Furan glucosyl triazole type compound and preparation method and bactericide thereof
A glucofuranose and triazole-based technology, which is applied in the preparation of sugar derivatives, botanical equipment and methods, fungicides, etc., to achieve good antibacterial activity and remarkable antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
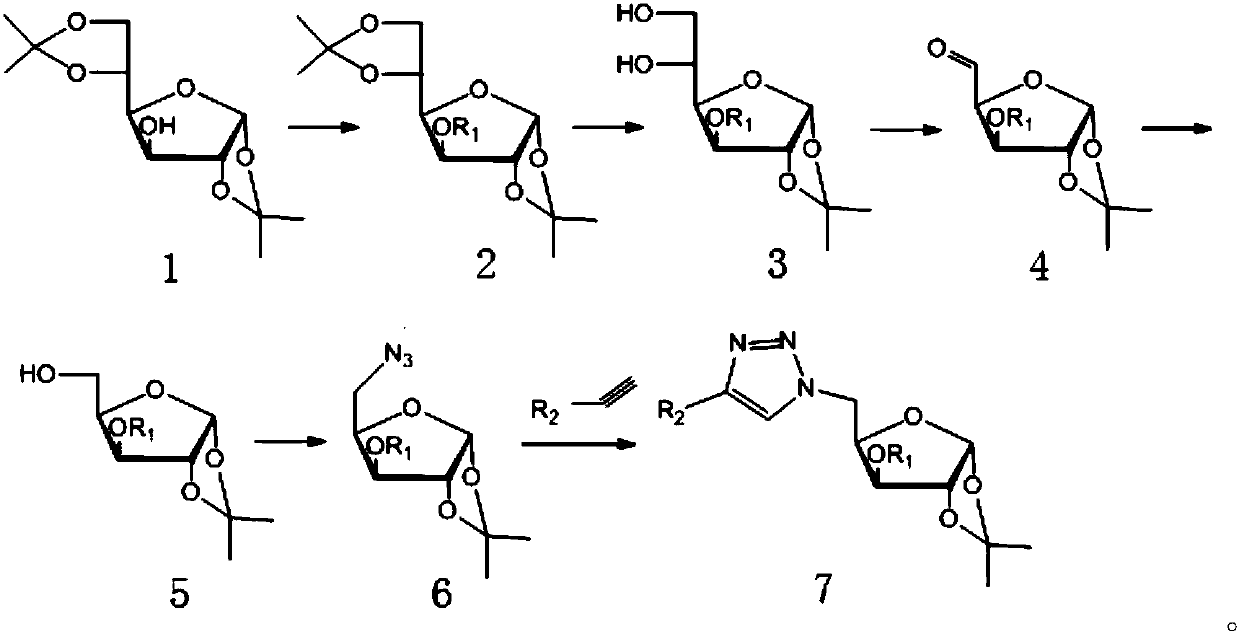
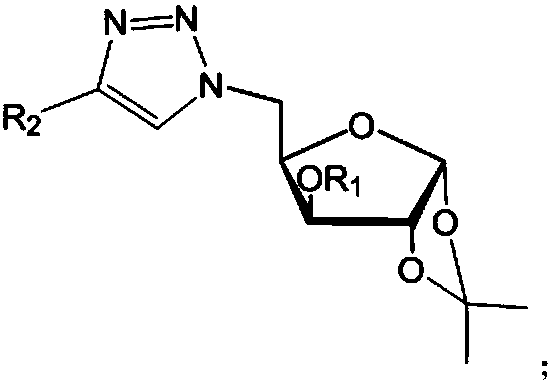
[0051] Glucofuranosyl triazole compounds, with target (R 1 = Me,R 2 =C 6 h 4 The synthesis of -) is an example, and the preparation method is as follows:
[0052] 1. Synthesis of Compound 2
[0053] Add 20 g of diacetone glucose into a 500 mL round bottom flask, dissolve in anhydrous DMF, slowly add 3.7 g of NaH at room temperature, react for a while, then slowly add 12 mL of methyl iodide dropwise, and react for 6 h at room temperature, TLC [V (petroleum ether): V (ethyl acetate) = 4:1] detection + complete reaction, use diatomaceous earth to filter to remove insoluble matter, dissolve with dichloromethane after concentration under reduced pressure, wash with water, separate liquid, dry, and obtain white powdery solid after concentration The target product compound 2. Yield 98%.
[0054] 2. Synthesis of compound 3
[0055] Add 15g of compound 2 and 50mL of 60% acetic acid into a 500mL round bottom flask, stir at room temperature for 10h, TLC [V (petroleum ether): V (et...
Embodiment 2
[0065] R 1 is methyl, R 2 4-Cl-C 6 h 4 -or 4-F-C 6 h 4 -;
[0066] Other steps are the same as in Example 1, except that in the synthesis method of compound 7, phenylacetylene is replaced by 4-Cl-phenylacetylene or 4-F-phenylacetylene.
Embodiment 3
[0068] R 1 is methyl, R 2 4-NO 2 -C 6 h 4 -;
[0069] Other steps are the same as in Example 1, except that in the synthetic method of compound 7, phenylacetylene is replaced by 4-NO 2 - Phenylacetylene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com