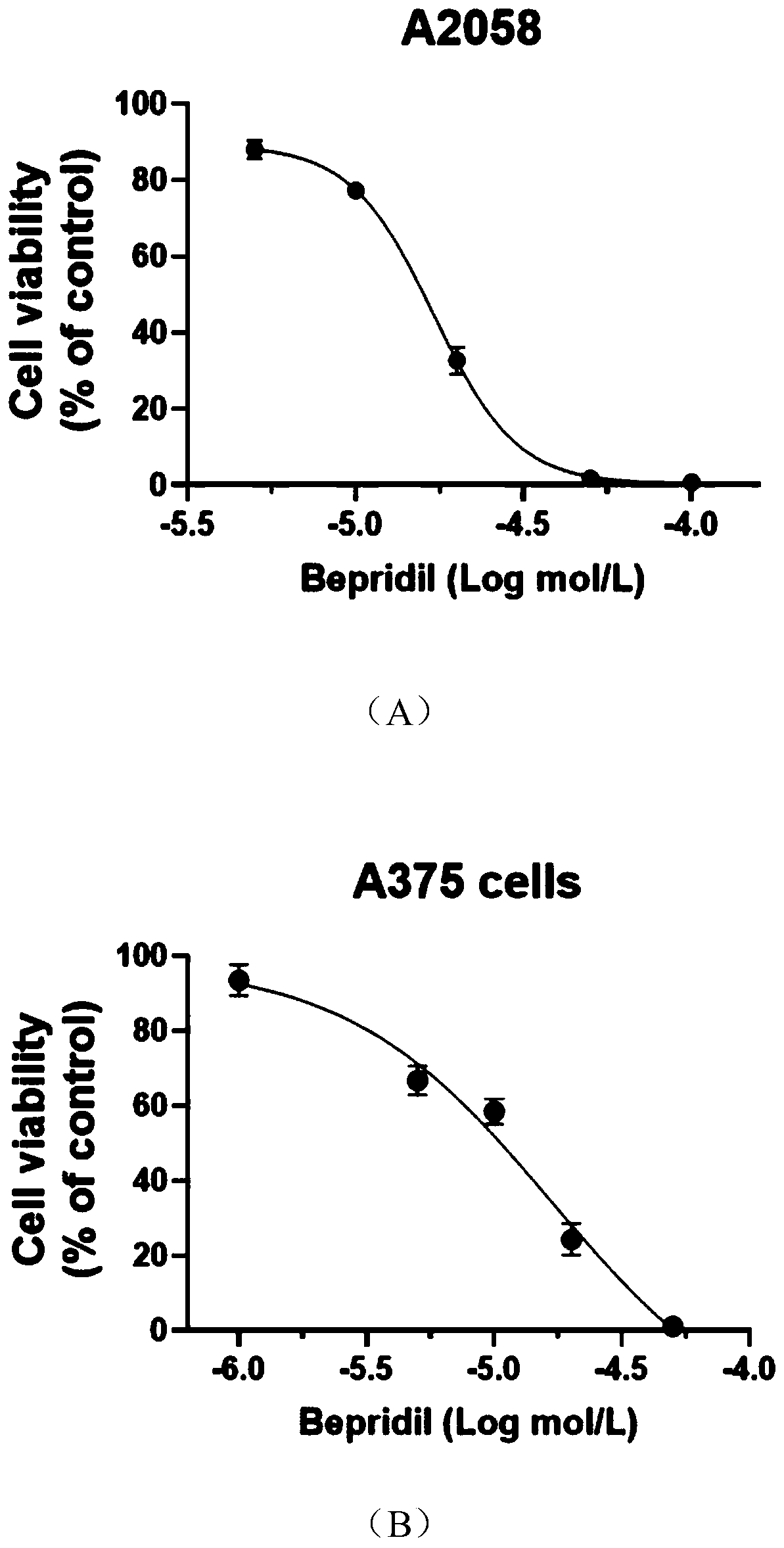
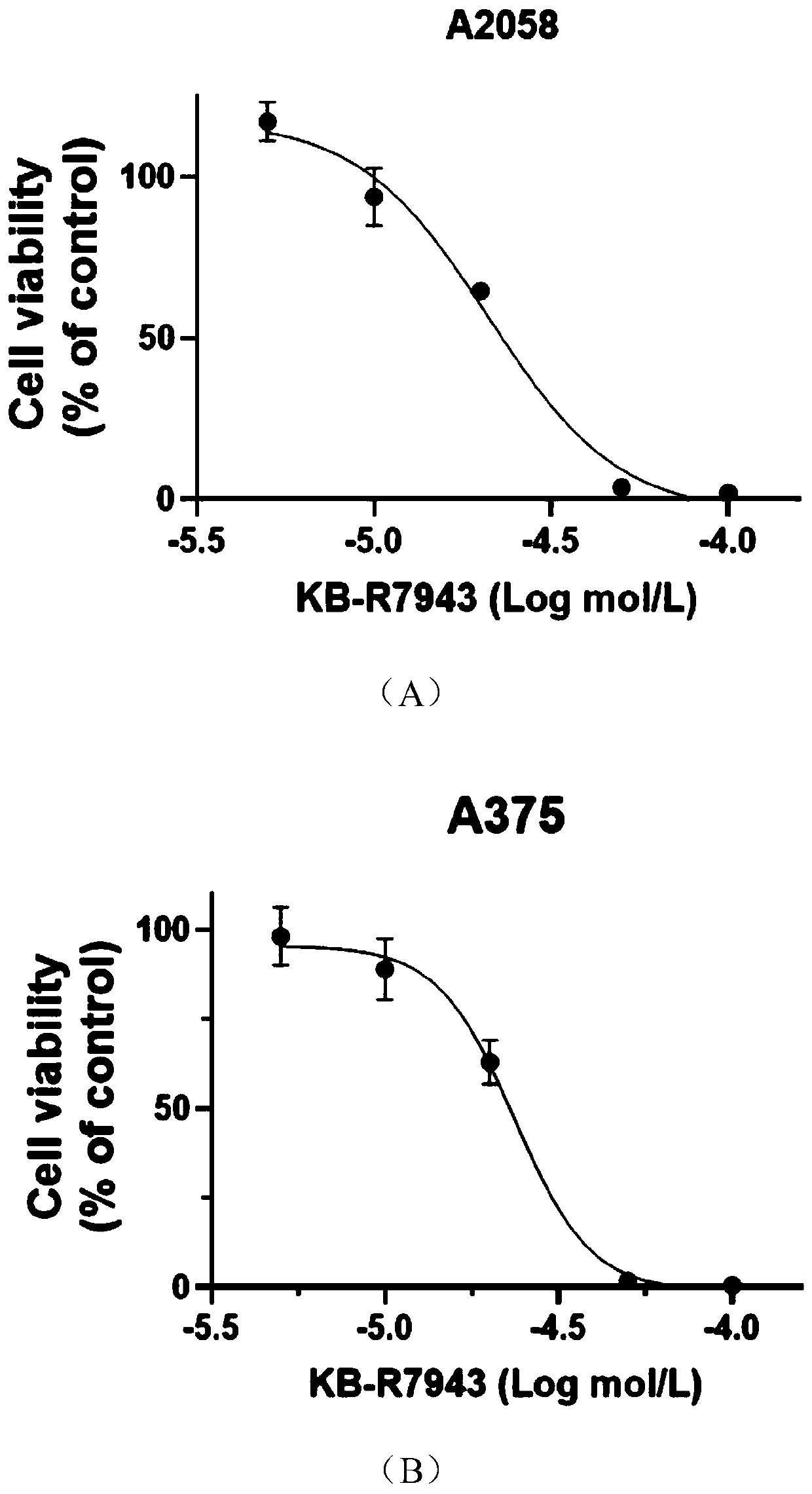
Application of Bepridil or KB-R7943 in preparation of a medicine for treating melanoma
PendingCN111450089ADrug resistanceGood curative effectAmide active ingredientsAntineoplastic agentsConventional medicineNew medications
The invention relates to the technical field of biological medicines, and particularly relates to application of Bepridil or KB-R7943 in preparation of a medicine for treating melanoma. The inventionprovides a new strategy for resisting melanoma, namely the inhibitor Bepridil or KB-R7943 of a sodium-calcium exchanger is applied to treat melanoma, and belongs to new application of an old medicine.The mechanism of Bepridil or KB-R7943 for killing tumor cells is completely different from that of the conventional medicine, and drug resistance is not generated. Due to the use of the two new medicines, prognosis of a patient suffering from melanoma is expected to be improved, the survival rate of the patient is increased, the social burden is relieved, and bright prospect is brought to treatment of melanoma.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Non-total-nutrient formula food for melanoma
InactiveCN104855982AIncrease supplementHighly absorbentSugar food ingredientsVitamin food ingredientsFormularyStage melanoma
The invention belongs to the technical field of formulas of edible-medicinal foods and new resource foods, and particularly provides a non-total-nutrient formula food, specific total-nutrient formula food or total-nutrient formula food for patients suffering from the melanoma. The formula is made according to the requirements of the <General Rules for Formula Foods for Special Medical Purposes>, and is combined with the physical characteristics of patients suffering from the melanoma. The formula food provided by the invention is prepared by reasonably mixing various 'edible-medicinal' traditional Chinese medicines according to the quintessence theory of traditional Chinese medicine, and is prepared by mixing essences which are extracted from various edible-medicinal traditional Chinese medicines by adopting a prehydrolysis SBE technique, short peptides extracted from biological enzymolysis, prebiotics, amino acid, carbohydrate, grease with a function of keeping healthy, various vitamins and mineral substances and other technologies. The formula food can be used as a single nutrient source satisfying the nutrient demands of patients suffering from the melanoma, and has the efficacy of clearing away heat and toxic materials, removing toxic materials and blood stasis, strengthening body resistance and inhibiting cancers, benefiting qi and nourishing blood, and strengthening immunity.
Owner:JINSHANMEI BIOTECH
Low dose haptenized tumor cell and tumor cell extract immunotherapy
InactiveUS20050232951A1EffectiveEffective treatmentBiocideGenetic material ingredientsAbnormal tissue growthAdjuvant
This invention relates to compositions comprising haptenized tumor cells and extracts thereof, methods for preparing the compositions, vaccines comprising such haptenized tumor cells, and methods for treating cancer with such vaccines. In a specific embodiment, melanoma cells are haptenized with a dinitrophenyl group, and used for treatment of melanoma patients having metastatic disease. Preferably, patients are given a first vaccine dose containing haptenized cells to “prime” the immune system. Subsequently, patients are injected with an immunomodulatory compound such as cyclophosphamide. In a preferred embodiment, an appropriate time period after the “priming” vaccine dose, additional vaccine doses containing a mixture of haptenized cells and an adjuvant are administered. The described treatment plan is more effective for eliciting favorable anti-tumor immune responses.
