Use of anti-PD-1 antibody in tumor treatment
A technology of PD-1 and PD-L1, which is applied in the direction of anti-tumor drugs, antibody medical components, antibodies, etc., can solve the problems of limited research and poor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Example 1: Clinical research of anti-PD-1 antibody monotherapy in the treatment of melanoma
[0161] Inclusion Criteria: Eligible subjects must be (1) at least 18 years old, (2) have locally advanced or metastatic melanoma, (3) refractory to standard systemic therapy, (4) ECOG score 0 or 1, (5) no history of autoimmune disease or persistent infection, (6) no previous anti-PD-1 / or anti-PD-L1 immunotherapy.
[0162] Subjects must have evaluable lesions according to RECIST v 1.1 standards, and are not allowed to use anti-tumor drug therapy, systemic steroid drug therapy or have not used anti-CTLA4, anti-PD-1, anti-PD-L1 antibody therapy.
[0163] Test drug: anti-PD-1 antibody toripalimab (WO2014206107).
[0164] The dose of anti-PD-1 antibody used in this test is: 3mg / kg, administered intravenously once every two weeks (Q2W).
[0165] Clinical design:
[0166] This is a single-arm, Phase II, open-label clinical trial. This study is to evaluate the safety and anti-tumor...
Embodiment 2
[0194] Example 2: Research on the correlation between biomarkers and clinical efficacy
[0195] 2.1 Expression of PD-L1 in tumors
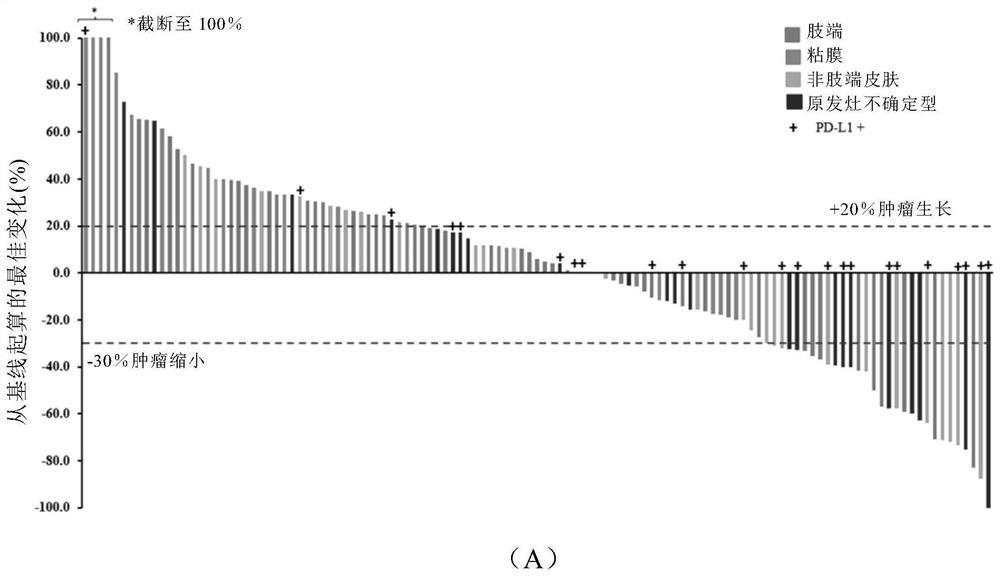
[0196] The tumor biopsy specimens of 127 patients were tested to analyze the correlation between tumor histology and anti-PD-1 antibody clinical efficacy. The rabbit anti-human PD-L1 antibody SP142 from Roche was used for detection. PD-L1 positive status was defined as the presence of ≥1% membrane staining intensity of tumor cells.
[0197] Such as Image 6 As shown in (A), 26 cases (20.5%) were positive for PD-L1, 84 cases (66.1%) were negative for PD-L1, and the expression of PD-L1 was unknown in 17 cases (13.4%). Such as Image 6 (B) In the four subtypes of melanoma, PD-L1 + The proportion of acral type (6.8%) and mucosal type (10.5%) is significantly lower than that of non-acral type (37.5%) and melanoma of uncertain primary focus (52.2%).
[0198] Such as Image 6 (C) and 6(D), PD-L1 + patients compared to PD-L1 - Patients treated wi...
Embodiment 3
[0205] Example 3: Gene sequencing analysis
[0206] In the experiment of Example 1, whole exome sequencing was performed on tumor biopsies and paired peripheral blood samples from patients using second-generation sequencing technology, and 19,278 gene mutations were identified from 98 patients, including 7964 missense mutations, 509 gene deletions, 482 rearrangements, 288 alternative splicing sites, 129 frameshift truncations and 8157 gene amplifications. After excluding frequently mutated genes in the public exome, the most frequently altered genes (≥10%) were BRAF (33%), TERT (32%), CDKN2A (12%), NRAS (16%) ), CDK4 (12%), APOB (11%), CCND1 (11%), AGAP2 (11%), NF1 (10%), LRP1B (10%), MDM2 (10%) and KIT (10%), see details Figure 7 .
[0207] As shown in Table 6, sequencing results from 98 patients revealed distinct patterns of genomic alterations in melanoma subgroups. BRAF mutations were more frequent in nonacral cutaneous subtypes (11 / 23, 48%) and melanomas of unknown p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com