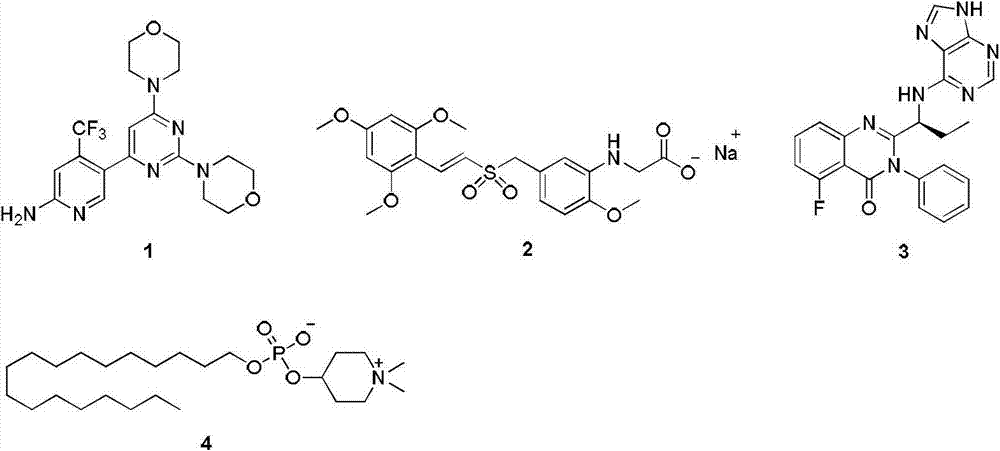
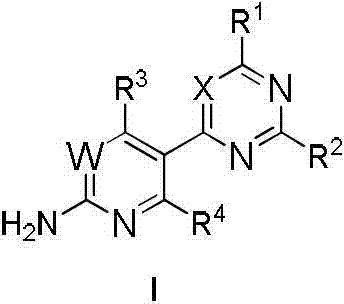
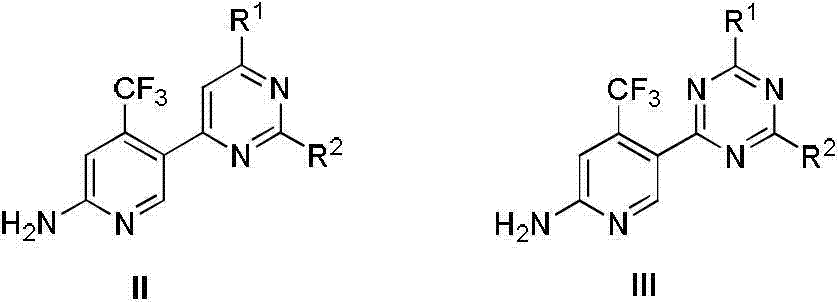
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "BRAF inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
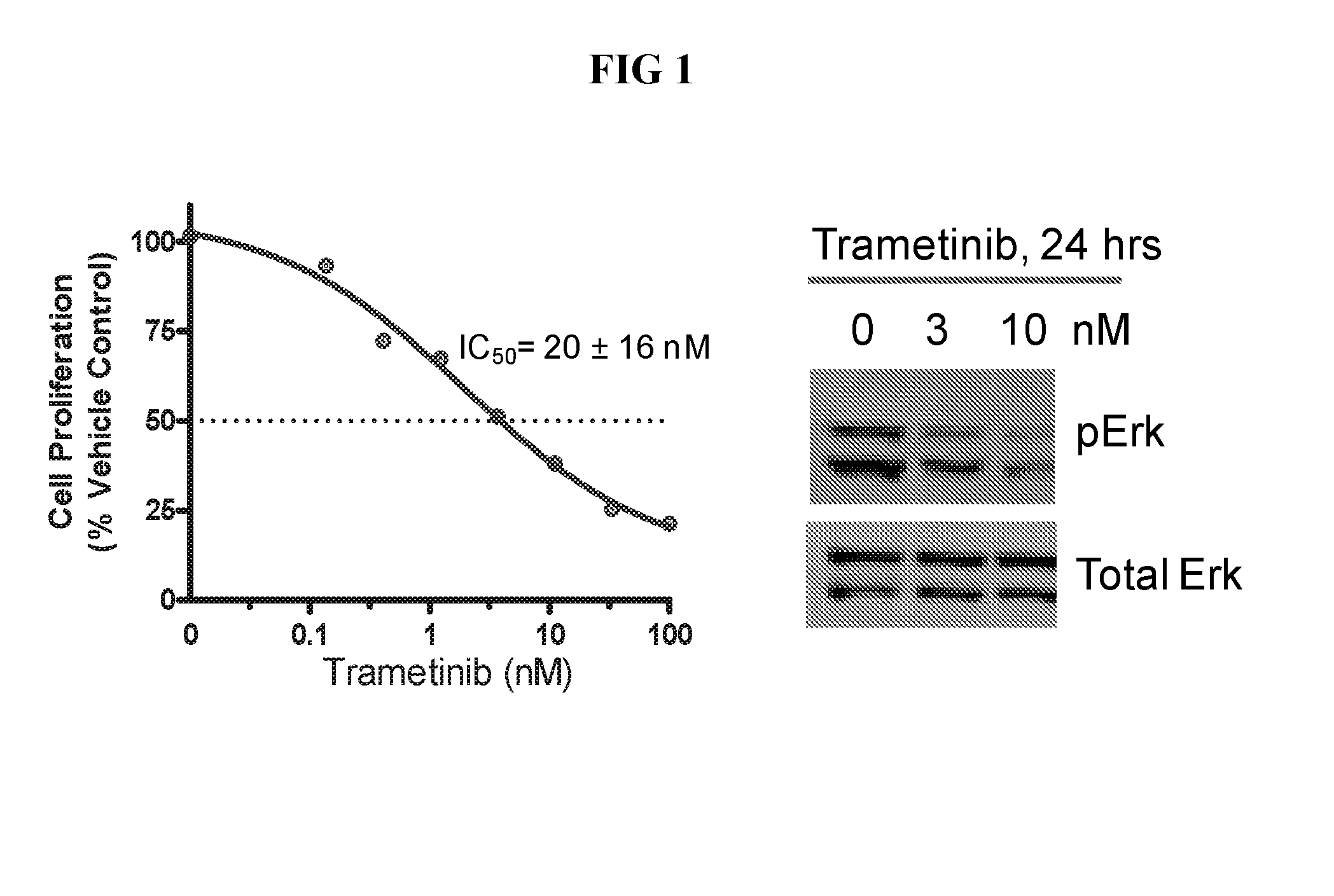
Trametinib (Mekinist) is a MEK inhibitor that was approved by the FDA in May 2013. It is indicated for unresectable or metastatic melanoma with BRAF V600E or V600K mutations confirmed by the THxID BRAF mutation test.
Phosphoinositide 3-kinase (P13K) inhibitor, pharmaceutical composition containing P13K inhibitor, and application of phosphoinositide kinase inhibitor and pharmaceutical composition
ActiveCN103483345AImprove effectivenessGood choiceOrganic active ingredientsOrganic chemistryKinase inhibitionEnzyme inhibitor
The invention discloses a phosphoinositide 3-kinase (P13K) inhibitor, a pharmaceutical composition containing the P13K inhibitor, and application of the P13K inhibitor and the pharmaceutical composition. The P13K inhibitor comprises a pyrimidine compound and a stereoisomer / hydrate / pharmaceutically-acceptable salt thereof. The pyrimidine compound has a general formula I of which the structure is shown in the specification. The P13K inhibitor and the pharmaceutical composition containing the same can be used for inhibiting PI3 Ks and treating proliferative diseases on which the PI3 Ks act. According to the invention, high-effectiveness and high-selectivity inhibitors for treating proliferative diseases on which PI3Ks act can be provided.
Owner:SUN YAT SEN UNIV
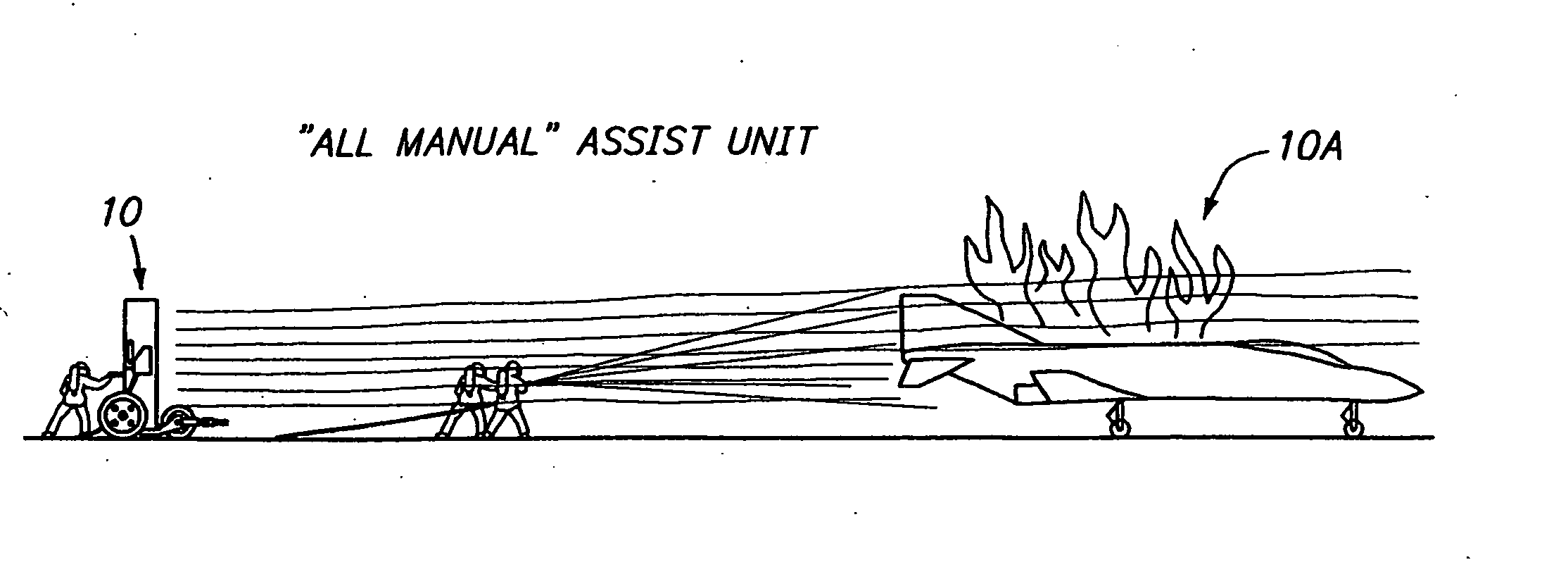
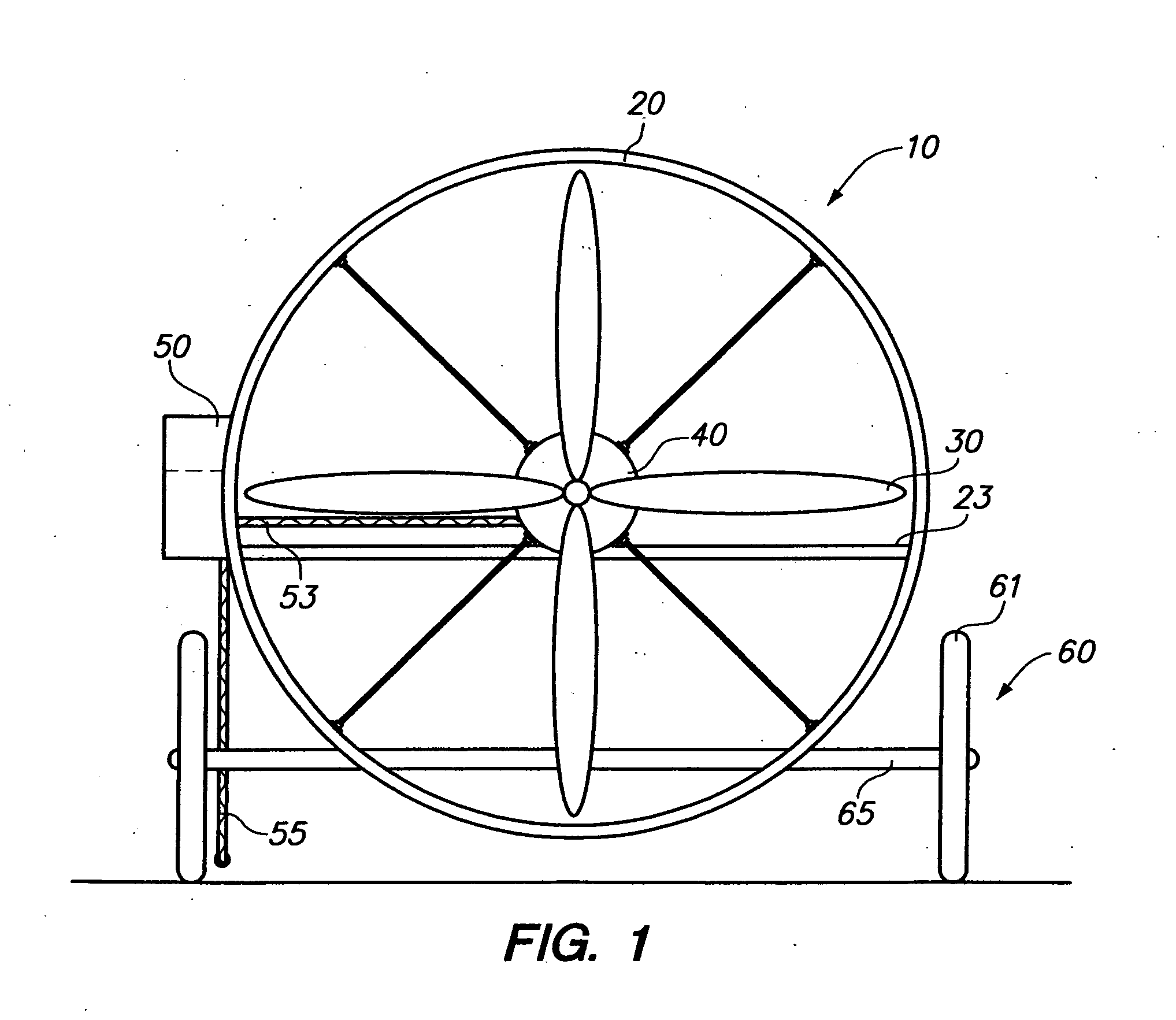
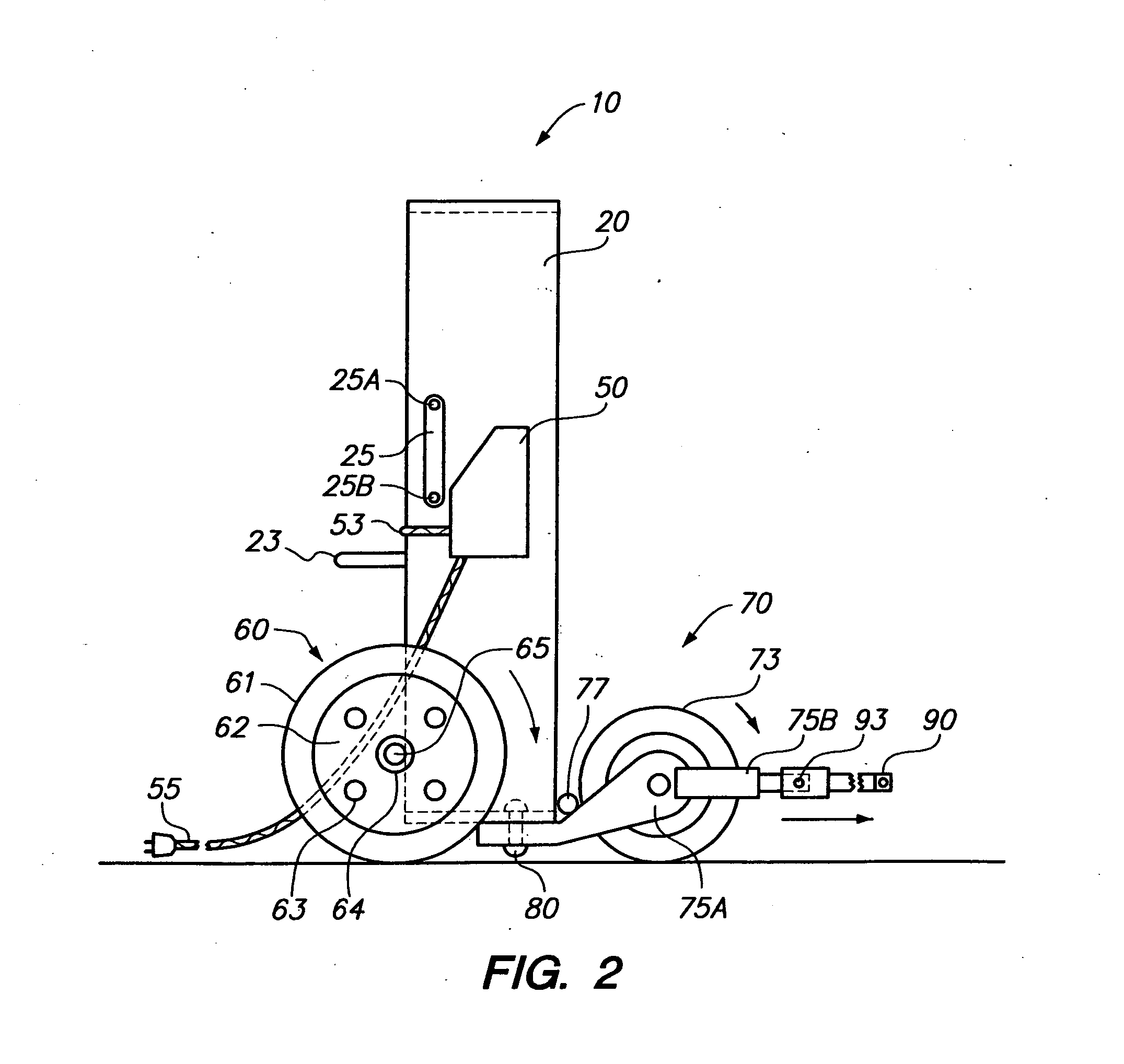
Assist unit for large outdoor fires
The invention is designed to be used for the large outdoor fires such as fires on the U.S. Navy's aircraft carriers, in forests and grasslands and at airports and chemical plants. The operators of an “assist unit” should always be behind the firefighters. At long range it can blow fire suppressors into a fire and smother it. The suppressors can be water or chemicals by the firefighters. Soot, ashes, dirt or sand around a fire itself are good suppressors. The “assist unit” will blow them into the fire and it blows away the smoke and fumes as well. There are two versions of the “assist unit”: the ALL MANUAL one and the VEHICLE MOUNTED one.
Owner:LEWIS NATHANIAL HENRY
Assist unit for large outdoor fires background of the invention
The invention is designed to be used for the large outdoor fires such as fires on the U.S. Navy's aircraft carriers, in forests and grasslands and at airports and chemical plants. The operators of an “assist unit” should always be behind the firefighters. At long range it can blow fire suppressors into a fire and smother it. The suppressors can be water or chemicals by the firefighters. Soot, ashes, dirt or sand around a fire itself are good suppressors. The “assist unit” will blow them into the fire and it blows away the smoke and fumes as well. There are two versions of the “assist unit”: the ALL MANUAL one and the VEHICLE MOUNTED one.
Owner:LEWIS NATHANIAL HENRY
Method to treat melanoma in braf inhibitor-resistant subjects
InactiveUS20120053185A1Better respondEffective treatmentCompound screeningBiocideBRAF inhibitorTyrosine