Owner:BERD DAVID
Methods and materials for cancer treatment
InactiveUS20080119636A1Facilitate inductionFacilitate persistenceImmunoglobulin superfamilyTumor rejection antigen precursorsAbnormal tissue growthEpitope
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Biomarker for predicting tumor drug sensitivity and related product thereof
InactiveCN113981092AImprove accuracyHigh AUC valueChemical property predictionMicrobiological testing/measurementMetastatic melanomaIndividualized treatment
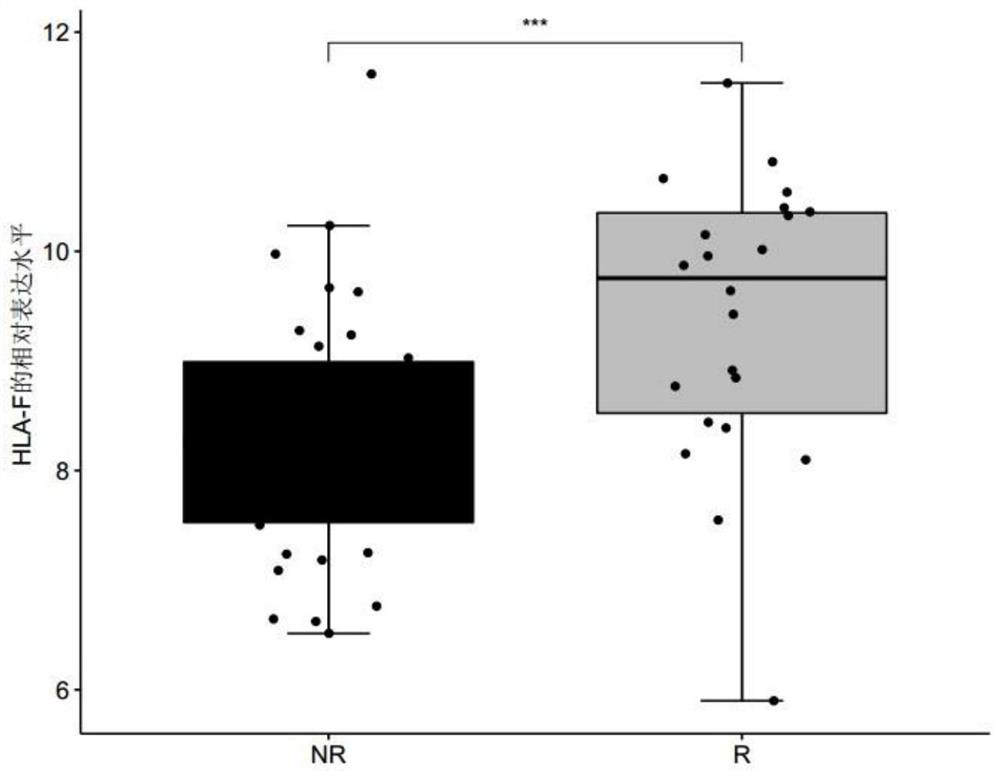
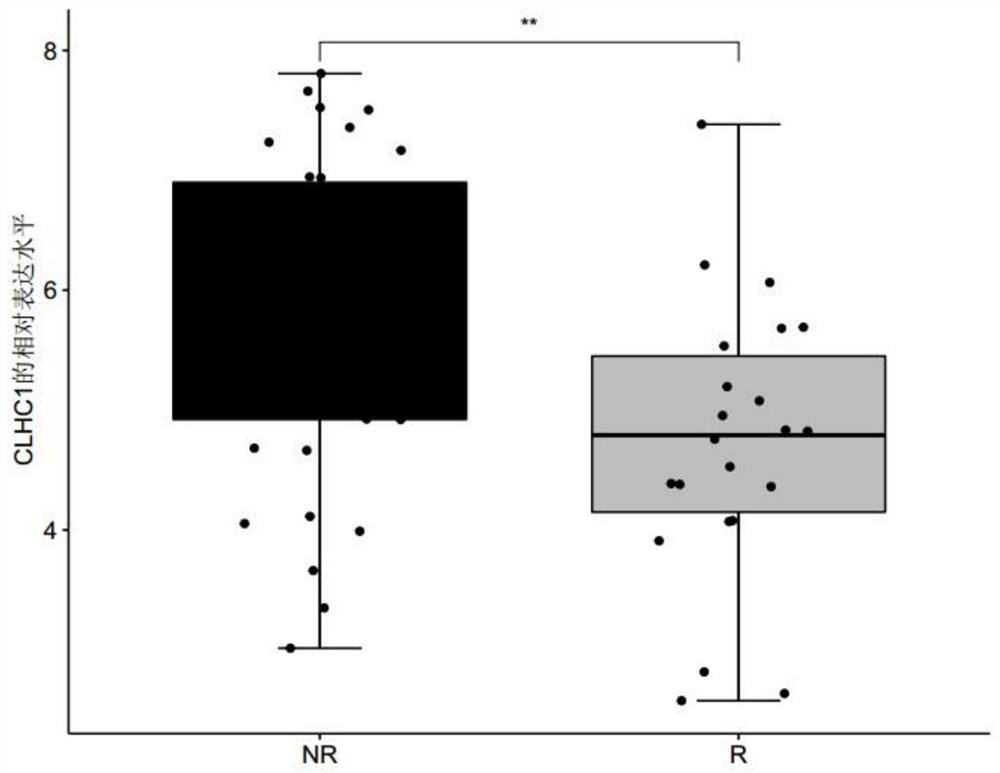
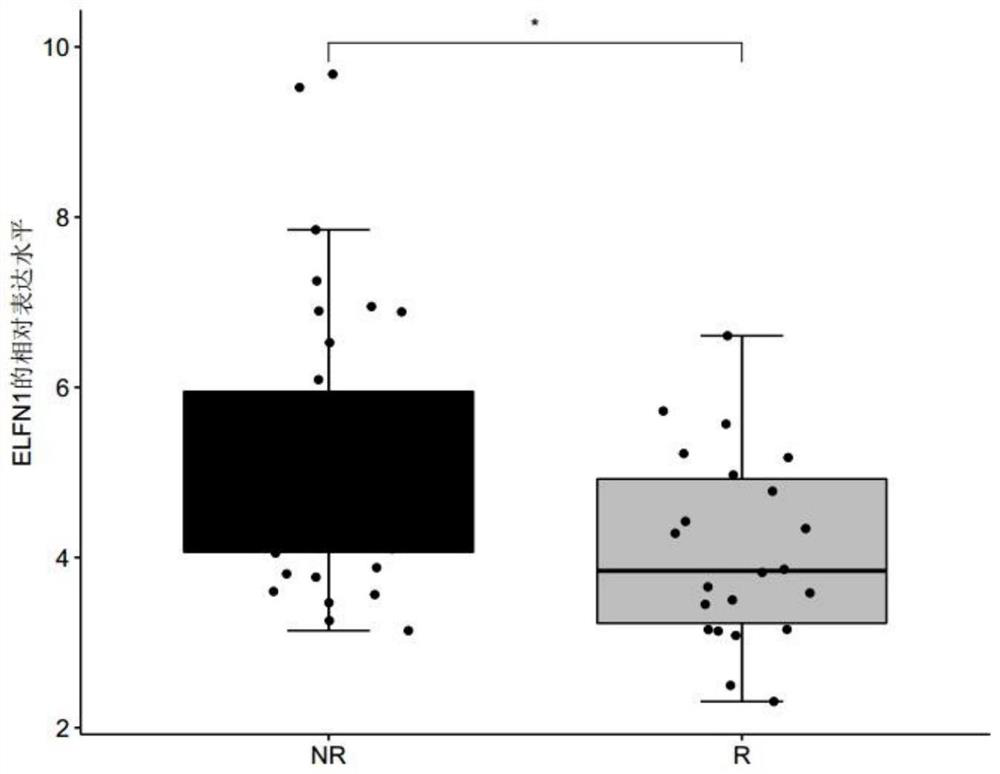
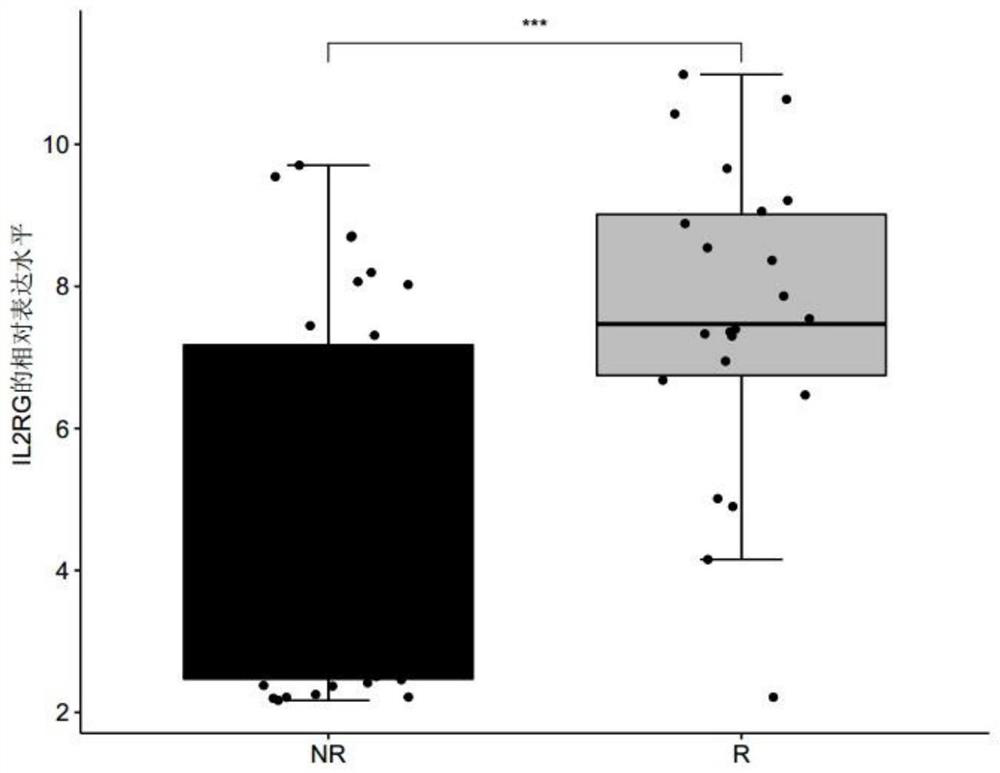
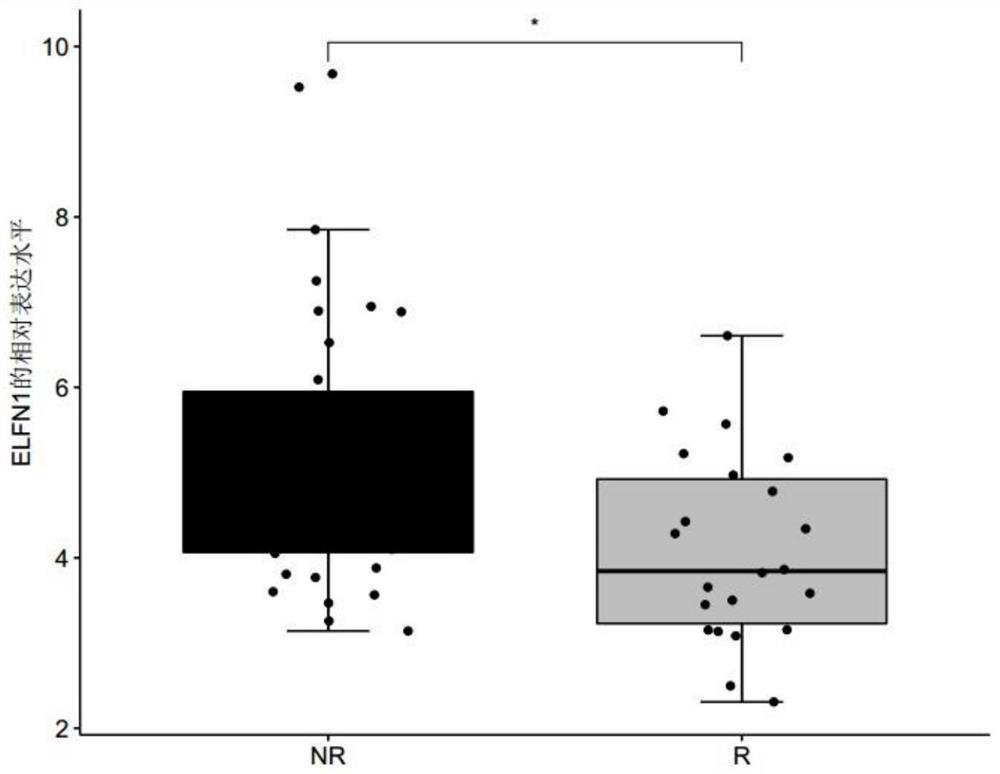
The invention discloses a biomarker for predicting tumor drug sensitivity and a related product thereof, wherein the biomarker is HLA-F, CLHC1 and / or ELFN1, and the product comprises a reagent for detecting the expression level of the biomarker. The biomarker and the related product thereof are used for predicting the sensitivity of metastatic melanoma patients to an immunotherapy drug MAGE-A3, clinical medication is guided by using the biomarker and the related product thereof provided by the technical scheme of the invention, not only can the blindness of drug selection be avoided, but also the treatment effect of the drug can be monitored in the treatment process, and the purposes of medication reasonability and individualized treatment are achieved.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
PCR kit for detecting human NRAS gene mutation
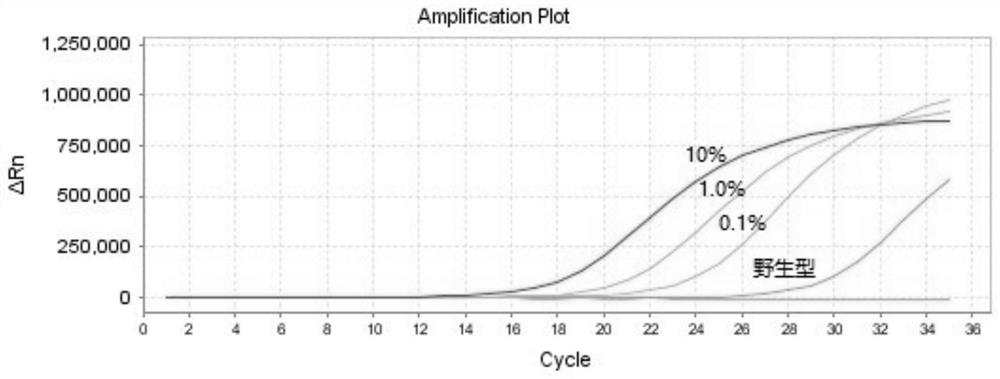
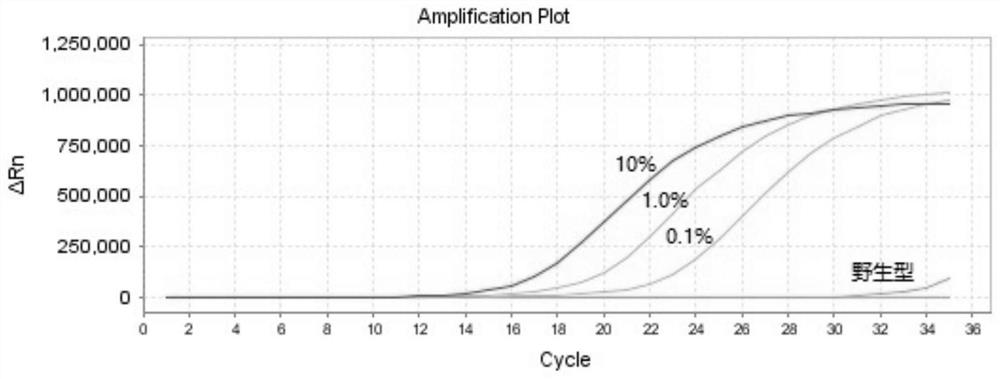
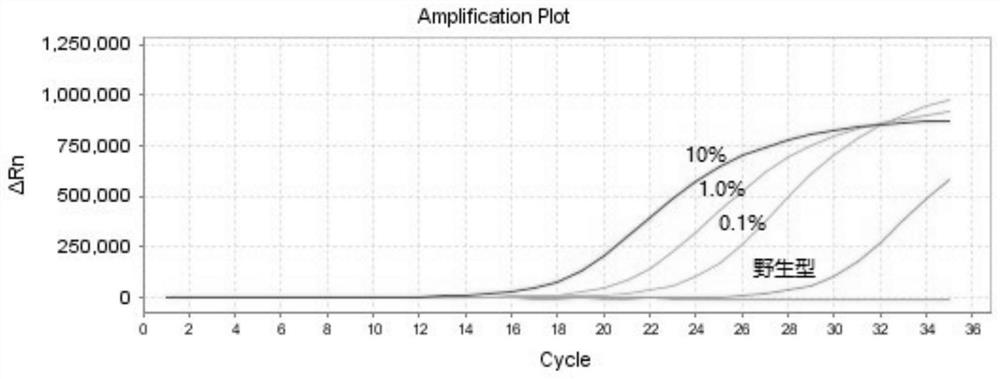
The invention discloses a PCR kit for detecting human NRAS gene mutation. The kit comprises a nucleic acid amplification reagent and a reference substance, wherein the nucleic acid amplification reagent comprises an NRAS Q61H reaction solution, an NRAS Q61K reaction solution, an NRAS Q61L reaction solution and an NRAS Q61R reaction solution, the reaction solutions respectively contain specific primers and probes for detecting of NRAS Q61H, Q61K, Q61L and Q61R mutations, and each reaction solution contains a pair of upper primer and lower primer, and a TaqMan fluorescent probe. The kit adopts an ARMS-PCR method, and the specific primers and probes aiming at Q61H, Q61K, Q61L and Q61R sites are respectively designed. The kit can be used for detecting mutations of Q61H, Q61K, Q61L and Q61R sites of NRAS genes of tumor tissue DNA samples of patients suffering from colorectal cancer, thyroid cancer and melanoma. The kit can be used for detecting mutations as low as 0.1% in the total amount of 50ng DNA.
