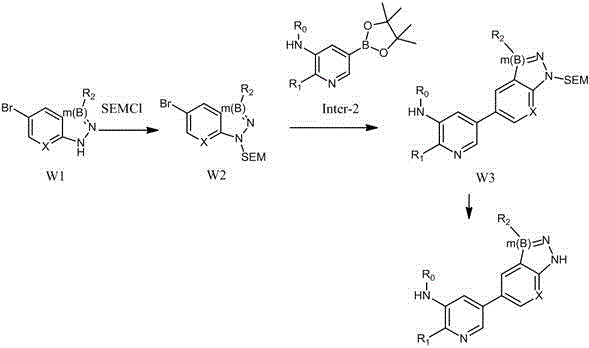
PI3K inhibitor, preparation method and application thereof in pharmacy
A technology of inhibitors and drugs, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] N-[2-Chloro-5-(quinolin-3-yl)pyridin-3-yl]benzenesulfonamide (Compound 1)
[0094]
[0095] 1: Preparation of N-(2-chloro-5-bromopyridin-3-yl)benzenesulfonamide (1-J)
[0096]
[0097] In a 100mL round bottom flask, add dichloromethane (DCM) (50mL), 3-amino-2-chloro-5-bromopyridine (B1) (5.0g, 24.0mmol), pyridine (3.8g, 48mmol), ice Under cooling in a water bath, benzenesulfonyl chloride (D1) (5.3 g, 31 mmol) was added dropwise. The reaction solution was slowly raised to room temperature, stirred for 24 h, followed by HPLC. After the reaction, pour it into water (30mL), separate the layers, wash the organic phase with saturated aqueous citric acid solution (50ml), saturated sodium bicarbonate (50ml), and saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate to remove DCM, a solid precipitated out. Recrystallization with petroleum ether (PE) / DCM gave light white solid product N-(2-chloro-5-bromopyridin-3-yl)benzenesulfonamide (1-J), MS (ESI...
Embodiment 2
[0105] N-[2-Chloro-5-(quinolin-3-yl)pyridin-3-yl]benzenesulfonamide (Compound 2)
[0106]
[0107] Preparation of N-[2-chloro-5-(quinolin-6-yl)pyridin-3-yl]benzenesulfonamide (compound 2)
[0108]
[0109] The experimental procedure is the same as the synthesis of compound 1 in Example 1, except that 3-bromoquinoline (Q3) is replaced by 6-bromoquinoline (Q6) to obtain compound 2. MS(ESI positive ion)m / z:396.86(M+1),1HNMR(400MHz,CDCl 3): δppm3.857(s, 1H), 7.504(t, J=7.6Hz, 2H), 7.597-7.616(m, 1H), 7.831-7.851(m, 2H), 7.903(d, J=8.0Hz, 1H), 8.010(d, J=8.0Hz, 1H), 8.236-8.284(m, 2H), 8.343(d, 2.8Hz, 2H), 8.477(d, J=2.0Hz, 1H), 8.991(d, J=4.0Hz, 1H).
Embodiment 3
[0111] N-{2-chloro-5-[1H-pyrrole(2,3-b)pyridin-5-yl]pyridin-3-yl}benzenesulfonamide (Compound 3)
[0112]
[0113] Preparation of N-{2-chloro-5-[1H-pyrrole(2,3-b)pyridin-5-yl]pyridin-3-yl}benzenesulfonamide (Compound 3)
[0114]
[0115] The experimental procedure is the same as the synthesis of compound 1 in Example 1, except that 3-bromoquinoline (Q3) is replaced by 5-bromo-1H-pyrrole [2,3-b] pyridine (B3) to obtain the product N-{ 2-Chloro-5-[1H-pyrrole(2,3-b)pyridin-5-yl]pyridin-3-yl}benzenesulfonamide (3). MS(ESI positive ion)m / z:385.84(M+1),1HNMR(400MHz,DMSO-d6):δppm6.551(s,1H),7.595(t,J=8.0Hz,3H),7.695(d ,J=6.8Hz,1H),7.783(d,J=7.2Hz,2H),7.954(d,J=2.4Hz,1H),8.224(d,J=2.0Hz,1H),8.451(d,J =2.0Hz, 1H), 8.613(d, J=2.0Hz, 1H), 10.440(s, 1H), 11.866(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com