Methods to treat cancer patients, especially melanoma patients, who have BRAF mutations and have become resistant to BRAF mutant kinase inhibitors employ inhibitors of multiple receptor tyrosine kinases. In addition, methods are described for identifying pharmaceutical compositions and drugs that will be successful in treating these patients.
Owner:GENEKEY CORP
Pyrimidine compound, PI3K inhibitor, pharmaceutical composition comprising PI3K inhibitor and application of inhibitor and pharmaceutical composition
ActiveCN103694218AImprove effectivenessGood choiceOrganic active ingredientsOrganic chemistryDiseaseAdjuvant
The invention discloses a pyrimidine compound of which the structure is shown in a general formula (I), and also discloses a PI3K inhibitor which is composed of at least one of the following substances: the pyrimidine compound shown in the general formula (I), a steric isomer of the pyrimidine compound, a hydrate of the pyrimidine compound and a pharmaceutically acceptable salt of the pyrimidine compound. The invention also discloses a pharmaceutical composition comprising the PI3K inhibitor, and also discloses application of the PI3K inhibitor in preparation of a drug for treatment and / or prevention and / or auxiliary treatment of a proliferative disease of PI3K action. The pharmaceutical composition is composed of the PI3K inhibitor, at least one pharmaceutically acceptable auxiliary material, an adjuvant or a carrier. The PI3K inhibitor and the pharmaceutical composition comprising the PI3K inhibitor can be applied to inhibition of P13 kinase and the proliferative disease of P13 kinase action, and the inhibitor with better validity and selectivity can be provided for treatment of the proliferative disease of P13 kinase action.
Owner:SUN YAT SEN UNIV
Pharmaceutical Combinations
InactiveUS20160339023A1Reduce morbidityInhibit progressNervous disorderRespiratory disorderDiseaseReceptor
A pharmaceutical combination comprising (a) an ALK inhibitor, or a pharmaceutically acceptable salt thereof, and (b) at least one HDMA-2 / p53 receptor inhibitor or a pharmaceutically acceptable salt, or at least one BRaf inhibitor or a pharmaceutically acceptable salt, and optionally a pharmaceutically acceptable carrier, for simultaneous, separate or sequential administration; the uses of such combination in the treatment of cancer; and methods of treating a subject suffering from a proliferative disease comprising administering a therapeutically effective amount of such combination.
Owner:NOVARTIS AG
Combinations of an Anti-pd-l1 antibody and a mek inhibitor and/or a braf inhibitor
InactiveUS20160089434A1Good anticancer effectHighly synergisticOrganic active ingredientsAntibody ingredientsAntiendomysial antibodiesReceptor
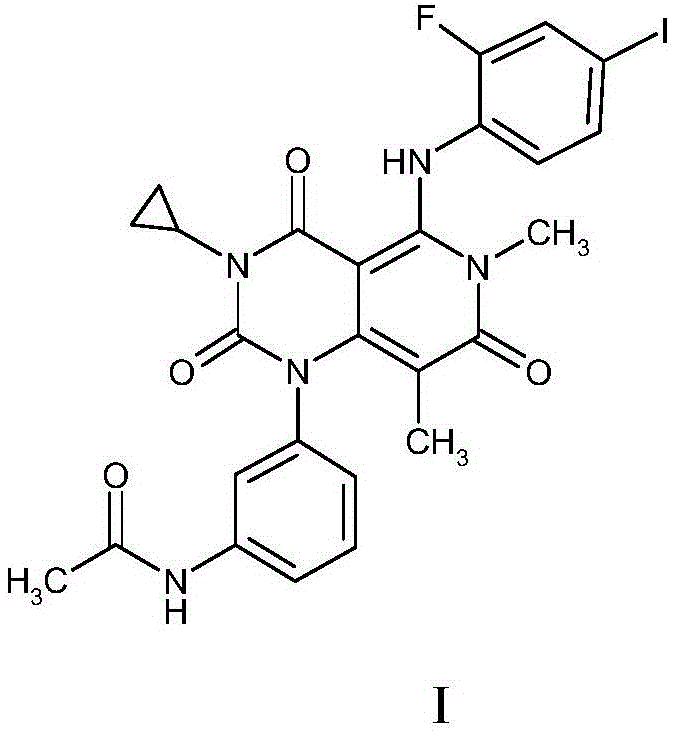
A novel combination comprising the MEK inhibitor N-{3-[3-cyclopropyl-5-(2-fluoro-4-iodo-phenylamino)6,8-dimethyl;-2,4,7-trioxo-3,4,6,7-tetrahydro-2H-pyrido[4,3-d]pyrimidin-1-yl]phenyl}acetamide, or a pharmaceutically acceptable salt or solvate thereof, and / or a B-Raf inhibitor, particularly N-{3-[5-(2-Amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide or a pharmaceutically acceptable salt thereof, and an anti-PD-L1 antibody; pharmaceutical compositions comprising the same and methods of using such combinations and compositions in the treatment of conditions in which the inhibition of MEK and / or B-Raf and / or neutralizing or inhibiting the interaction between PD-L1 and its receptor, e.g. PD-1, is beneficial, eg. cancer.