Owner:上海佰臻生物科技有限公司
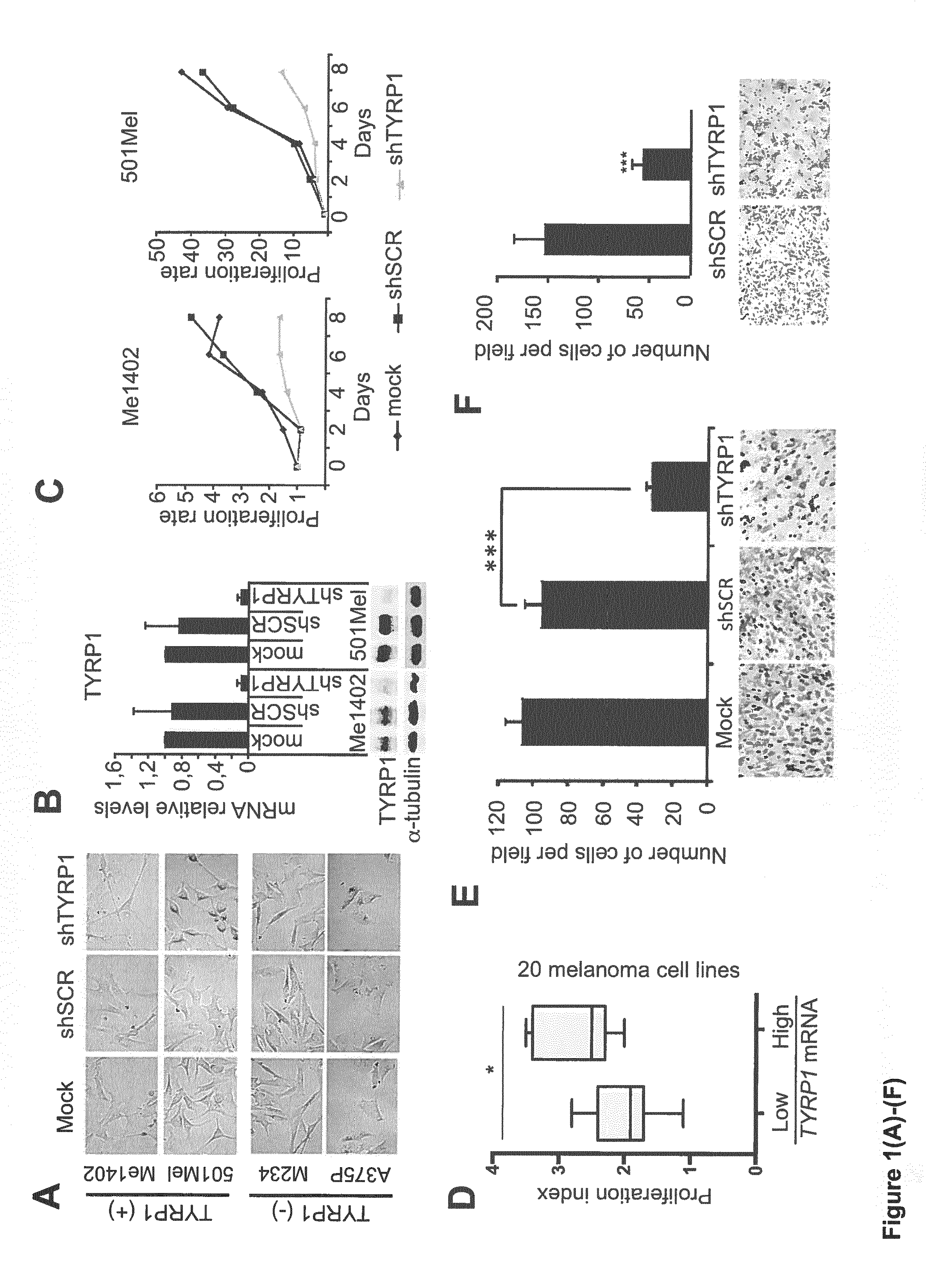
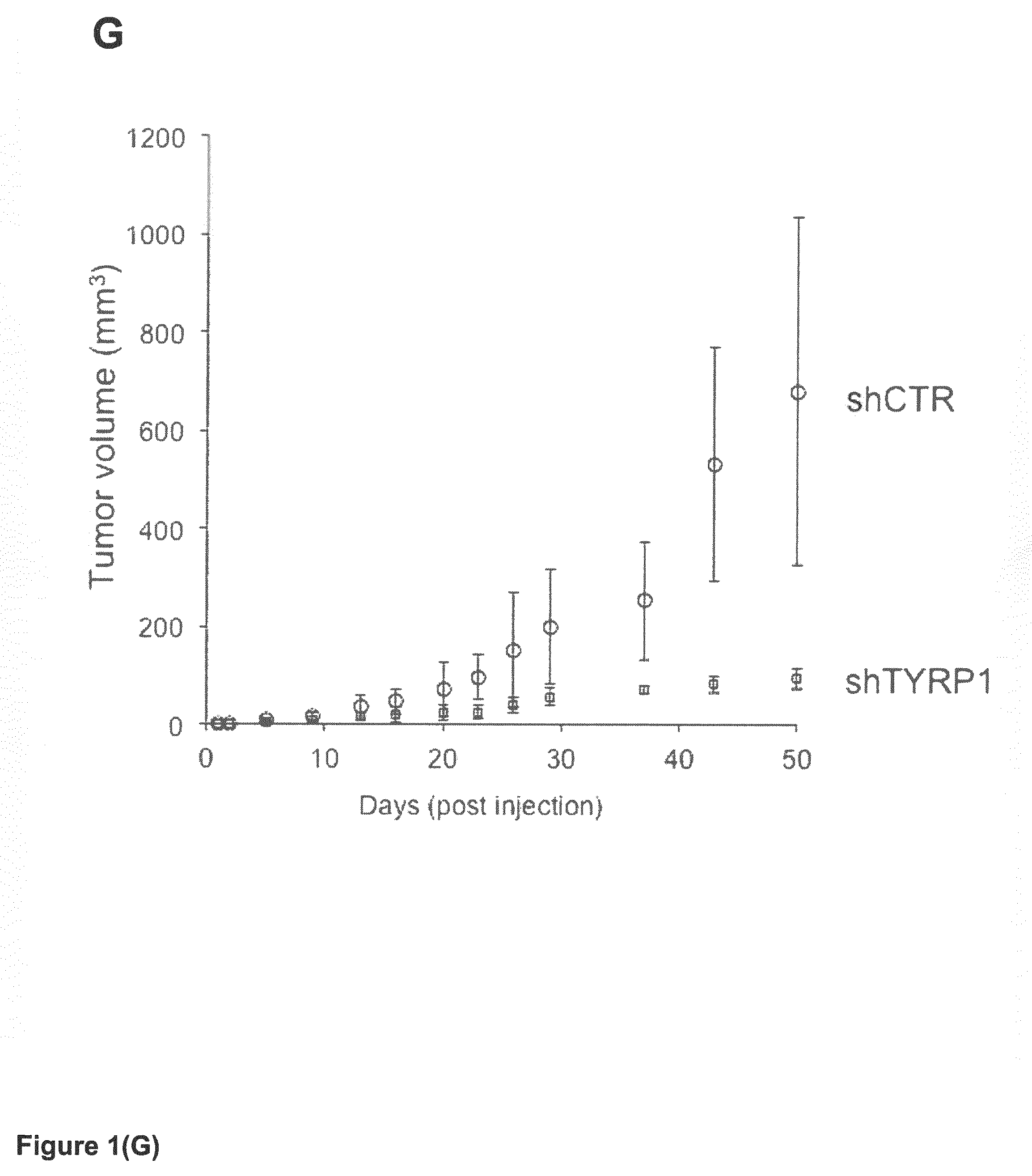
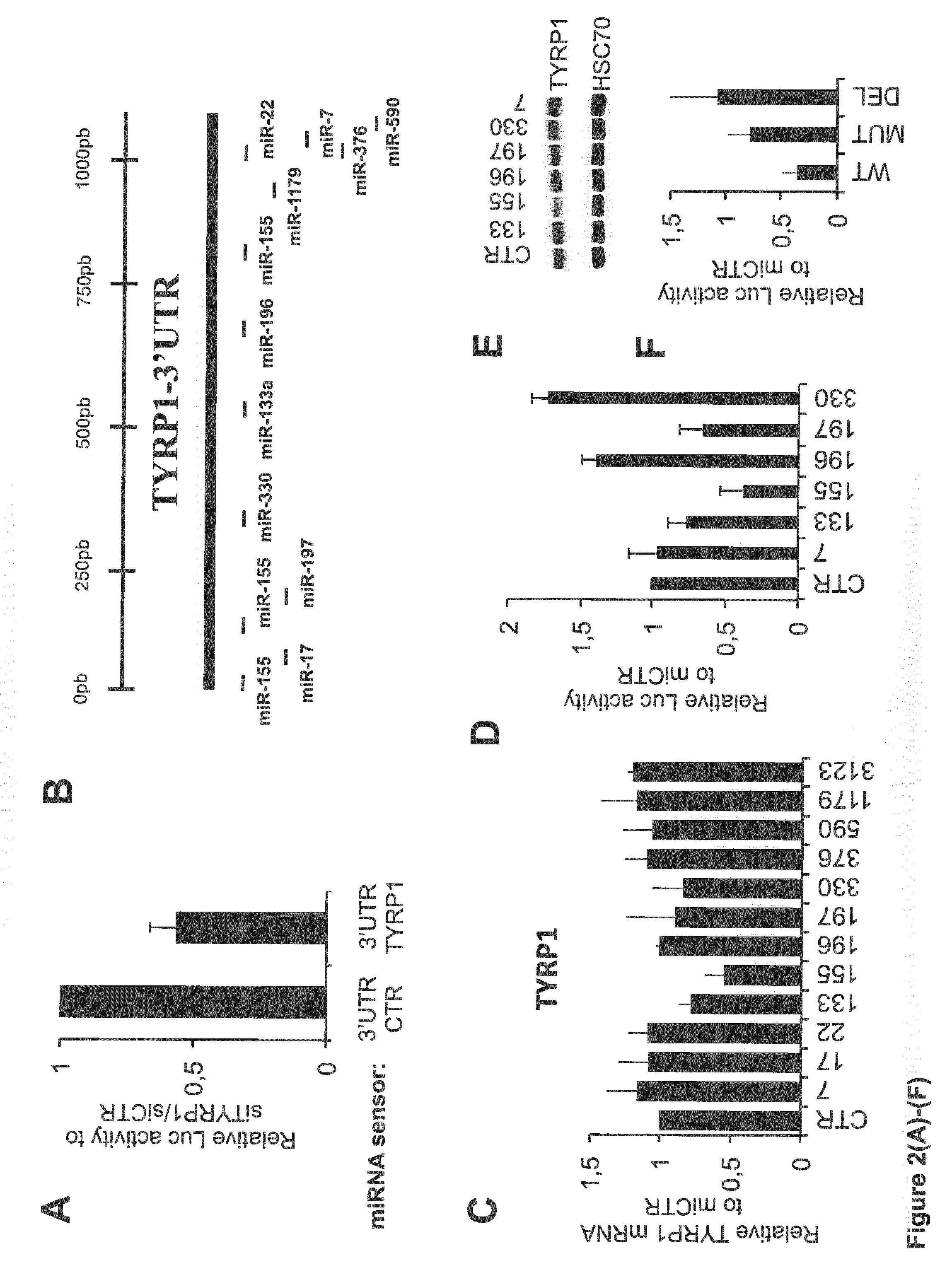
Tyrp1, a natural mirna sponge, and its use in managing human melanoma aggressiveness
ActiveUS20160265063A1Microbiological testing/measurementOxidoreductasesMetastatic melanomaHuman melanoma
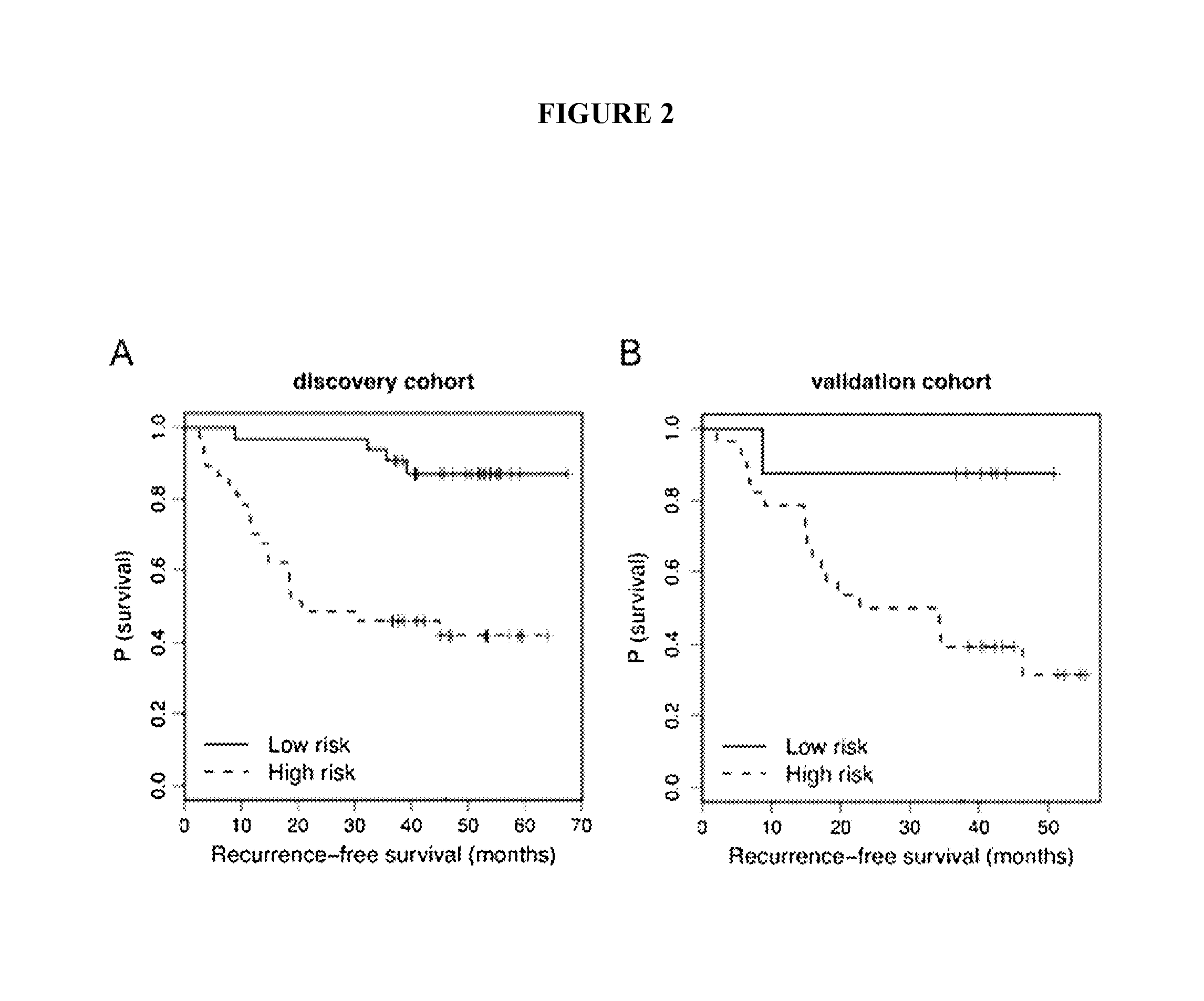
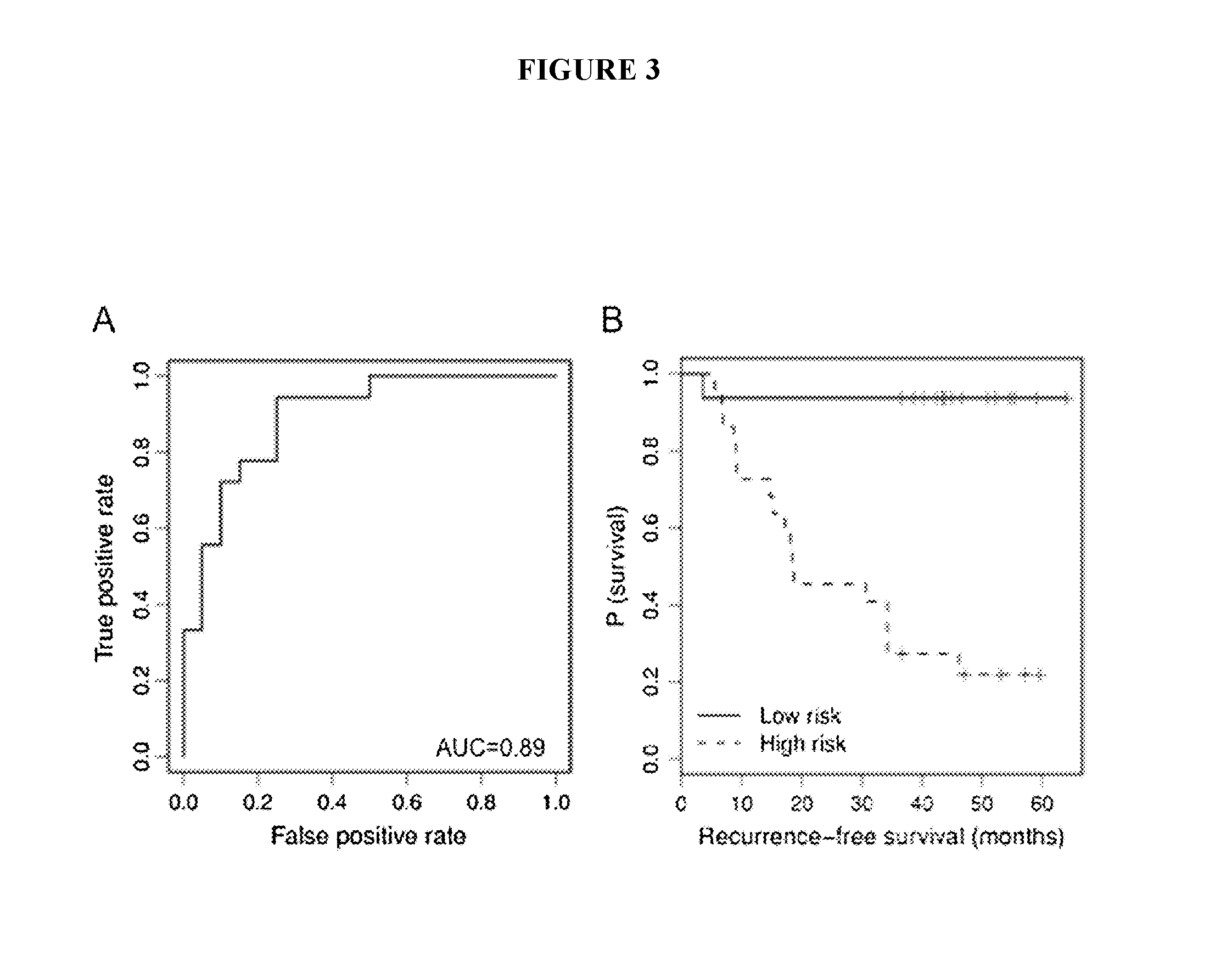
The present invention relates to methods and reagents for the treatment of melanoma and / or of metastatic melanoma that directly or indirectly target TYRP1 RNA transcript. The invention also relates to methods for predicting if a melanoma patient will be therapeutically responsive to such methods of treatment and reagents, and to methods for assessing the effectiveness of such methods of treatment and reagents. The invention further relates to a combination of biological markers that allows the prediction of melanoma patients' survival irrespective of the treatment administered.
Owner:UNIV DE RENNES I +2
Use of anti-PD-1 antibody in tumor treatment
InactiveCN113244386AOrganic active ingredientsMicrobiological testing/measurementAntiendomysial antibodiesAntigen Binding Fragment
The present invention relates to the use of an anti-PD-1 antibody in the treatment of melanoma. The invention also relates to application of reagents for detecting BRAF, NRAS and CDK4 / CCND1 gene mutations in a detection kit for predicting the treatment effect of a melanoma patient on independently applying the anti-PD-1 antibody and / or the antigen binding fragment thereof.
Owner:SHANGHAI JUNSHI BIOSCI
Product for predicting sensitivity of melanoma patient to MAGE-A3 immunotherapy
