Biomarker for predicting sensitivity of melanoma patient to immunotherapy, application and device
A biomarker and immunotherapy technology, applied in the field of biomarkers for predicting the sensitivity of melanoma patients to immunotherapy, can solve the problems of long work cycle, high sequencing cost, and high technical platform requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] 1. Data Acquisition
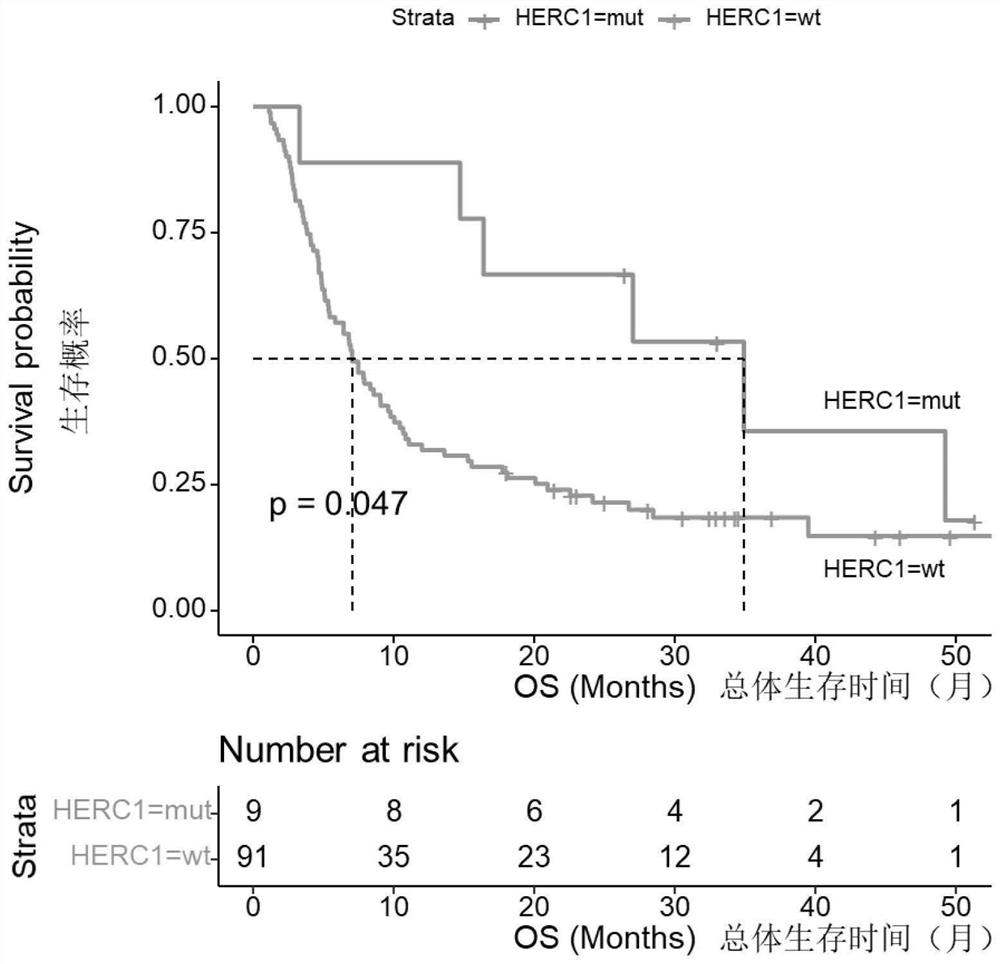
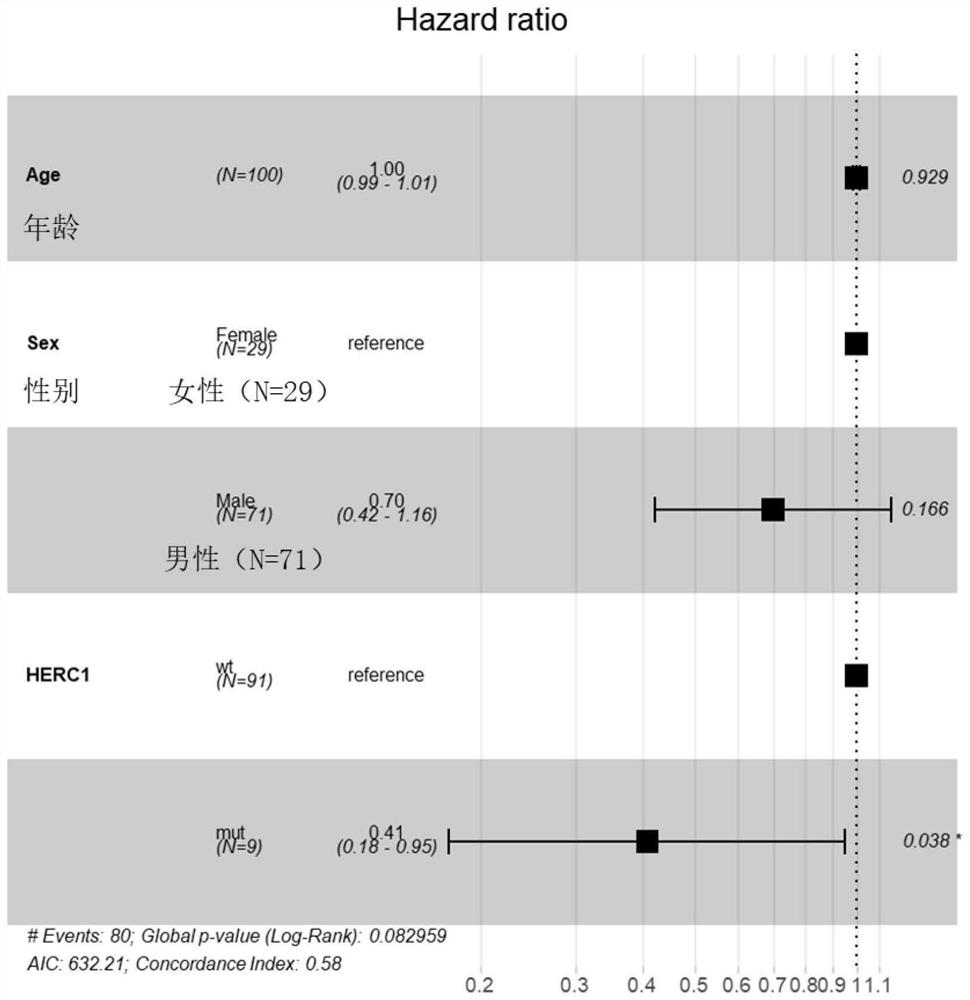
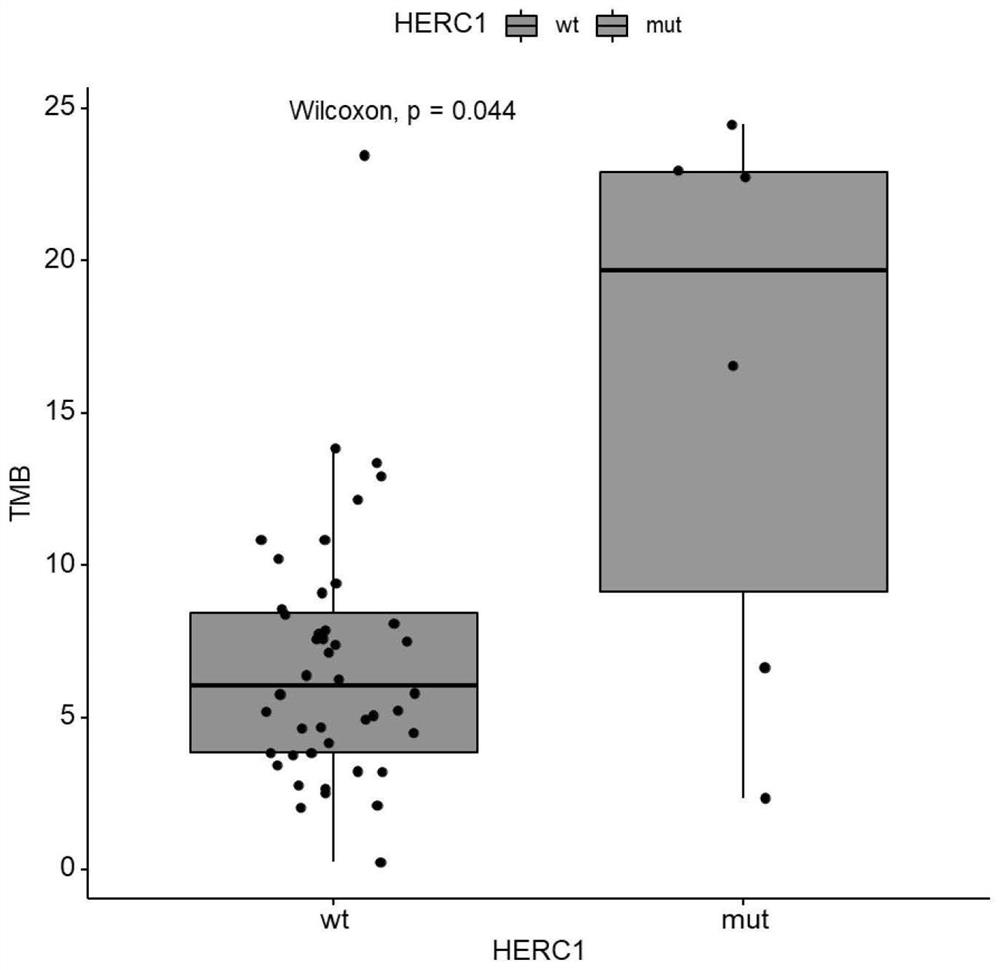
[0075] The data of 100 patients with advanced (Phase IV) skin melanoma (Skin Cutaneous Melanoma, SKCM) treated with immune checkpoint inhibitors (ipilimumab) were obtained from the DFCI (Dana-Farber Cancer Institute) database, including Gene mutation data, treatment plan data and overall survival (Overall Survival, OS) data are shown in Table 2.
[0076] Samples with somatic non-synonymous mutations in the HERC1 gene (including at least one of missense mutations, in-frame insertion mutations, in-frame deletion mutations, nonsense mutations, frameshift mutations, and splice site mutations) were classified as HERC1 =MUT group, wherein the number of samples is 9, and the specific mutation sites are p.G3682E, p.S2422F, p.T4807I, p.V374A, p.S463F, p.H1447Y, p.P216L, p.T22A or p. L1148F; The samples without any somatic non-synonymous mutations in the HERC1 gene or only mutations in the non-coding region of the HERC1 gene were classified as the HERC1=WT ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com