Owner:NOVARTIS AG
PI3K inhibitor, preparation method and application thereof in pharmacy
The invention belongs to the technical field of pharmaceuticals and particularly relates to a PI3K inhibitor, a preparation method and application thereof in the pharmacy. The PI3K inhibitor is a compound of the structure shown by the general formula I or medically acceptable salt of the inhibitor. After the PI3K inhibitor is tested with a PI3K biochemical activity test method, the compound has excellent inhibitory activity to PI3K alpha and PI3K gamma, wherein the IC50 values of a plurality of compounds to the PI3K alpha and PI3K gamma reach nanomole grades (smaller than 100 nM). The result shows that the compounds can provide the inhibitor with better effectiveness and selectivity for curing PI3K-acted proliferative disease, and further a targeted drug for curing No. I type diabetes mellitus, lung disease, breast cancer, prostatic cancer, solid tumor, lymphoma, cardiovascular disease, rheumatoid arthritis, leukemia and the like can be hopefully developed. (Please see the general formula I in the description.).
Owner:FUDAN UNIV
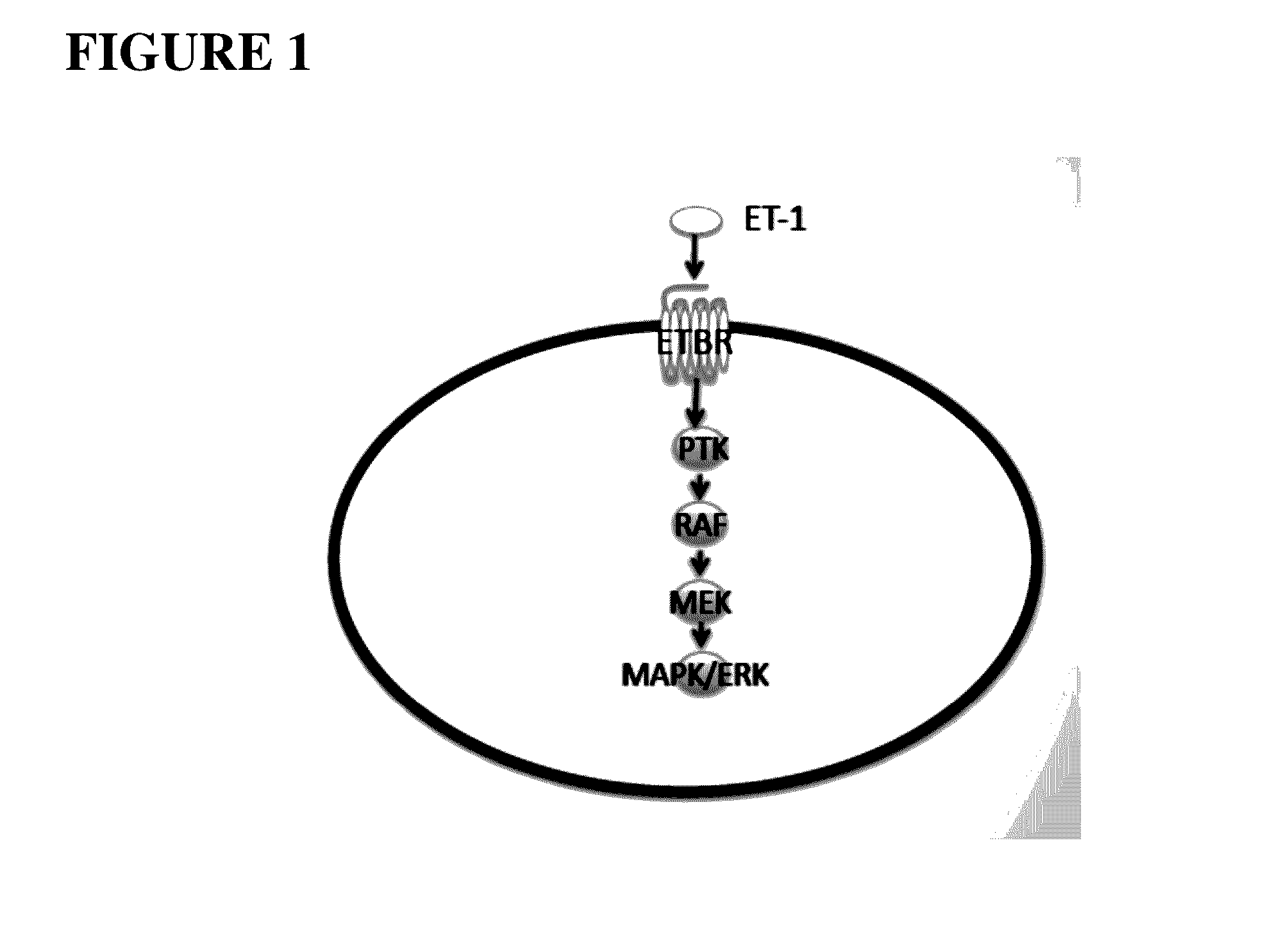
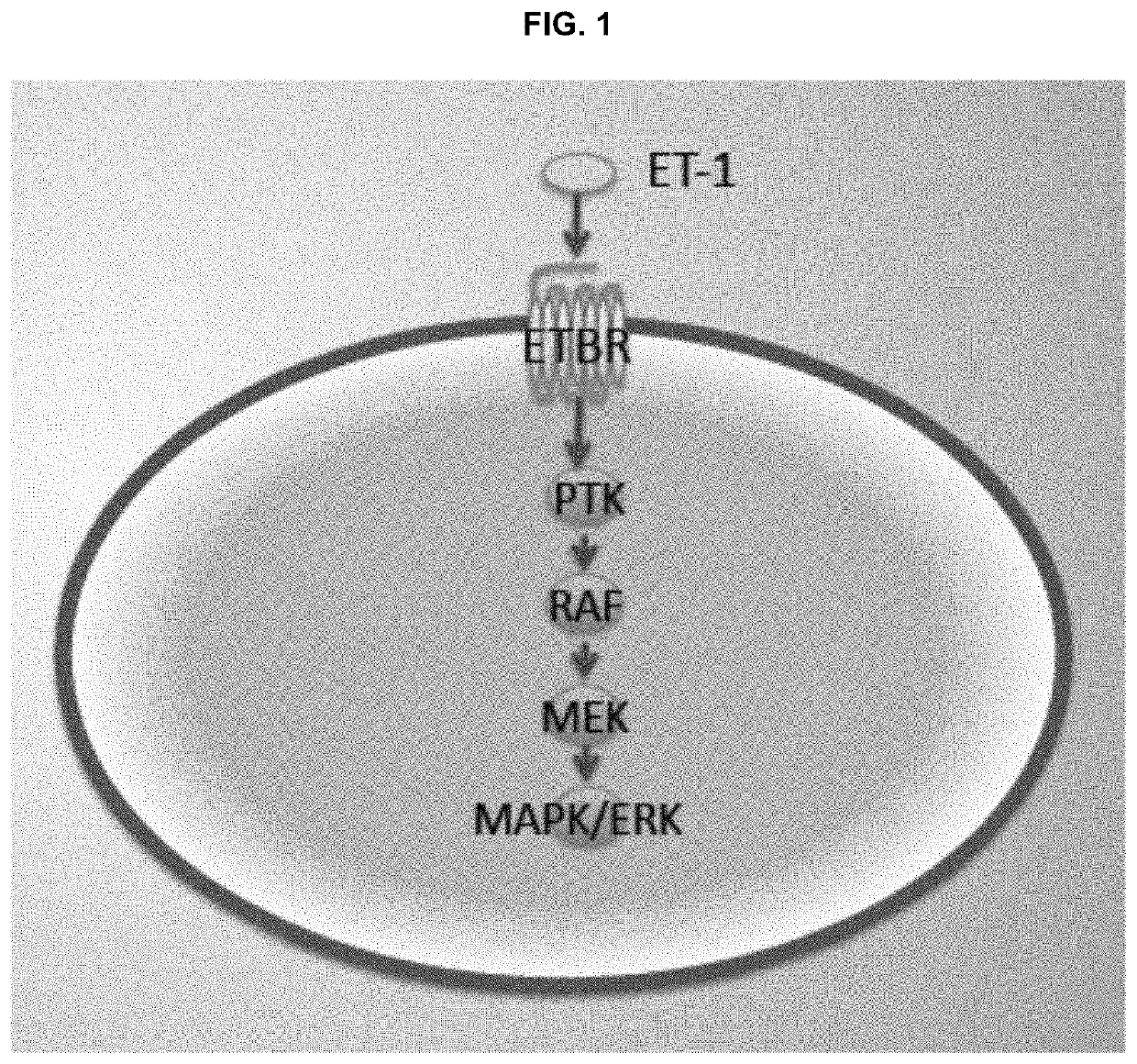
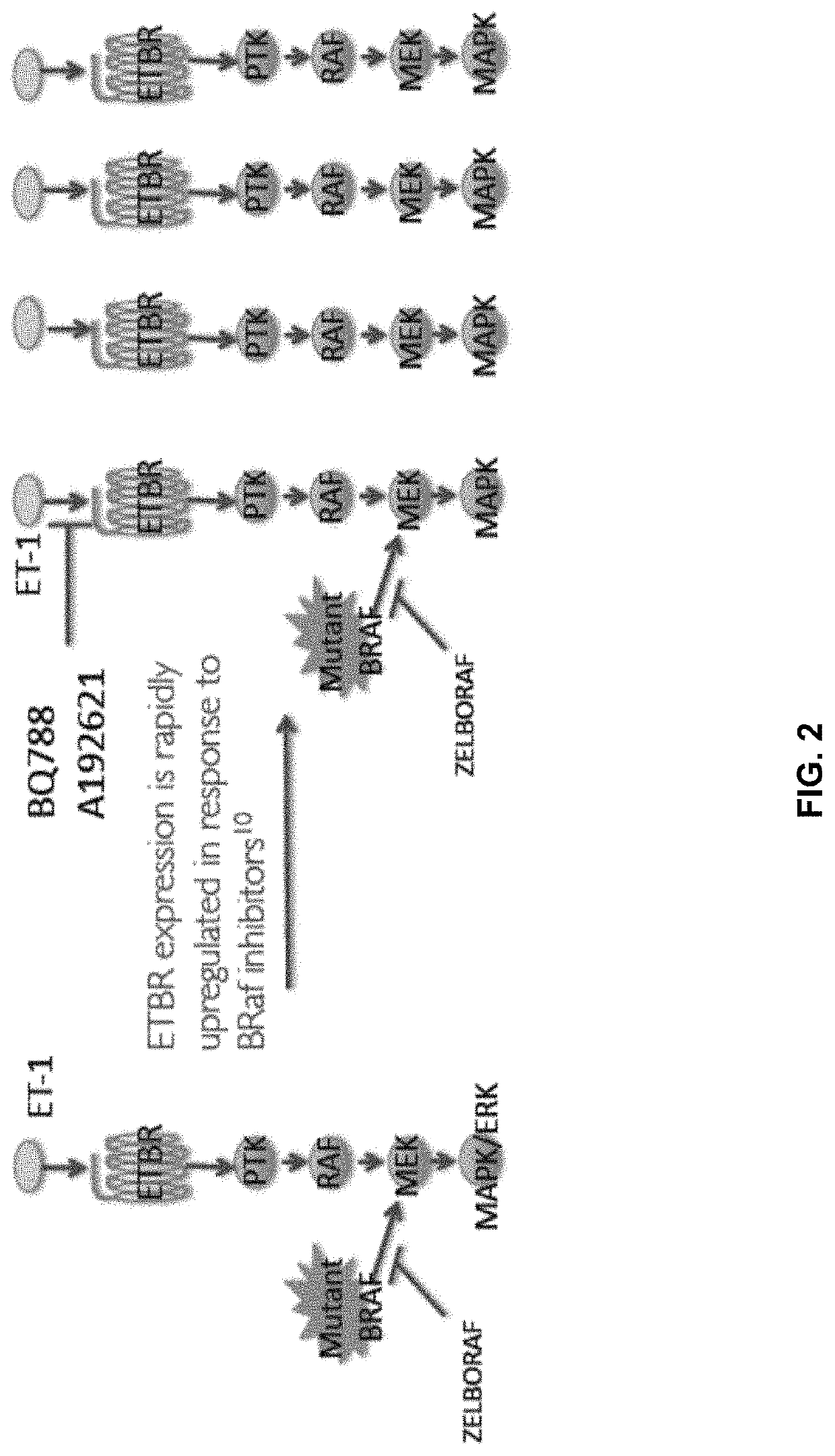
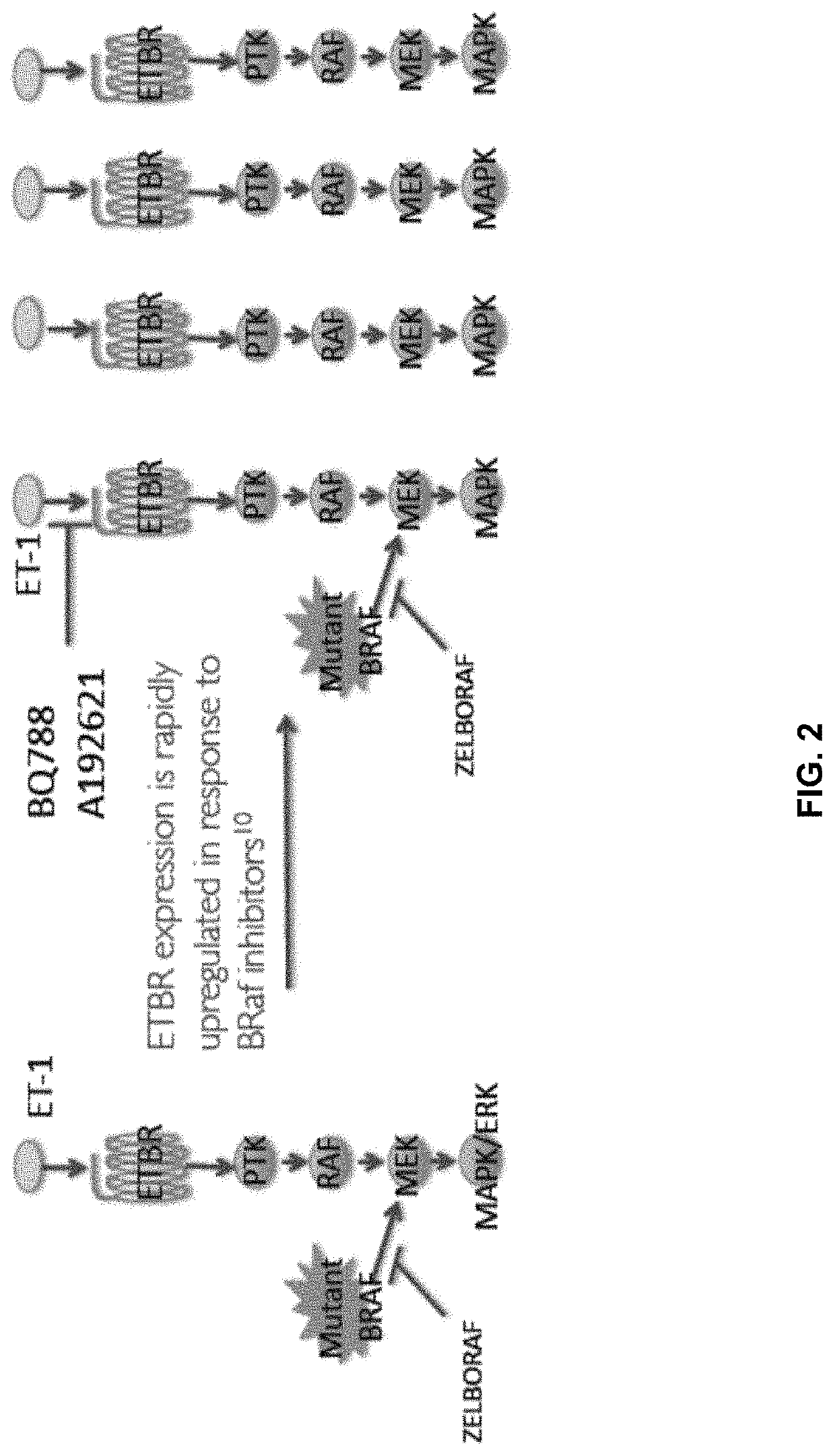
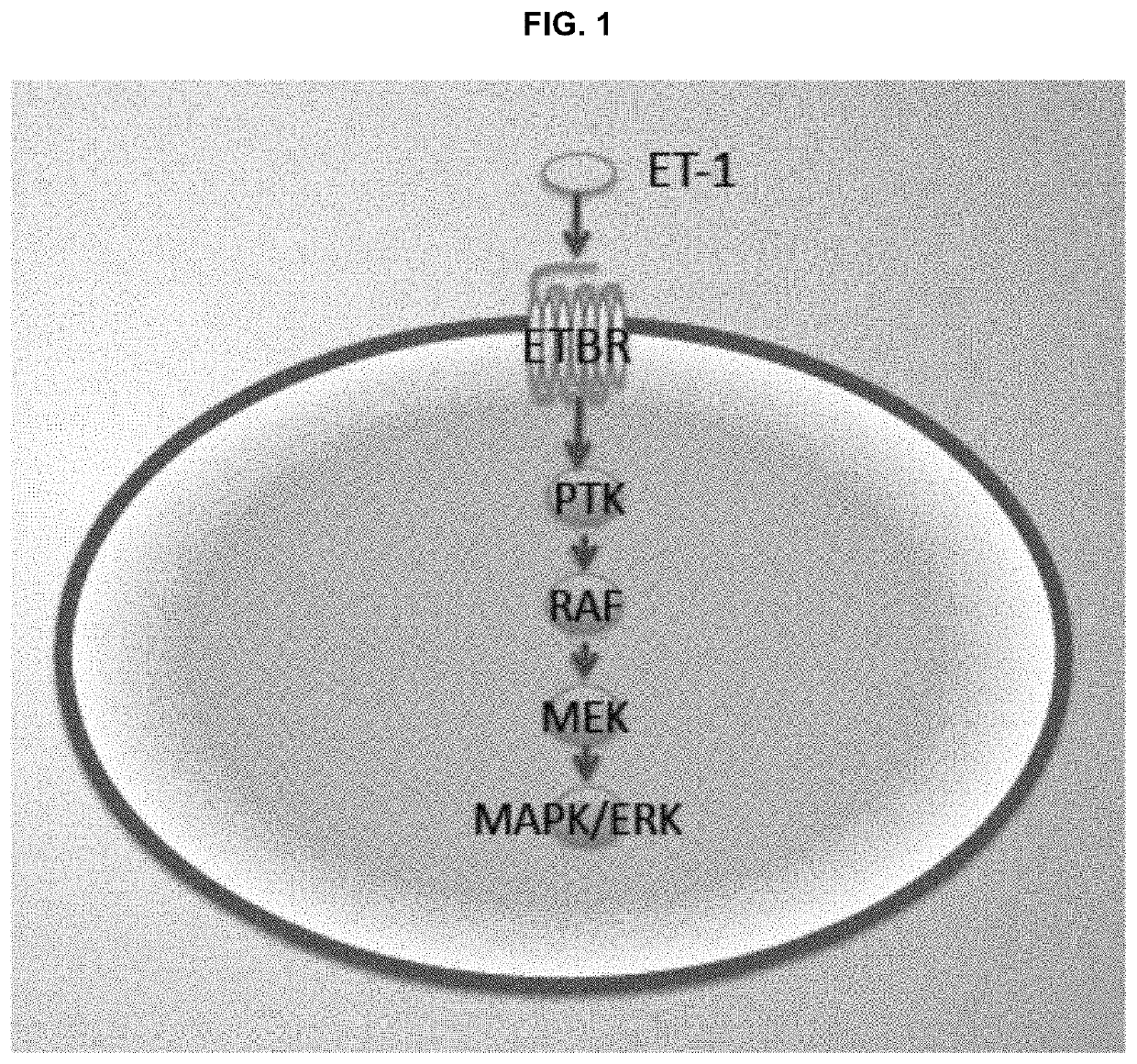
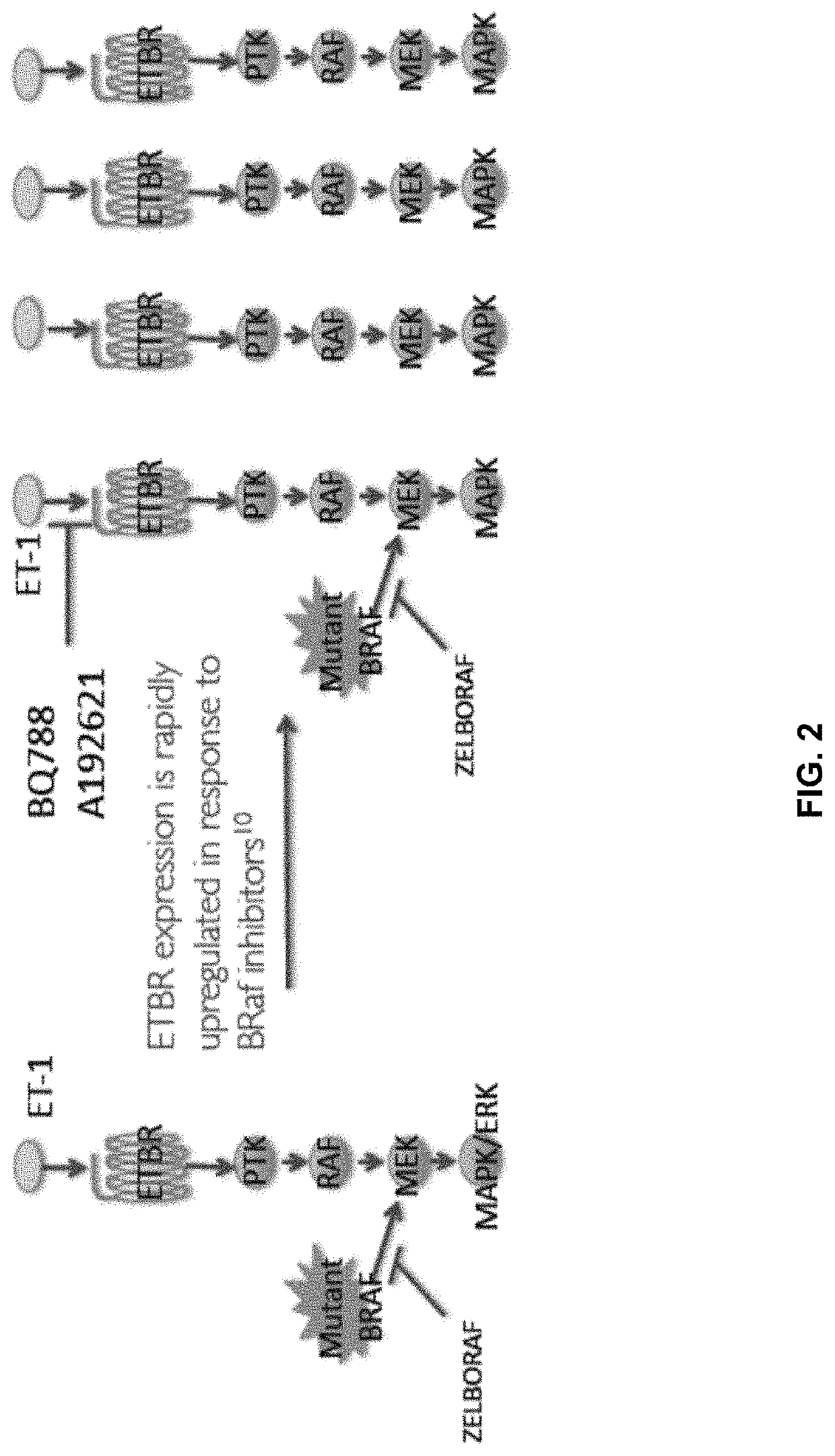
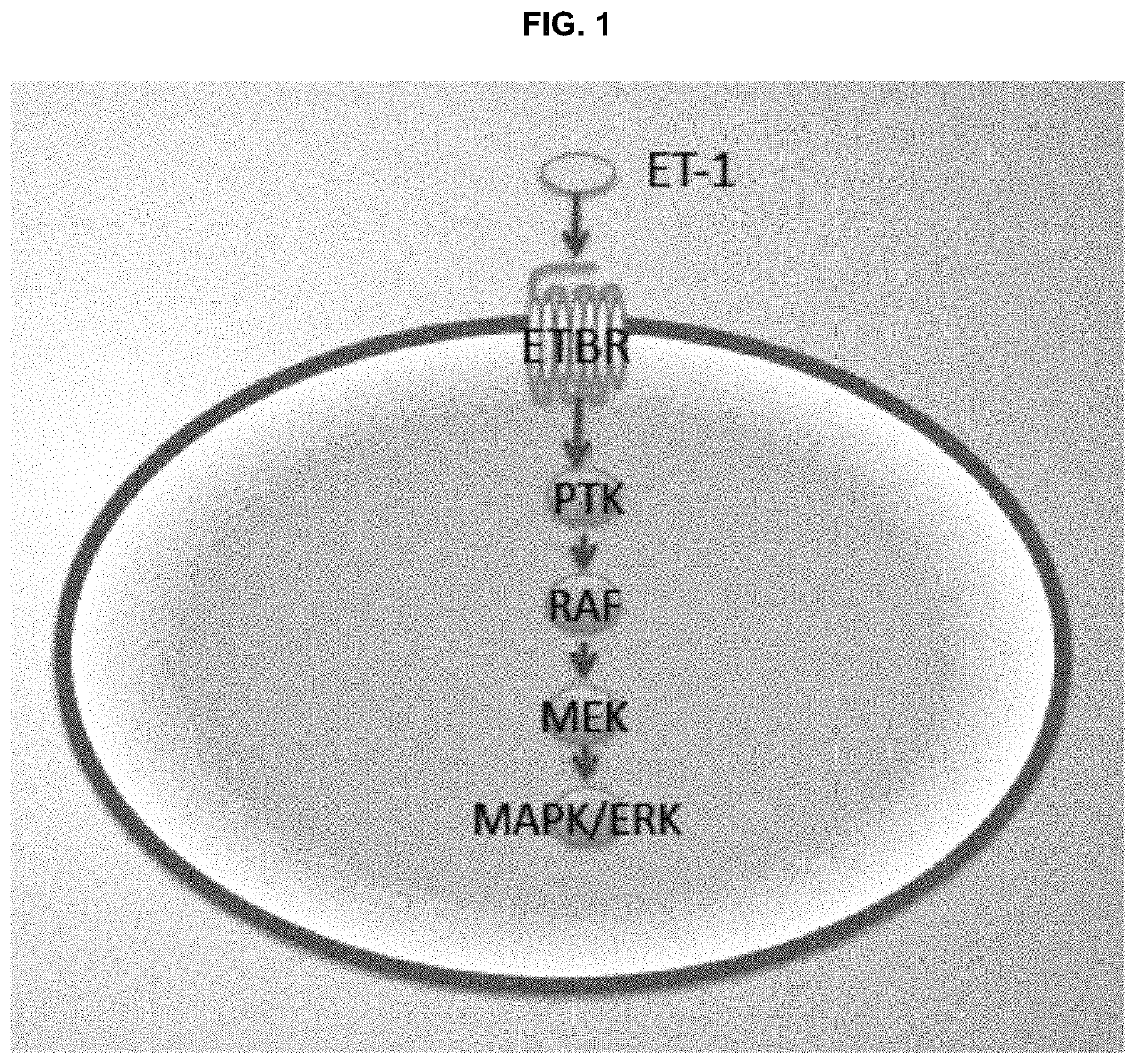
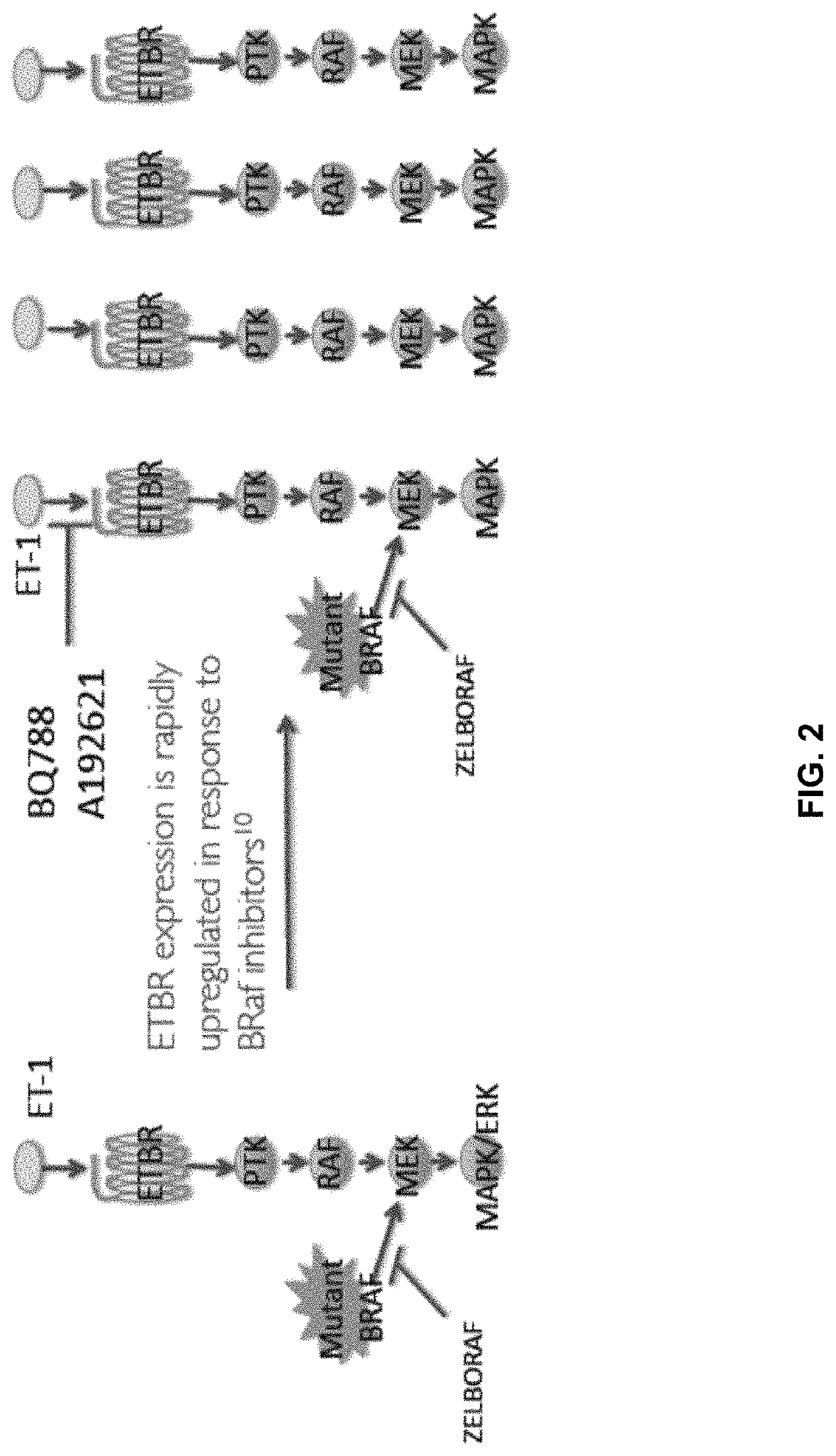
Compositions and methods for treating cancers associated with etbr activation
The description provides compositions and methods for treating ETBR-related cancer. In certain aspects, the description provides a delivery system for the controlled, systemic release of at least one of ETBR antagonists, caspase-8 inhibitors, or a combination thereof, optionally including an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide or a combination thereof. The compositions described are useful for the treatment of certain cancers, including, e.g., breast cancer, malignant melanoma, squamous cell