InactiveCN113913527AImprove accuracyHigh AUC valueMicrobiological testing/measurementBiological testingOncologyImmunotherapy
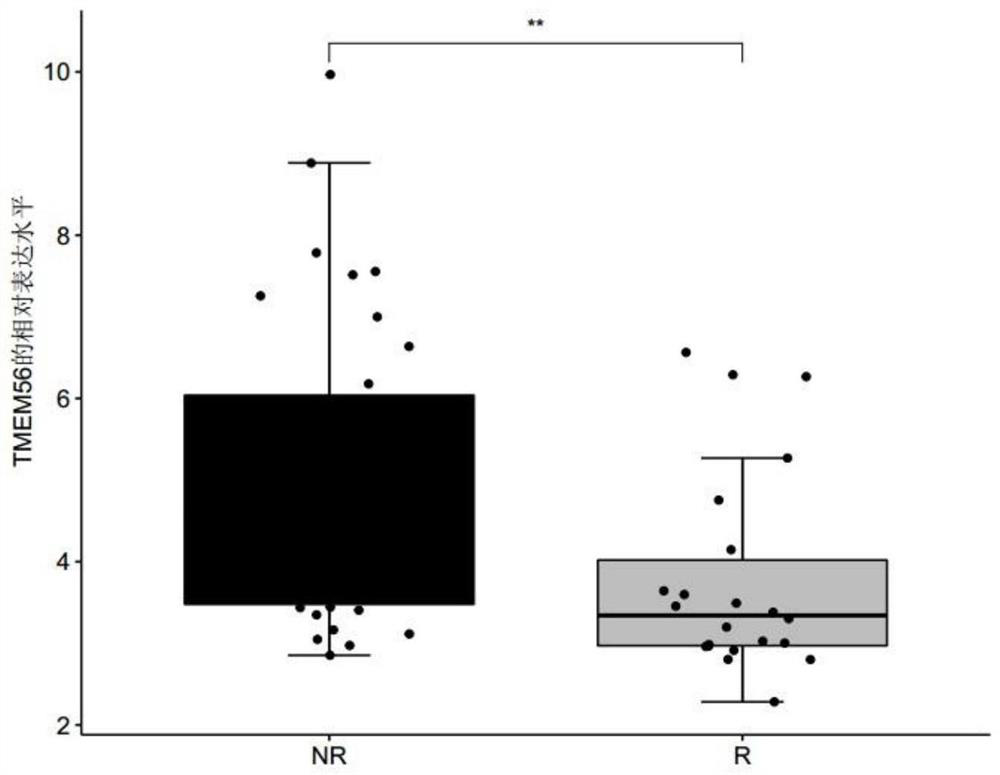
The invention discloses a product for predicting sensitivity of a melanoma patient to MAGE-A3 immunotherapy. The product comprises a kit. The kit contains reagents for detecting expression levels of markers IL2RG, TMEM56 and / or ELFN1. The provided method and product predict sensitivity of a melanoma patient to MAGE-A3 immunotherapy. The method is simple, has short time and high efficiency, and can provide guidance for individualized medication and selection of treatment schemes.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
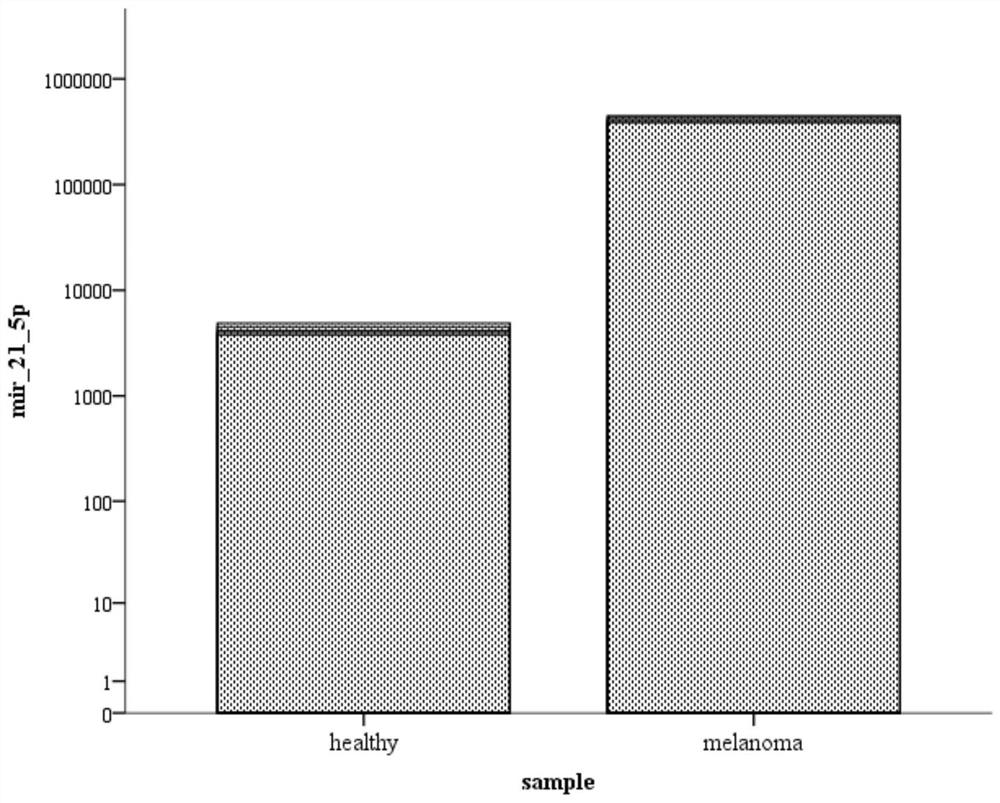
Sera Based miRNAs as Non-Invasive Biomarkers in Melanoma
InactiveUS20150344961A1Modify stabilityModify cellularNucleotide librariesMicrobiological testing/measurementSerum markersPatient risk
The present invention relates to serum marker microRNAs (miRNAs) which are associated with cancer, particularly melanoma, and to the assessment thereof in the prognosis, treatment and management of cancer. Embodiments include methods, compositions, kits and isolated nucleic acids. The present invention is directed to methods and compositions for prognosing melanoma and monitoring for recurrence by monitoring serum miRNAs, and the use of miRNAs and antagonists thereof, particularly antagomirs, for predicting and assessing risk and / or likelihood of recurrence in a melanoma patient. The present invention relates to biomarkers for melanoma, particularly serum markers and sets thereof which are relevant and significant as prognostic indicators of melanoma disease and patient risk for recurrence.