carcinoma, glioblastoma, as well as others. In addition, the description provides a delivery system for the controlled release of at least one of ETBR antagonists, caspase-8 inhibitors or a combination thereof, optionally including at least one of an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide, or a combination thereof, to the central nervous system that are useful for treating cancers that have spread to the brain.
Owner:ENB THERAPEUTICS INC
Compounds for treatment of cancer
ActiveUS10022356B2Reduce severityReduce riskDermatological disorderAntineoplastic agentsMEK inhibitorBRAF inhibitor
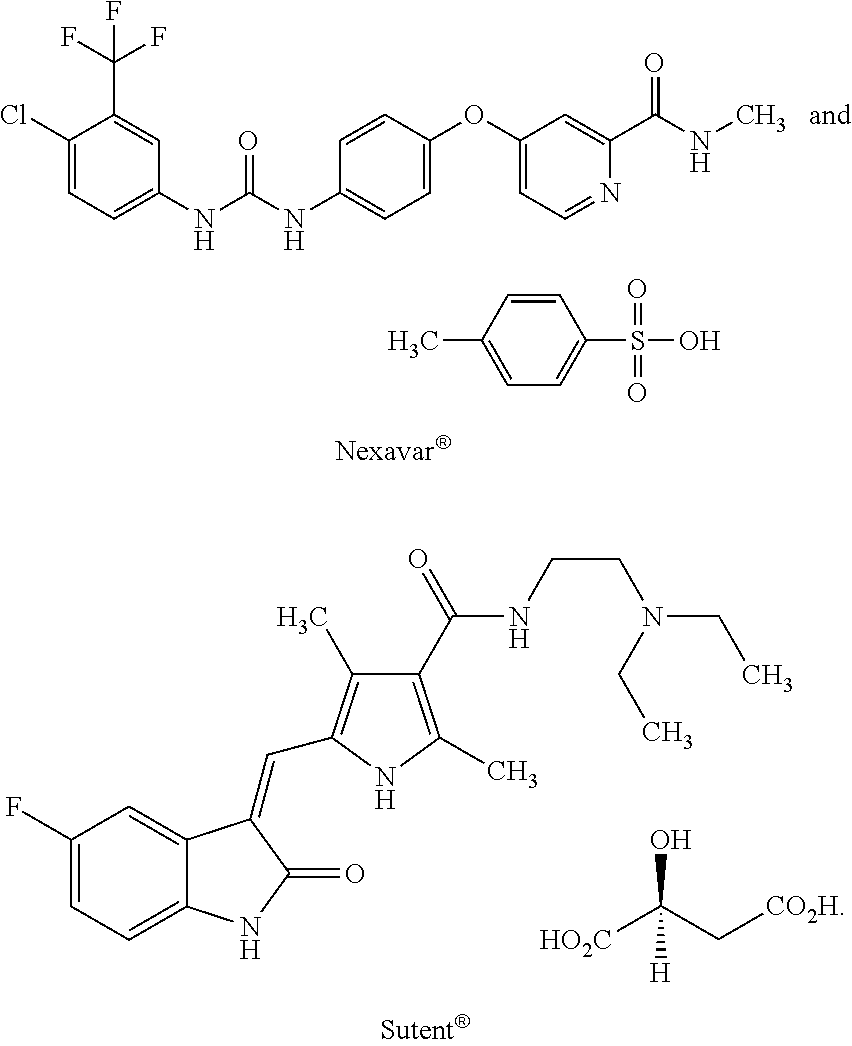
The present invention relates to pharmaceutical compositions for treating cancer comprising BRAF inhibitors, (e.g. vemurafenib) and / or MEK inhibitor, (e.g. trametinib, RO5068760), in combination with anti-tubulin compounds of the invention or other known tubulin inhibitors, and using such compositions for treating cancer such as melanoma, drug-resistant cancer, and cancer metastasis.