Owner:NEW YORK UNIV
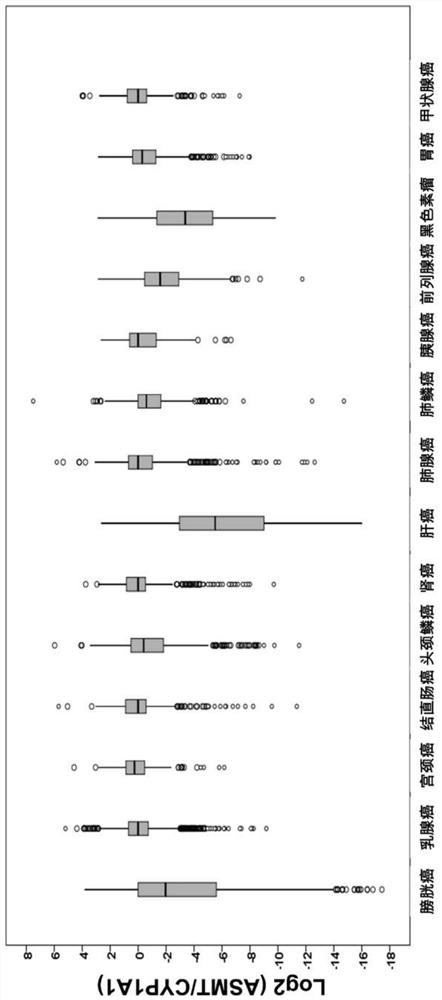
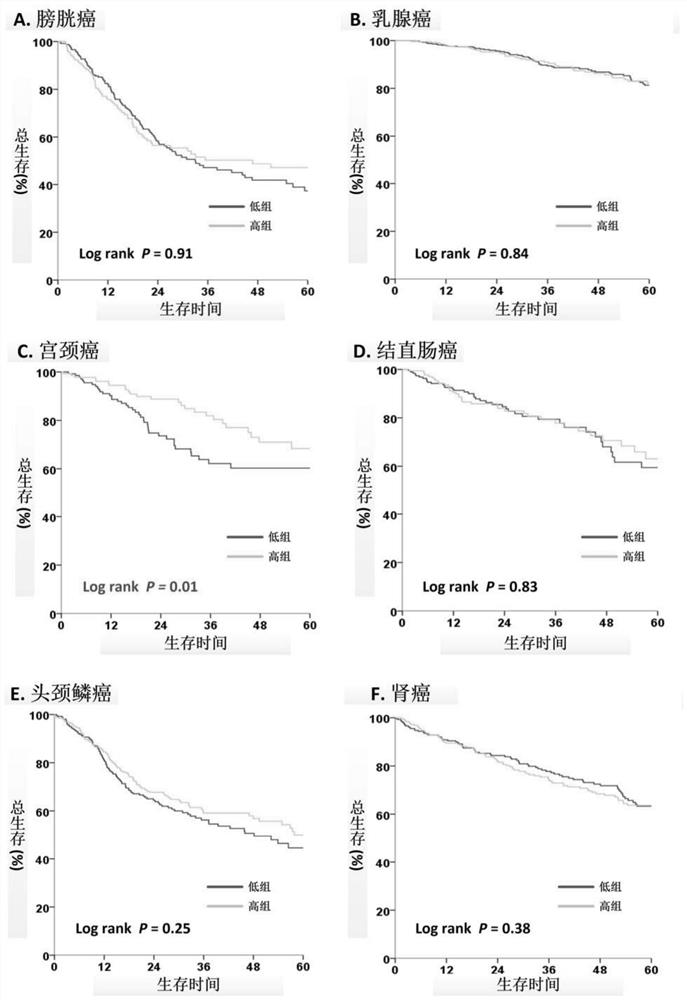
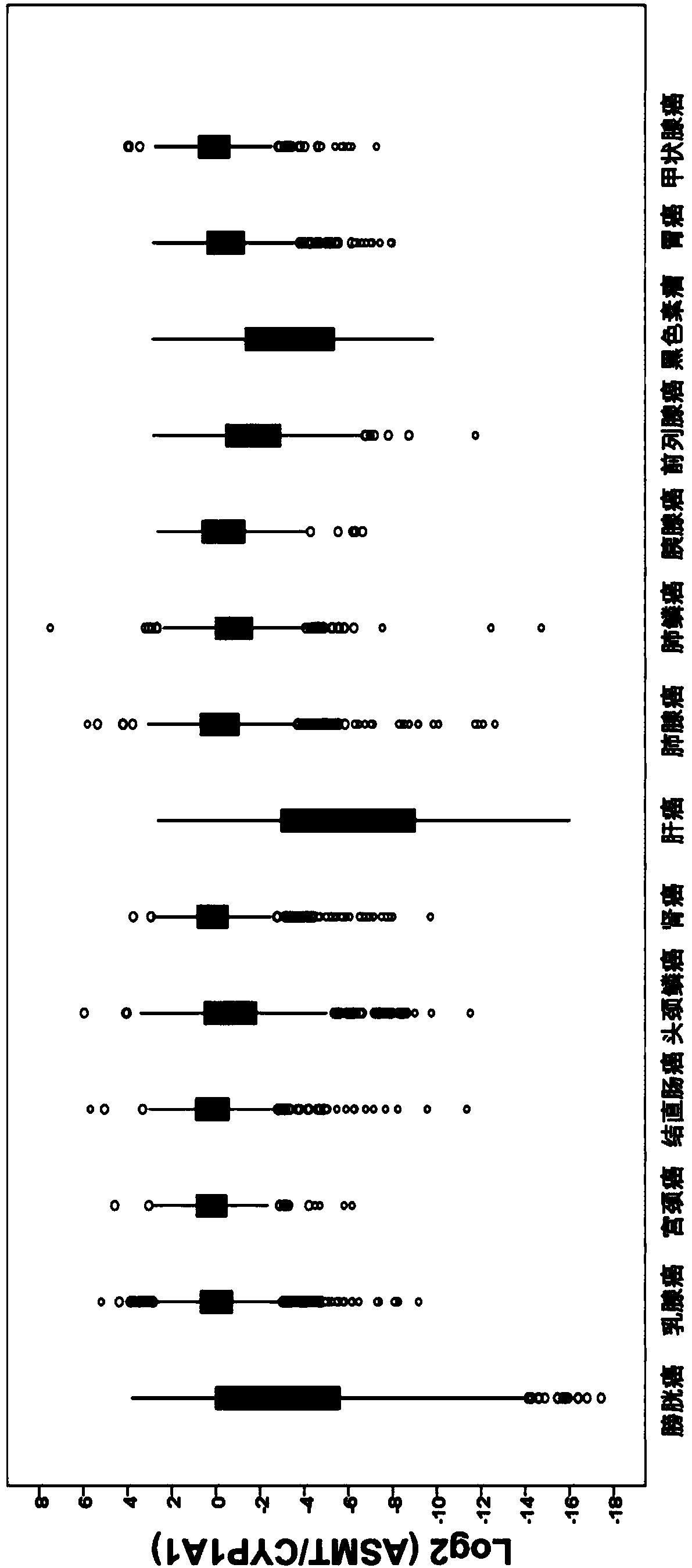
Application of ASMT/CYP1A1 molecular signature in predicting clinical prognosis or immune characteristics of solid tumors
ActiveCN109652547BEffectively predict the benefit of immunotherapyPredicting benefit from immunotherapyMicrobiological testing/measurementAntigenProstate carcinoma
Owner:SUN YAT SEN UNIV CANCER CENT
CircRNA related to malignant melanoma and application of circRNA
PendingCN111850128AImplement diagnosticsImprove survival rateMicrobiological testing/measurementBiological testingOncologyMalignancy
The invention discloses circRNA related to malignant melanoma and application of the circRNA, and belongs to the technical field of biological medicine. The circRNA in the application is circRNA _ 0012891, and it is found for the first time that the expression level of circRNA _ 0012891 is related to melanoma metastasis. The invention provides a circRNA diagnostic marker which is circRNA _ 0012891, and also provides a product which can be used for diagnosing whether melanoma metastasis exists or not, and improving the survival rate and the life quality of melanoma patients through advanced detection.
Owner:云南省肿瘤医院
Application of ASMT/CYP1A1 molecular tag in predicting clinical prognosis or immune characteristics of solid tumors
ActiveCN109652547AEffectively predict clinical prognosisEfficient prediction of immune body mutation levelsMicrobiological testing/measurementAntigenInformation analysis
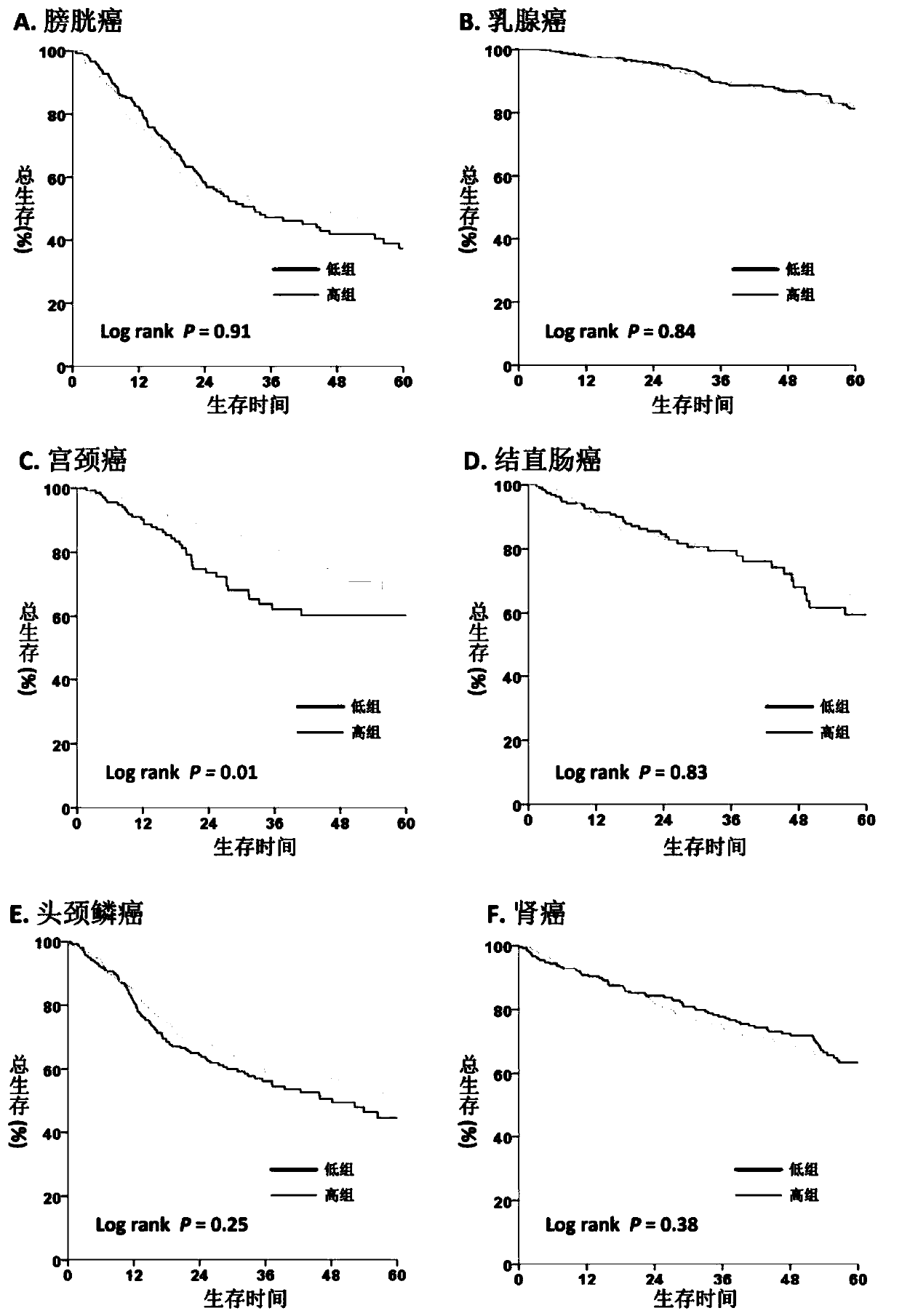
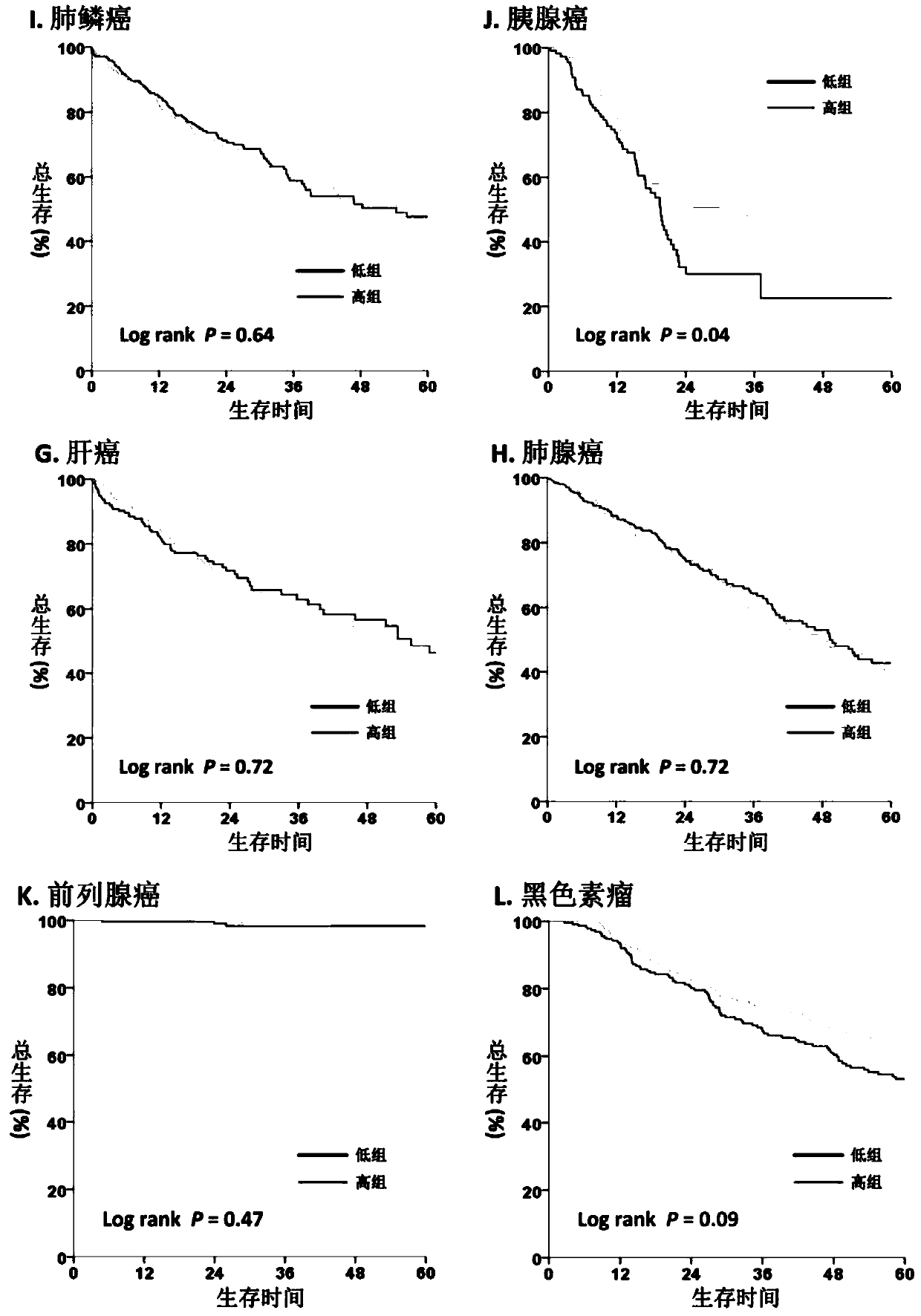
The invention discloses an application of an ASMT / CYP1A1 molecular tag in predicting clinical prognosis or immune characteristics of solid tumors. By studying the transcription group and genome data of 6658 tumor samples of 14 solid tumors and carrying out biological information analysis of large samples, the relationships between the ASMT / CYP1A1 ratio levels in different solid tumors and the clinical prognosis, tumor mutation level of and tumor neogenesis antigen load of patients are confirmed, wherein the clinical prognosis of patients with cervical cancer, gastric cancer and melanin in theHigh Index group is more considerable. The results of multivariate analysis show that the results of ASMT / CYP1A1 are independent prognostic indexes for the clinical prognosis of patients with tumors.In addition, in patients with colorectal cancer, head and neck squamous cell carcinoma, renal cancer, liver cancer, prostate cancer and melanoma, the prognosis of the LowIndex group is poor, but moremutation load and / or tumor neogenesis antigen exist in the LowIndex group, suggesting that the patients in the LowIndex group are more likely to benefit from immunotherapy.
Owner:SUN YAT SEN UNIV CANCER CENT
Methods for predicting survival in metastatic melanoma patients
InactiveUS9410205B2Prolong survival timeStimulate immune responseMicrobiological testing/measurementDisease diagnosisDifferential survivalAbnormal tissue growth
Cellular and genetic signatures and methods of using same for subcategorizing stage III melanoma tumors are described herein. The signatures and methods are particularly useful with regard to establishing more distinct criteria on which basis to differentiate stage IIIB and IIIC melanoma patients. Assessment of the cellular and genetic signatures of a melanoma sample using methods described herein yields information on which basis differential survival duration and sensitivity to various cancer therapies can be predicted for a Stage IIIB or Stage IIIC melanoma patients. As described herein, gene expression profiling, determination of mitotic index (MI), and quantification of tumor infiltrating leukocytes (TILs) and CD3+ cells in metastatic lesions may be utilized to predict or assess drug response, drug sensitivity, and clinical outcome in metastatic melanoma patients.
Owner:NEW YORK UNIV
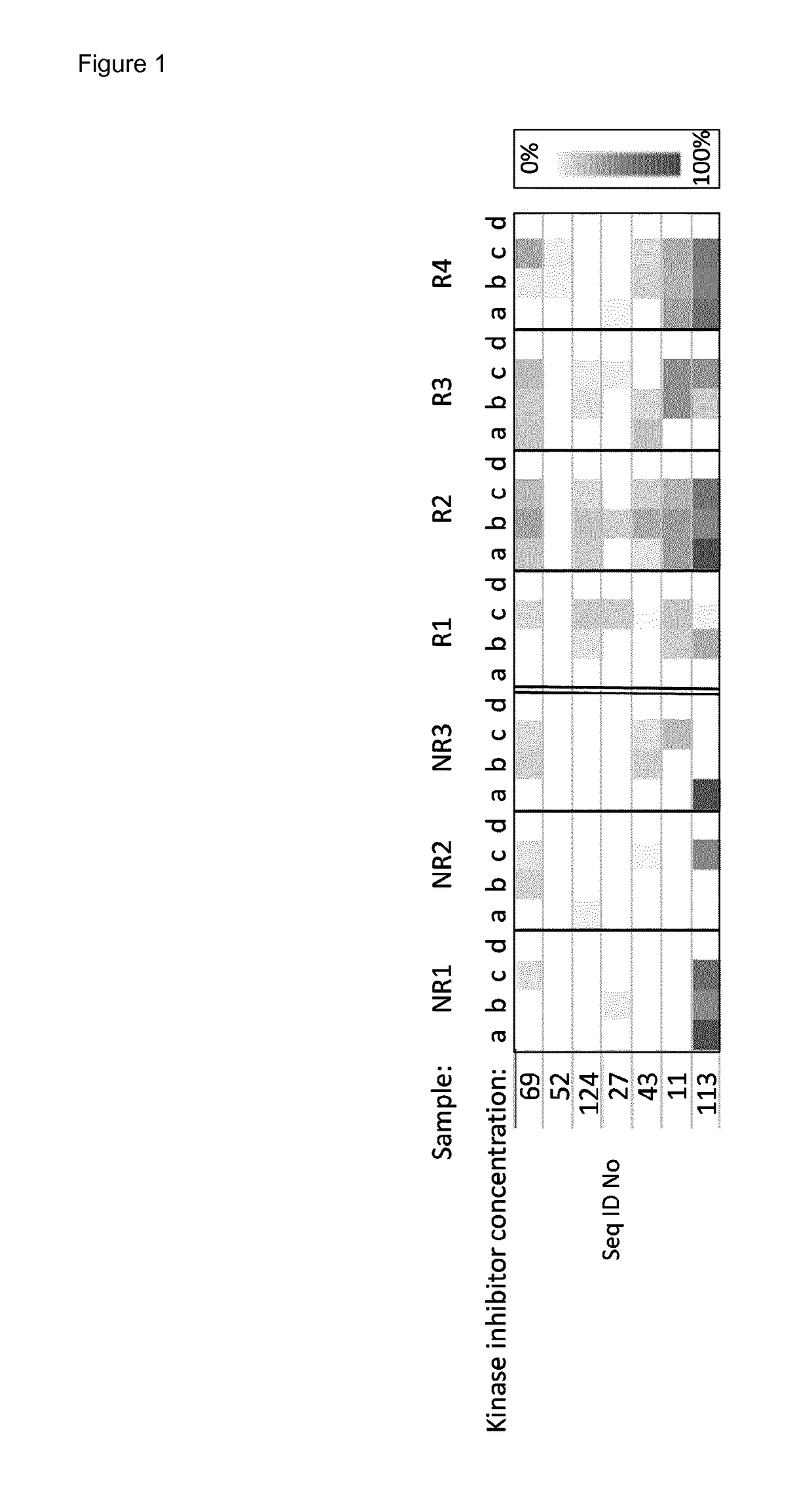
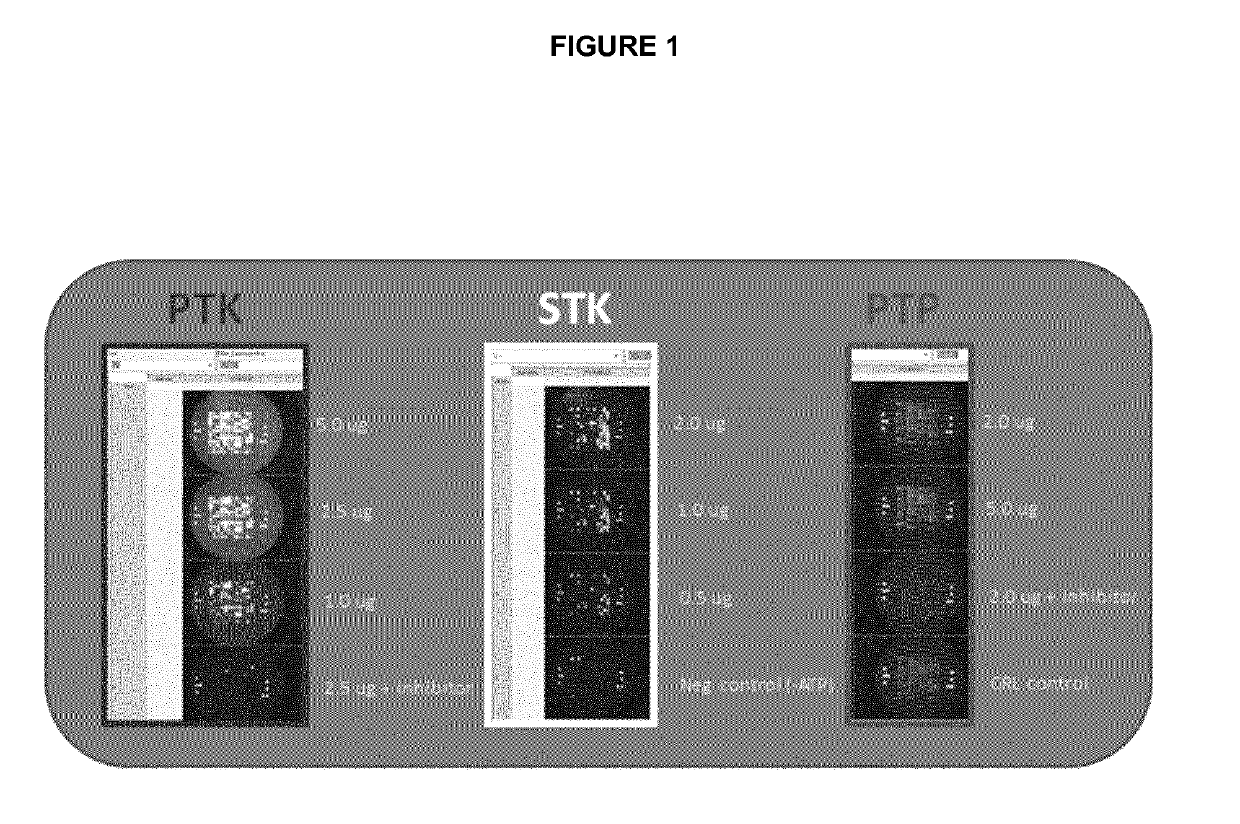
Method for predicting the response of melanoma patients to targeted pharmacotherapy
The present invention relates to a method for determining or predicting the response of a patient diagnosed with melanoma to targeted pharmacotherapy. The present invention also aims to provide methods and devices for predicting the response of patients diagnosed with melanoma to specific medicaments. More specifically, the present invention provides methods which measure kinase activity by studying phosphorylation levels and profiles and inhibitions thereof by drugs in samples of said patients.
Owner:PAMGENE
Method for predicting the response of melanoma patients to a medicament
PendingUS20190112633A1Induce adverse drug reactionInduce side effectMicrobiological testing/measurementDisease diagnosisPhosphorylationKinase
The present invention relates to a method for determining or predicting the response of a patient diagnosed with melanoma to targeted pharmacotherapy. The present invention also aims to provide methods and devices for predicting the response of patients diagnosed with melanoma to specific medicaments. More specifically, the present invention provides methods which measure kinase and / or phosphatase activity by studying phosphorylation levels and profiles and inhibitions thereof by drugs in samples, preferably blood samples, of said patients.
Owner:PAMGENE +1
Melanoma biomarker of urine sample and application of melanoma biomarker
PendingCN114807375AApply for early screeningApplicable to large-scale screeningMicrobiological testing/measurementDNA/RNA fragmentationHigh level expressionBiologic marker
The invention discloses a melanoma biomarker of a urine sample and application of the melanoma biomarker. The melanoma biomarker of the urine sample disclosed by the invention is miRNA with a sequence as shown in Seq ID No. 1. The melanoma biomarker of the urine sample is expressed at a high level in the urine of a patient with melanoma, and the expression level in the urine of a normal healthy person is remarkably low; therefore, the melanoma can be judged by detecting the expression level of the melanoma biomarker in a urine sample. The biomarker provides a new scheme and approach for melanoma detection, and is especially suitable for early screening and large-scale screening of melanoma.