Owner:UNIV OF TENNESSEE RES FOUND
Method to treat melanoma in BRAF inhibitor-resistant subjects
Owner:GENEKEY CORP
Combinations of anti-pd-l1 antibody and mek inhibitor and/or braf inhibitor
InactiveCN105658206AImprove bioavailabilityImprove anti-cancer effectOrganic active ingredientsAntibody ingredientsMEK inhibitorBRAF inhibitor
A novel combination comprising the MEK inhibitor N-{3-[3-cyclopropyl-5-(2-fluoro- 4-iodo-phenylamino)6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydro-2H-pyrido[4,3- d]pyrimidin-1 -yl]phenyl}acetamide, or a pharmaceutically acceptable salt or solvate thereof, and / or a B-Raf inhibitor, particularly N-{3-[5-(2-Amino-4-pyrimidinyl)-2-(1,1 - dimethylethyl)-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide or a pharmaceutically acceptable salt thereof, and an anti-PD-L1 antibody; pharmaceutical compositions comprising the same and methods of using such combinations and compositions in the treatment of conditions in which the inhibition of MEK and / or B-Raf and / or neutralizing or inhibiting the interaction between PD-L1 and its receptor, e.g. PD-1, is beneficial, eg. cancer.
Owner:NOVARTIS AG
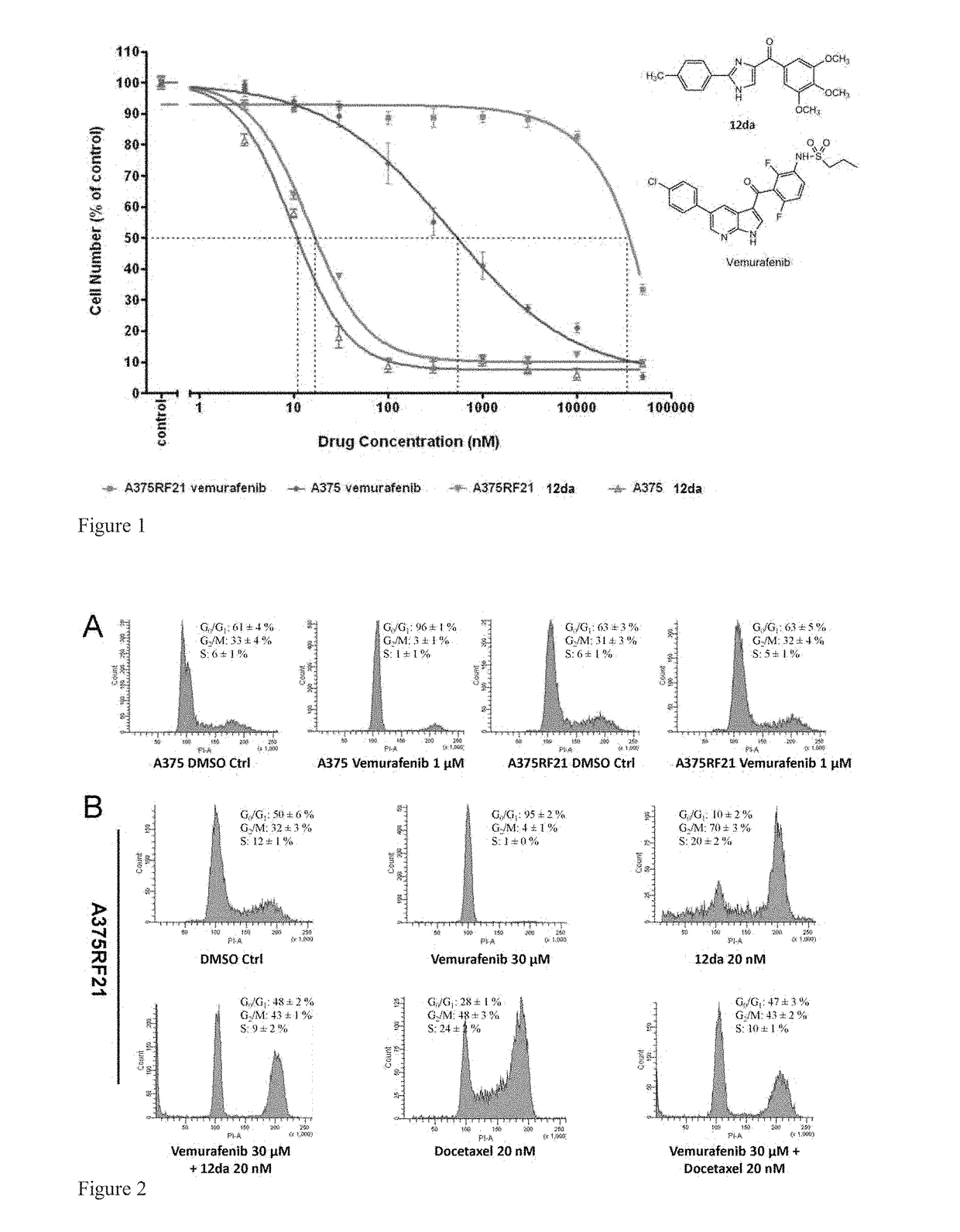
Compound preparation for treating BRAF inhibitor-resistant melanoma
InactiveCN107569485AEasy to takeSolve the problem of acquired drug resistance caused by long-term useOrganic active ingredientsAntineoplastic agentsAdditive ingredientBRAF inhibitor
The invention discloses a compound preparation for treating BRAF inhibitor-resistant melanoma. Medicinal ingredients in the compound preparation include vemurafenib and propranolol, wherein the weightratio of the vemurafenib to the propranolol is at (5-15) to 1. The compound preparation provided by the invention is more economic and effective, so that family burdens of patients can be reduced.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Compounds for treatment of cancer
The present invention relates to pharmaceutical compositions for treating cancer comprising BRAF inhibitors, (e.g. vemurafenib) and / or MEK inhibitor, (e.g. trametinib, RO5068760), in combination with anti-tubulin compounds of the invention or other known tubulin inhibitors, and using such compositions for treating cancer such as melanoma, drug-resistant cancer, and cancer metastasis.
Owner:UNIV OF TENNESSEE RES FOUND
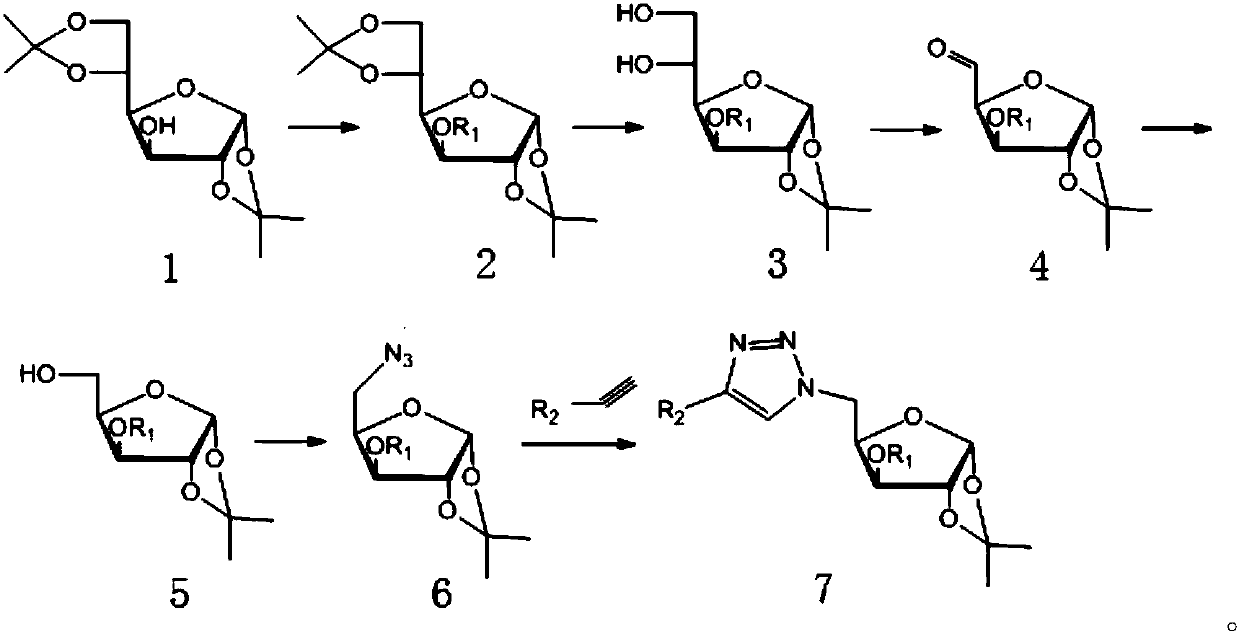
Furan glucosyl triazole type compound and preparation method and bactericide thereof
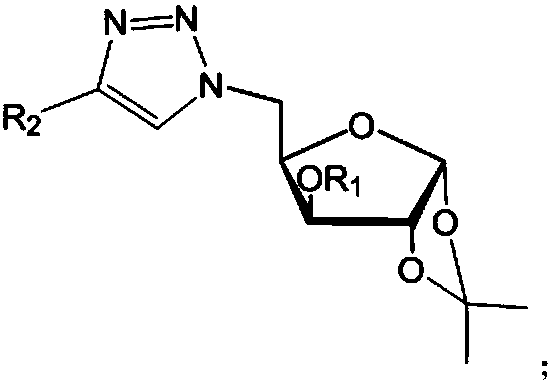
ActiveCN107857782AHigh antibacterial activityGood antibacterial effectBiocideSugar derivativesFuranPhenyl group
The invention relates to the field of bactericidal compounds, in particular to a furan glucosyl triazole type compound and a preparation and a bactericide thereof. The molecular formula of the furan glucosyl triazole type compound is shown in the following description, wherein R1 is methyl or benzyl, and R2 are phenyl and derivative of the phenyl or ethyl derivatives. Based on structural characteristics of the substrate fructose-6-phosphate and an ISOM catalytic hypothesis mechanism, the inventor adopts a five-membered furan glucose derivative with a similar structure as a basic skeleton, introduces an effective active group triazole structure of pesticides, designs a series of novel furan glucosyl triazole type compounds for the first time, studies the biological activities of the furan glucosyl triazole type compound, examines structure-activity relationships of the furan glucosyl triazole type compound, and lays the foundation for selecting better inhibitors.