Owner:SHENZHEN HAPLOX BIOTECH
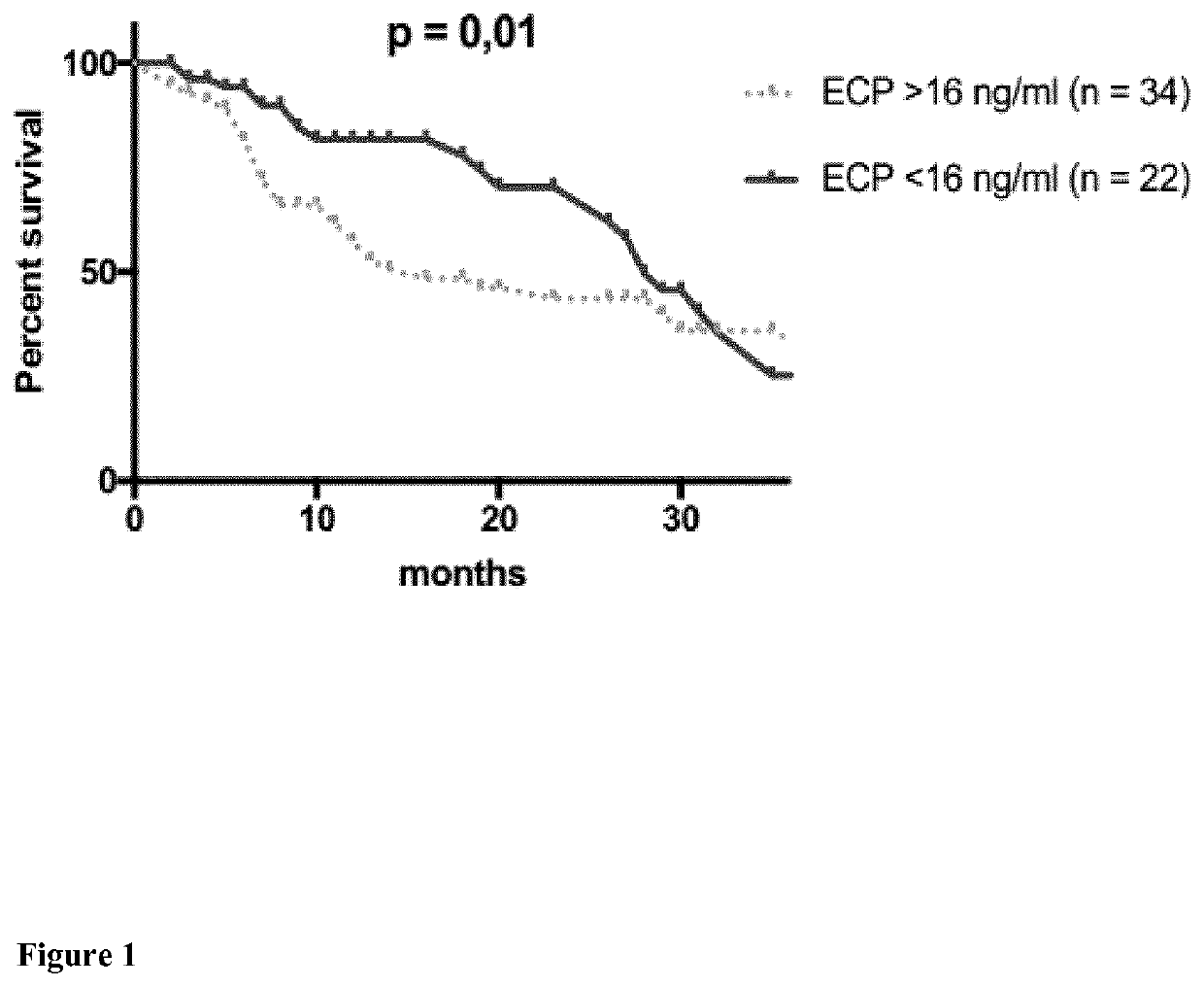
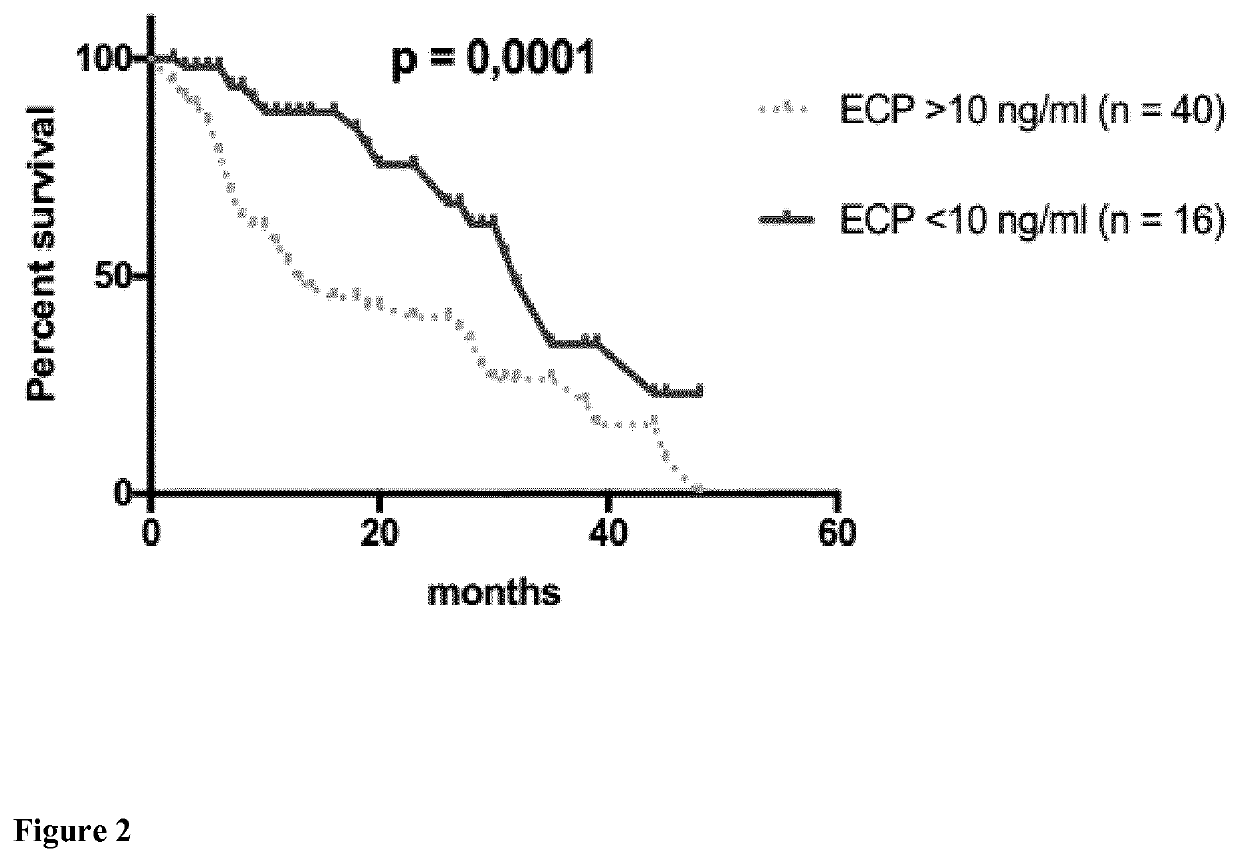
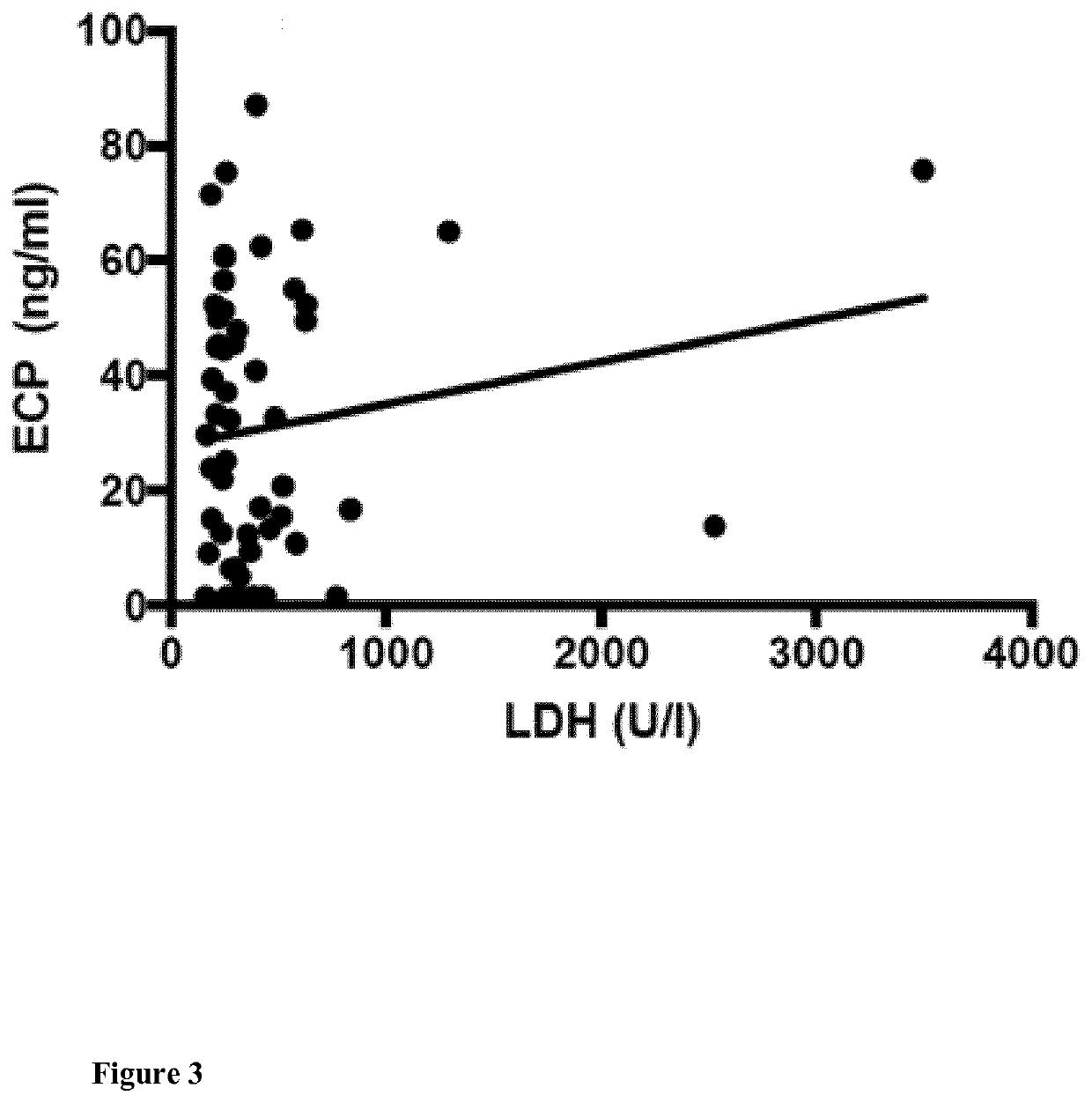
Eosinophil Cationic Protein (ECP) as a Tumor Marker for Malignant Tumors
The present invention relates to methods of prognosis of survival, of classifying a disease stage, and of selecting the mode of treatment of a patient diagnosed with malignant tumors such as melanoma, of a predictive marker for response to therapy, a kit for determination of the level of eosinophil cationic protein (ECP) in a sample of a tumor patient, such as a melanoma patient, and the use of this kit for methods of prognosis of survival, of classifying a disease stage and of selecting the mode of treatment and monitoring disease and response to treatment of a patient diagnosed with malignant tumors such as melanoma.
Owner:FRIEDRICH ALEXANDER UNIV ERLANGEN NURNBERG
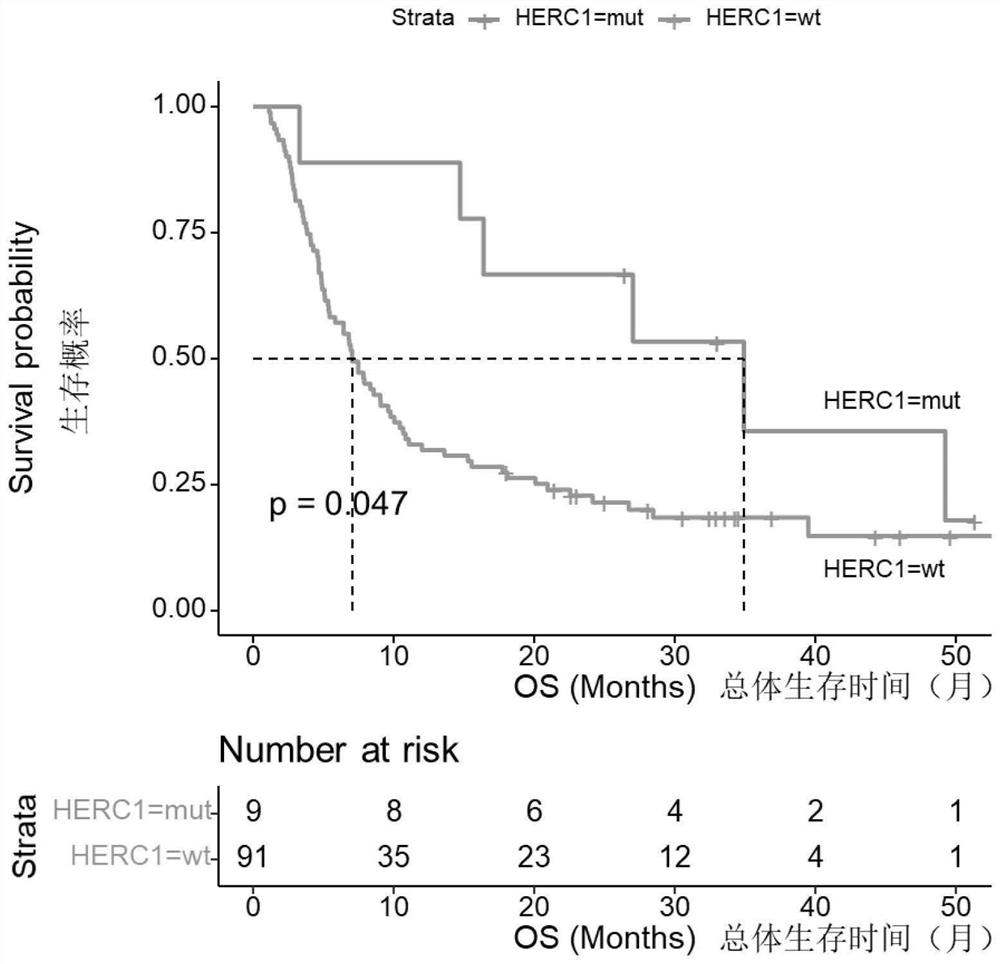
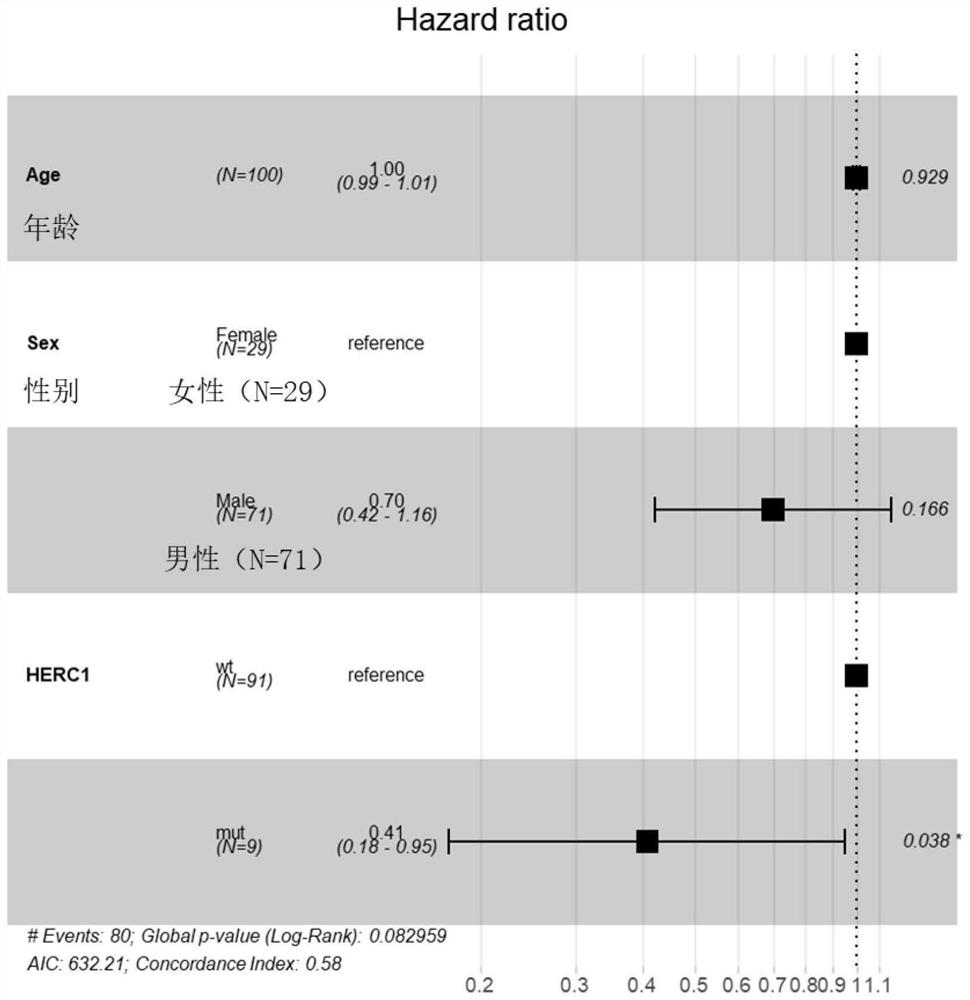
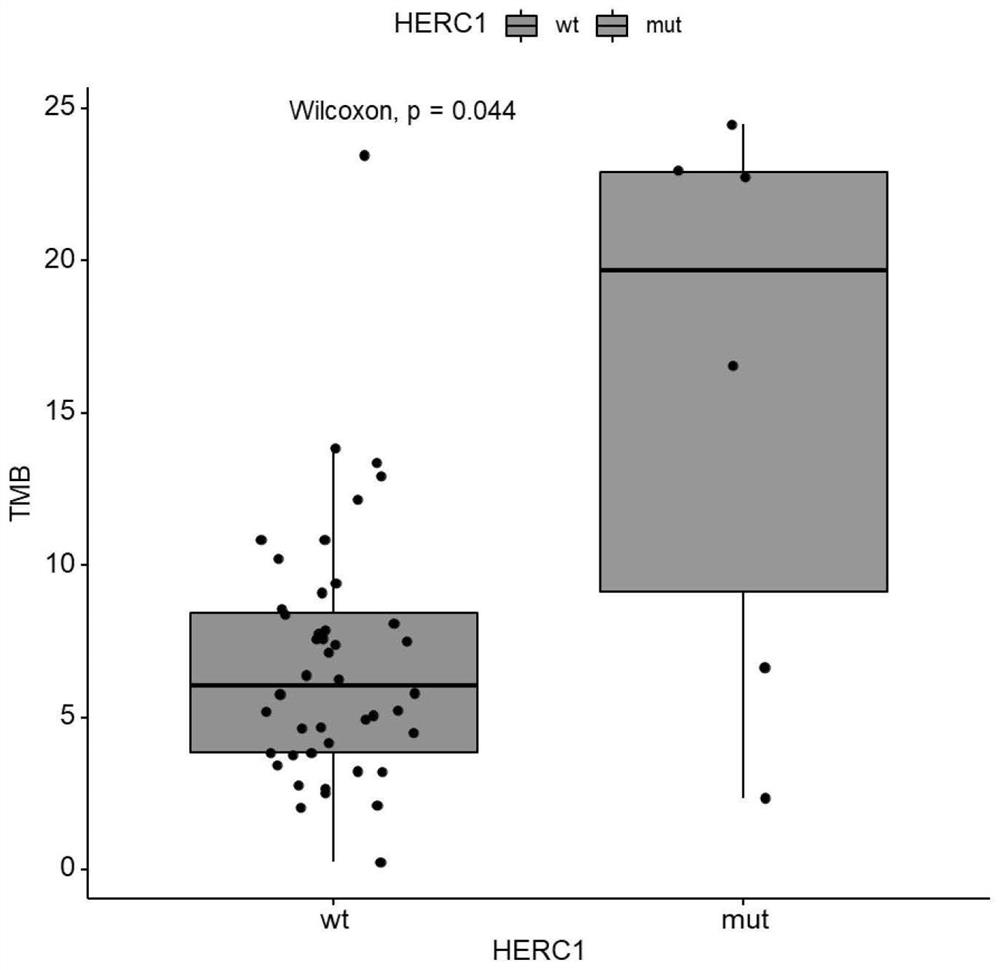
Biomarker for predicting sensitivity of melanoma patient to immunotherapy, application and device
PendingCN114410788AFully understandMicrobiological testing/measurementBiological material analysisBiologic markerMutant
The invention relates to a biomarker for predicting sensitivity of a melanoma patient to immunotherapy, application and a device. The biomarker for predicting the sensitivity of the melanoma patient to the immunotherapy comprises a mutant HERC1 gene, the mutation of the gene has significant correlation with a TMB value, the gene can be used as a new biomarker for predicting the sensitivity of the melanoma patient to the immunotherapy, people beneficial to the immunotherapy can be rapidly screened, the accuracy rate is high, the detection cost is saved, and the method is suitable for popularization and application. The population coverage of the melanoma patients which can benefit from immunotherapy is expanded and recognized, and the method has important significance on treatment of the melanoma patients.
Owner:SHANGHAI ORIGIMED CO LTD +2
Melanoma biomarkers
PendingUS20220317125A1Improve responsivenessImprove survivalDisease diagnosisBiological testingAutoantibodyOncology
The present invention relates to autoantibody biomarkers associated with melanoma. The autoantibody biomarkers can be used to detect or diagnose melanoma and can also be used to inform treatment of melanoma patients, particularly treatment with checkpoint inhibitors. The autoantibody biomarkers can be used in a variety of methods including: methods of selecting melanoma patients for treatment; methods of predicting responsiveness to treatment; methods of predicting survival responsive to treatment; and methods of predicting the risk of immune-related adverse events (irAEs) in patients treated with checkpoint inhibitors.
Owner:ONCIMMUNE GERMANY GMBH
Methods and materials for cancer treatment
InactiveUS7795381B2Immunoglobulin superfamilyTumor rejection antigen precursorsTumor responseCancer therapy
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Melanoma leptomeningeal metastasis model construction method
Owner:FUDAN UNIV
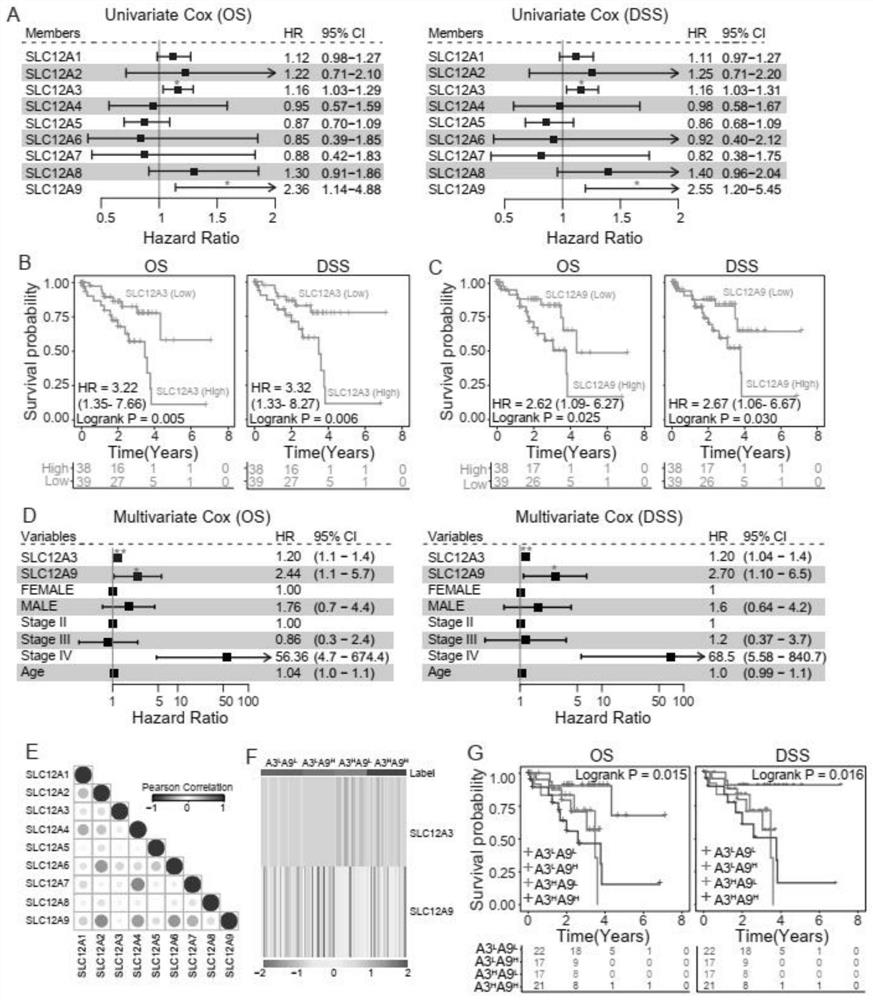
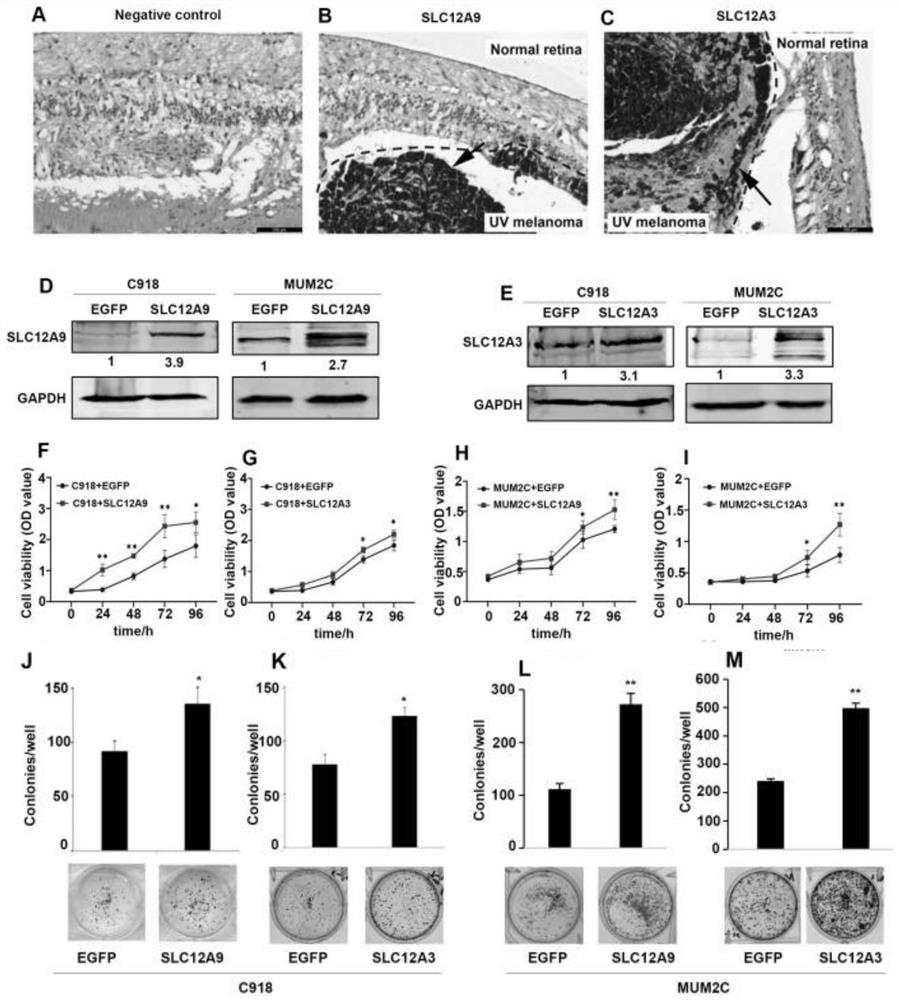
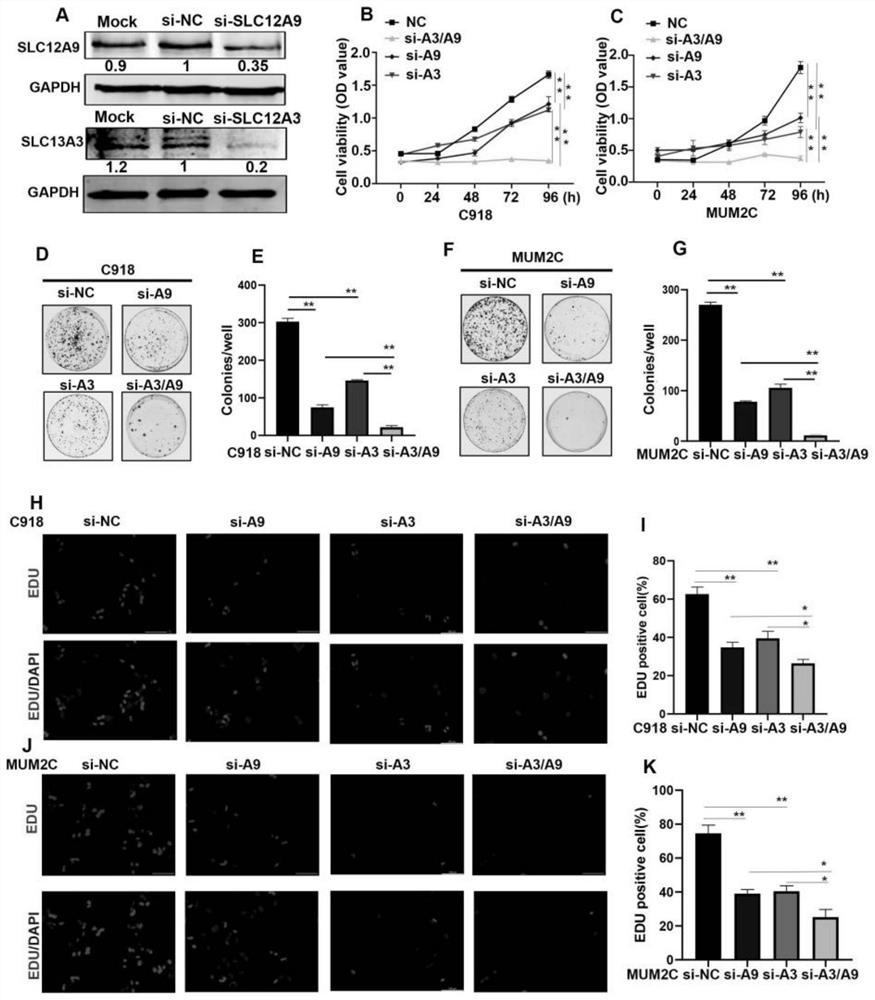
Application of SLC12A3 and/or SLC12A9 as detection index for treatment and prognosis of uveal melanoma
The invention discloses an application of SLC12A3 and / or SLC12A9 as an index for treatment and prognosis detection of uveal melanoma. The invention further discloses a calculation model for predicting uveal melanoma prognosis and a medicine for preventing or treating uveal melanoma. The invention provides a new method for prognosis monitoring and treatment of uveal melanoma patients.
Owner:THE EYE HOSPITAL OF WENZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com