Owner:BEIJING UNIV OF AGRI
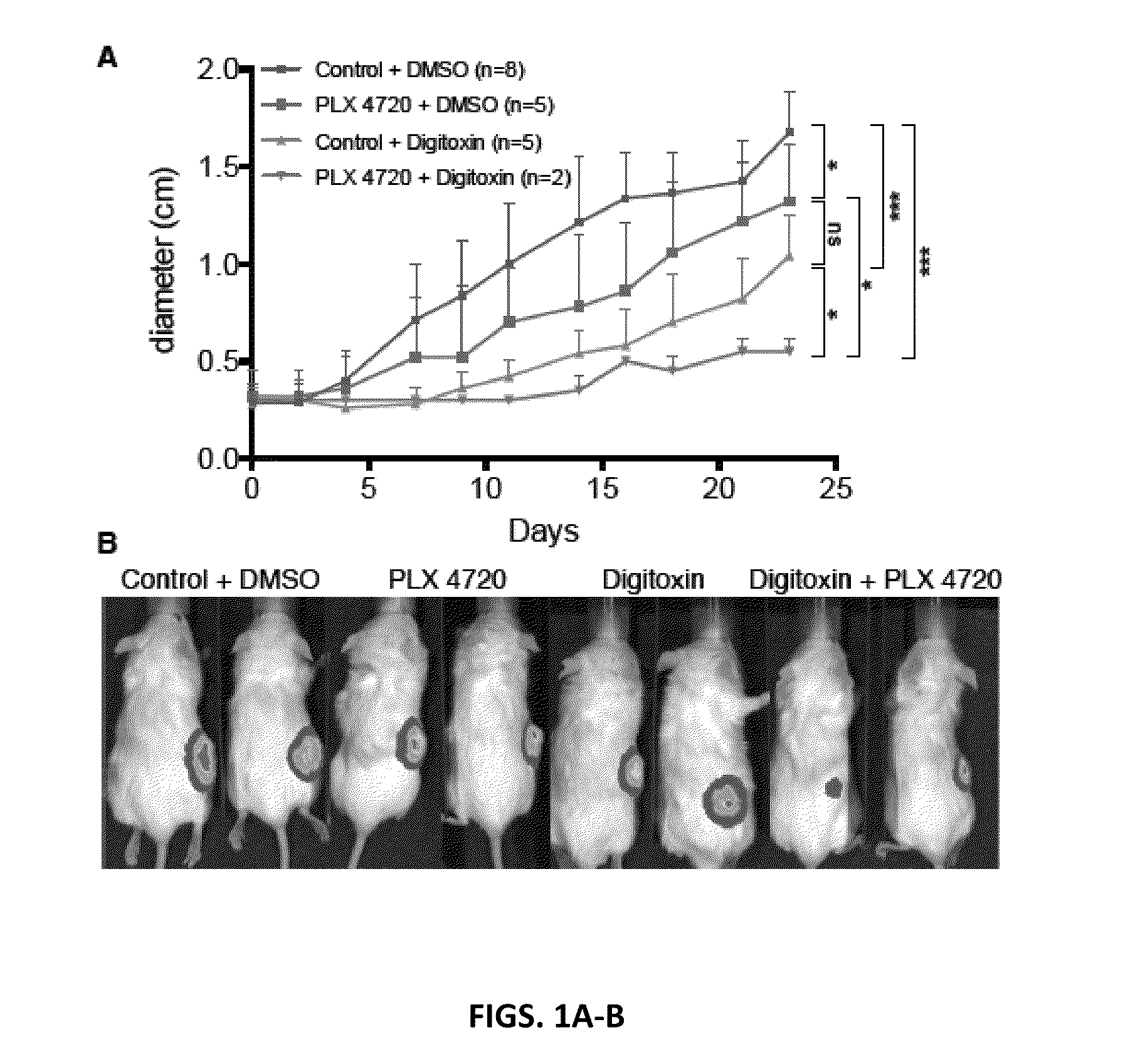
Combination treatments for melanoma
InactiveUS20140066392A1Inhibiting melanomaSufficient inhibitionBiocideAnimal repellantsBRAF inhibitorCardiac glycoside
The present invention relates to combination therapies for melanoma, and in particular, metastatic melanoma. Drugs for use in such therapies in include BRAF inhibitors such as PLX 4720 and PLX 4734 in combination with RO 31-8220, bafetinib or cardiac glycosides.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Treatment for melanoma
InactiveUS20170112865A1Growth inhibitionExtending remissionPeptide/protein ingredientsAntineoplastic agentsMEK inhibitorBRAF inhibitor
The present invention relates to combination therapies for melanoma, and in particular, metastatic melanoma. Drugs for use in such combination in include MEK inhibitors, BRAF inhibitors and cardiac glycosides.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for sensitizing BRAF inhibitor
InactiveCN107789625AImprove anti-tumor effectOrganic active ingredientsGenetic material ingredientsMelanomaSOX4
The invention belongs to the field of biomedicine, and relates to a method for sensitizing a BRAF inhibitor. An SOX4 gene can be used to adjust the killing effect of a BRAF inhibitor on a drug resistant melanoma cell strain and can be taken as a maker of apoptosis-inducing effect of the BRAF inhibitor on the drug resistant melanoma cell strain. The SOX4 gene can be regulated, the expression of SOX4 is inhibited, the killing effect of the BRAF inhibitor on the drug resistant melanoma cell strain is enhanced, and the apoptosis-inducing effect of the BRAF inhibitor on the drug resistant melanomacell strain is also strengthened. Furthermore, the antitumor effect of the BRAF inhibitor can be enhanced by inhibiting the expression of SOX4.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Methods of inhibiting endothelin b receptor expressing tumor metastases
InactiveUS20200289495A1Organic active ingredientsMammal material medical ingredientsBlastomaAntiendomysial antibodies
The description provides compositions and methods for treating ETBR-related cancer. In certain aspects, the description provides a delivery system for the controlled, systemic release of at least one of ETBR antagonists, caspase-8 inhibitors, or a combination thereof, optionally including an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide or a combination thereof. The compositions described are useful for the treatment of certain cancers, including, e.g., breast cancer, malignant melanoma, squamous cell carcinoma, glioblastoma, as well as others. In addition, the description provides a delivery system for the controlled release of at least one of ETBR antagonists, caspase-8 inhibitors or a combination thereof, optionally including at least one of an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide, or a combination thereof, to the central nervous system that are useful for treating cancers that have spread to the brain.
Owner:ENB THERAPEUTICS INC
Methods and compositions for treatment of endothelin b receptor expressing tumors
ActiveUS20200316049A1Dipeptide ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsBlastomaAntiendomysial antibodies
The description provides compositions and methods for treating ETBR-related cancer. In certain aspects, the description provides a delivery system for the controlled, systemic release of at least one of ETBR antagonists, caspase-8 inhibitors, or a combination thereof, optionally including an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide or a combination thereof. The compositions described are useful for the treatment of certain cancers, including, e.g., breast cancer, malignant melanoma, squamous cell carcinoma, glioblastoma, as well as others. In addition, the description provides a delivery system for the controlled release of at least one of ETBR antagonists, caspase-8 inhibitors or a combination thereof, optionally including at least one of an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide, or a combination thereof, to the central nervous system that are useful for treating cancers that have spread to the brain.
Owner:ENB THERAPEUTICS INC
A pharmaceutical combination for the treatment of melanoma
The present invention relates to a pharmaceutical combination comprising a cyclin dependent kinase (CDK) inhibitor represented by a compound of formula I (as described herein) or a pharmaceutically acceptable salt thereof; and at least one anticancer agent selected from a BRAF inhibitor or a MEK inhibitor, for use in the treatment of melanoma. The present invention also relates to a method for the treatment of melanoma comprising administering to a subject in need thereof, a therapeutically effective amount of a CDK inhibitor and a therapeutically effective amount of at least one anticancer agent selected from a BRAF inhibitor or a MEK inhibitor.
Owner:PIRAMAL ENTERPRISES LTD
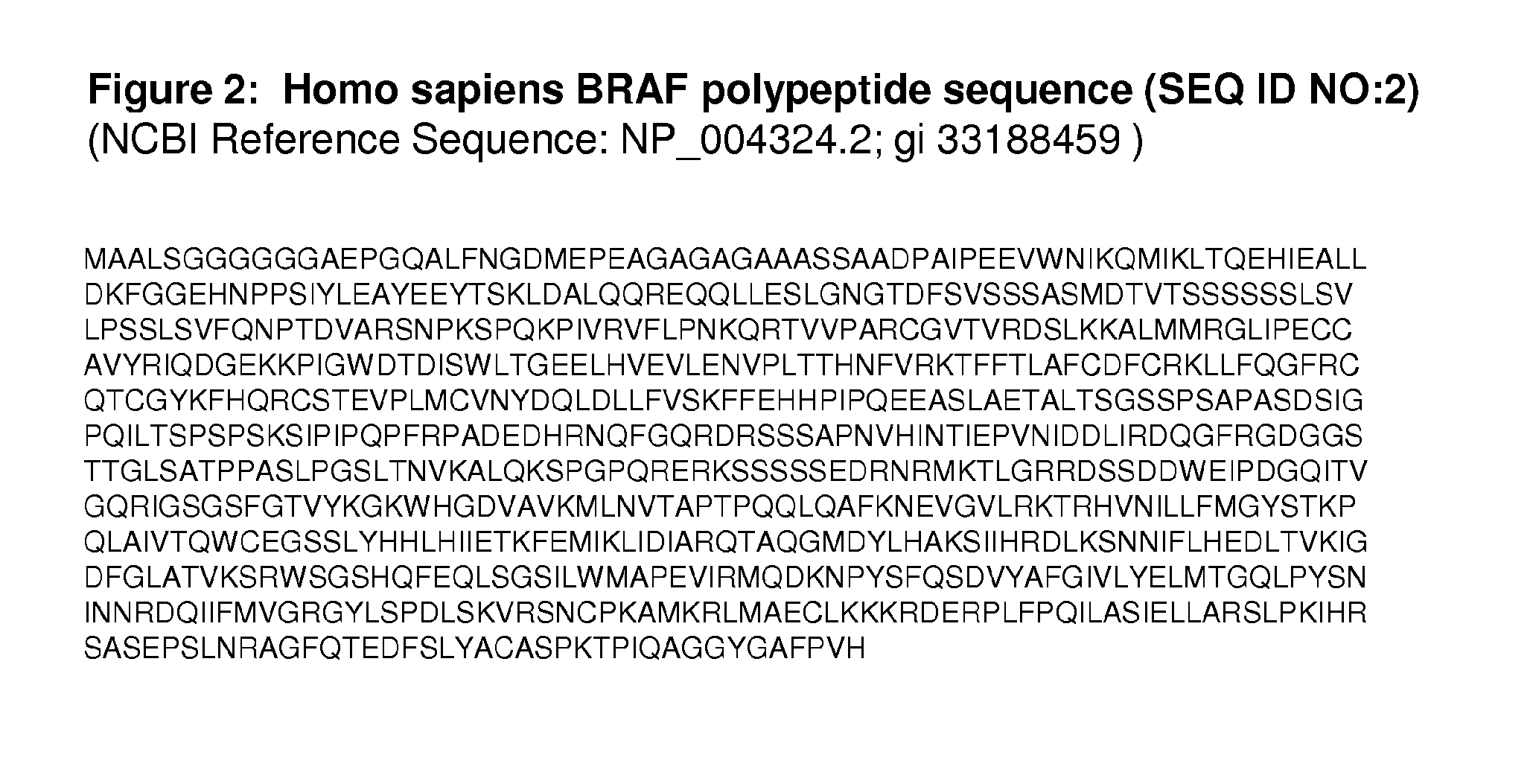
BRAF Mutations Conferring Resistance to BRAF Inhibitors
ActiveUS20130004509A1High riskConfer resistanceOrganic active ingredientsBacteriaAnticarcinogenMedicine
The present invention relates to methods, compositions and kits concerning resistance to treatment with an anti-cancer agent, specifically an inhibitor of BRAF. In particular embodiments, the invention concerns mutations in a BRAF sequence that confer resistance to a BRAF inhibitor. Identification of such mutations in a BRAF sequence allows the identification and design of second-generation BRAF inhibitors. Methods and kits for detecting the presence of a mutant BRAF sequence in a sample are also provided.
Owner:DANA FARBER CANCER INST INC
BRaf inhibitors and use thereof for treatment of cutaneous reactions
Owner:LUTRIS PHARMA LTD
Preparation method of near-infrared response nanocage and application of near-infrared response nanocage in tumor immune combined therapy
ActiveCN112870354AIncrease loading capacityGood biocompatibilityOrganic active ingredientsDrug photocleavageLight energyTumor therapy
The invention relates to a preparation method of a near-infrared response nanocage and application of the near-infrared response nanocage in tumor immune combined therapy. The preparation method of the near-infrared response nanocage comprises the four steps of preparing AuNC, preparing AuNC-coated mSiO2, preparing ASP and preparing an ASP intersection V. The near-infrared response nanocage has the advantages of good biocompatibility, safety, near-infrared region photothermal conversion, high drug loading capacity and the like. The drug-loaded nanocage can effectively deliver drugs into tumor cells and convert light energy into heat energy, light heat and a BRAF inhibitor are combined to inhibit tumor growth, induce immunogenic necrosis of the tumor cells, promote release of tumor-related antigens and generate systematic anti-tumor immune response, and the drug-loaded nanocage cooperates with an anti-PD-1 antibody to inhibit tumor growth. According to the gold nanocage, MAPK molecular targeting and anti-PD-1 immune combined treatment is realized, and a new strategy is provided for tumor treatment.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Nanoparticle compositions of endothelin b receptor antagonists
The description provides compositions and methods for treating ETBR-related cancer. In certain aspects, the description provides a delivery system for the controlled, systemic release of at least one of ETBR antagonists, caspase-8 inhibitors, or a combination thereof, optionally including an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide or a combination thereof. The compositions described are useful for the treatment of certain cancers, including, e.g., breast cancer, malignant melanoma, squamous cell carcinoma, glioblastoma, as well as others. In addition, the description provides a delivery system for the controlled release of at least one of ETBR antagonists, caspase-8 inhibitors or a combination thereof, optionally including at least one of an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide, or a combination thereof, to the central nervous system that are useful for treating cancers that have spread to the brain.
Owner:ENB THERAPEUTICS INC
Methods and compositions for treatment of endothelin B receptor expressing tumors
ActiveUS11338014B2Dipeptide ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsBlastomaAntiendomysial antibodies
The description provides compositions and methods for treating ETBR-related cancer. In certain aspects, the description provides a delivery system for the controlled, systemic release of at least one of ETBR antagonists, caspase-8 inhibitors, or a combination thereof, optionally including an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide or a combination thereof. The compositions described are useful for the treatment of certain cancers, including, e.g., breast cancer, malignant melanoma, squamous cell carcinoma, glioblastoma, as well as others. In addition, the description provides a delivery system for the controlled release of at least one of ETBR antagonists, caspase-8 inhibitors or a combination thereof, optionally including at least one of an ETAR antagonist, an anti-PD-1 antibody, a bRAF inhibitor, niacinamide, or a combination thereof, to the central nervous system that are useful for treating cancers that have spread to the brain.
Owner:ENB THERAPEUTICS INC
Use of topical braf inhibitor compositions for treatment of radiation dermatitis
ActiveUS20210346389A1Pharmaceutical delivery mechanismDermatological disorderBRAF inhibitorRadical radiotherapy
The present invention discloses methods of treatment, prevention and / or amelioration of radiation dermatitis caused by radiotherapy, by administration to a subject in need thereof of a topical composition comprising a therapeutically or prophylactically effective amount of at least one BRaf inhibitor of this invention, thus treating, preventing and / or ameliorating the effects of radiation dermatitis.
Owner:LUTRIS PHARMA LTD
Novel braf inhibitors and use thereof for treatment of cutaneous reactions
Owner:LUTRIS PHARMA LTD
Methods and compositions for treating melanoma resistant
PendingUS20210072244A1Dampen anti-proliferative effectDampened antiproliferative effectCompound screeningOrganic active ingredientsStage melanomaTricarboxylic acid
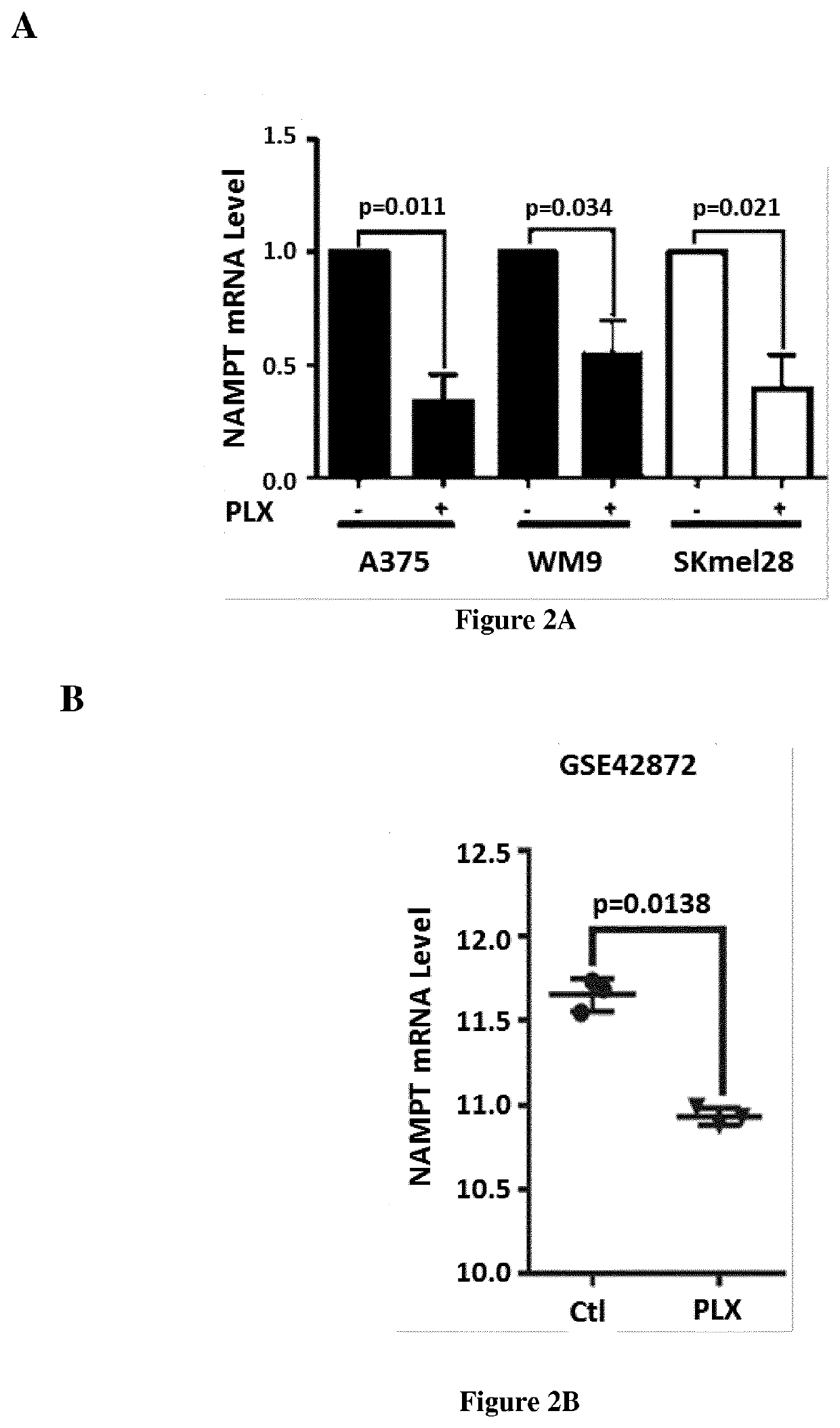
The present invention relates to a method for treating a subject suffering from melanoma resistant by administering to said subject an inhibitor of NAMPT. Using a global metabolic profiling, inventors have showed that in addition to glycolysis, the BRAF inhibitor, PLX4032, promoted a complex metabolic rewiring of melanoma cells, including protein catabolism and fatty acid synthesis. Importantly, they observed that PLX4032 reduced the levels of nicotinamide adenine dinucleotide (NAD+), an important redox co-factor in numerous metabolic processes, including glycolysis, tricarboxylic acid cycle (TCA) cycle, glutamate metabolism and fatty acid betaoxidation. Pharmacological or genetic inhibition of NAMPT impaired melanoma cell growth, whereas the overexpression of NAMPT dampened the antiproliferative effect of PLX4032. In vivo, the inhibition of NAMPT also prevented the xenograft development of PLX4032-sensitive and -resistant melanoma cells, identifying NAMPT as a potential target for BRAFi-resistant melanomas.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Application of BRAF inhibitor to preparation of novel drugs for treating programmed necrotic diseases and screening method of BRAF inhibitor
InactiveCN109568584AGood effect on inhibiting necrosisGood water solubilityMicrobiological testing/measurementDrug compositionsSolubilityScreening method
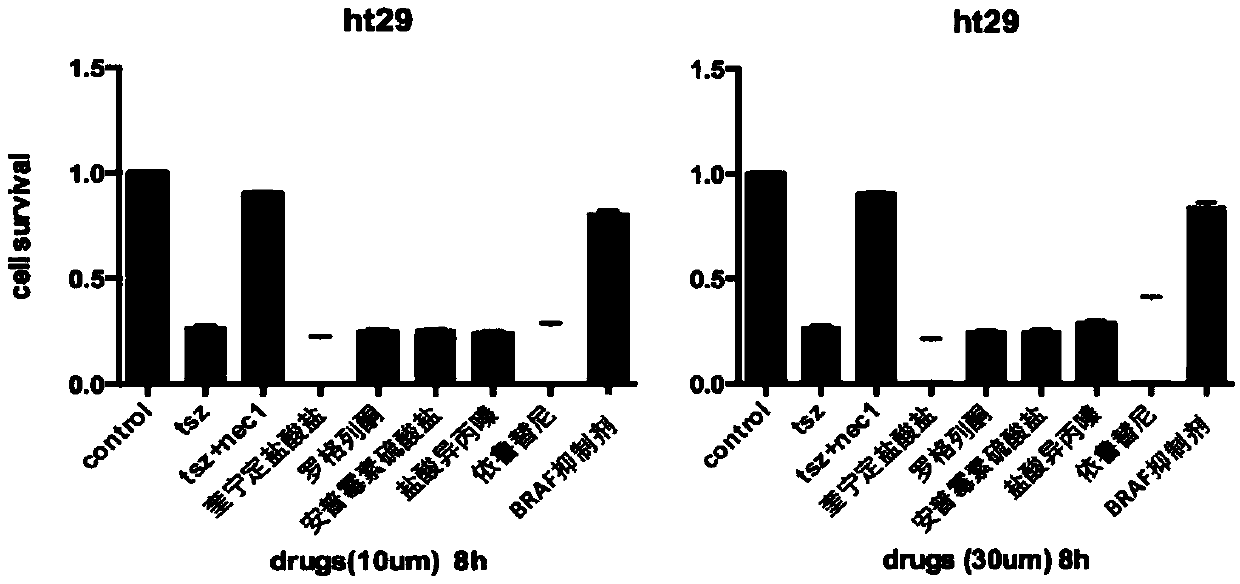
The invention discloses application of a BRAF inhibitor to preparation of novel drugs for treating programmed necrotic diseases and a screening method of the BRAF inhibitor. The method is characterized by taking necrotic pathway sensitive cell line L929-FADD gene knockout cell strains as model cells of cell proliferation and necrosis, screening out small-molecule compounds influencing and inhibiting cell death from an FDA drug library, detecting different drug concentration of the small-molecule compounds obtained by screening, determining toxicity-effect concentration, and performing comprehensive evaluation from toxicity and recovery efficiency to obtain the BRAF inhibitor; and through inhibition of the BRAF inhibitor on cell necrosis, the BRAF inhibitor can be used for preparing novel drugs for treating the programmed necrotic diseases. The BRAF inhibitor has excellent necrosis inhibition effects on necrotic sensitive cell line L929-FADD knockout strains, human cell line HT29 and animal models; the water solubility of the BRAF inhibitor is better than that of Nec-1; the BRAF inhibitor is an FDA-approved clinical drug; the targeting property and the safety of the BRAF inhibitor are guaranteed; and the BRAF inhibitor is easy for clinical promotion.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com