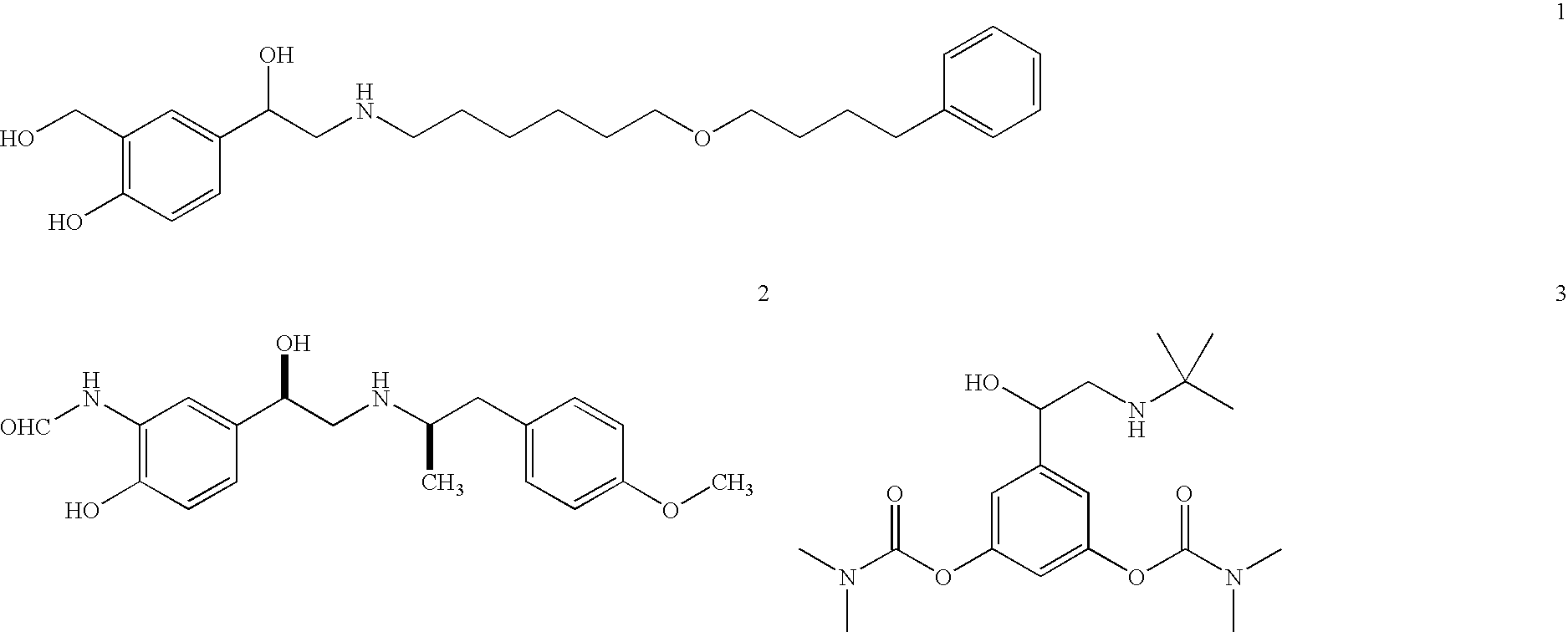
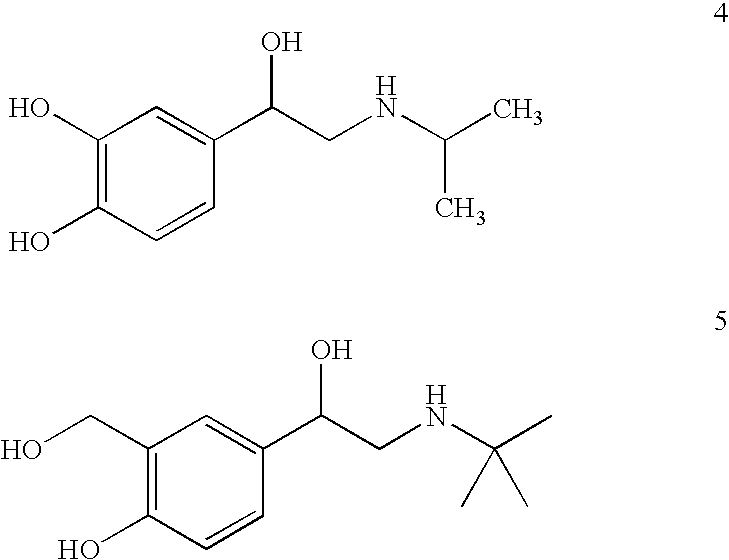
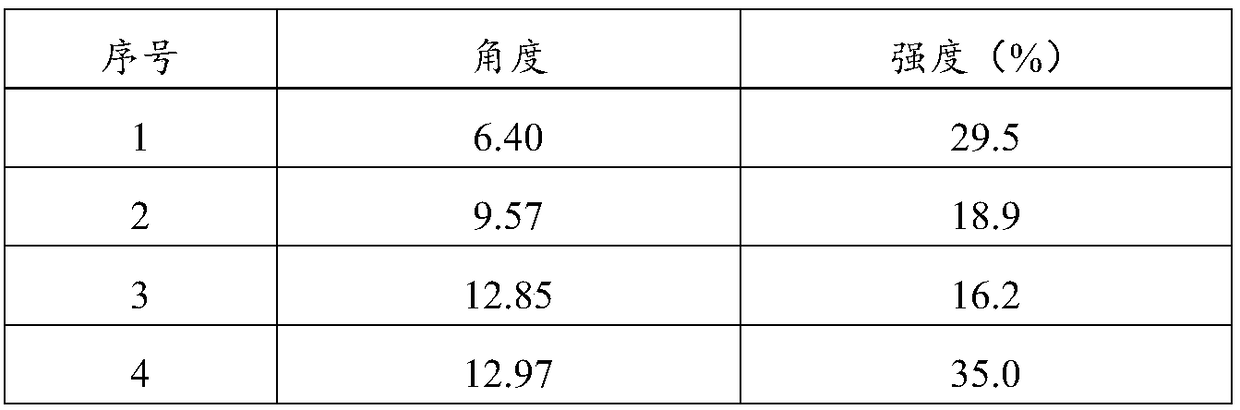
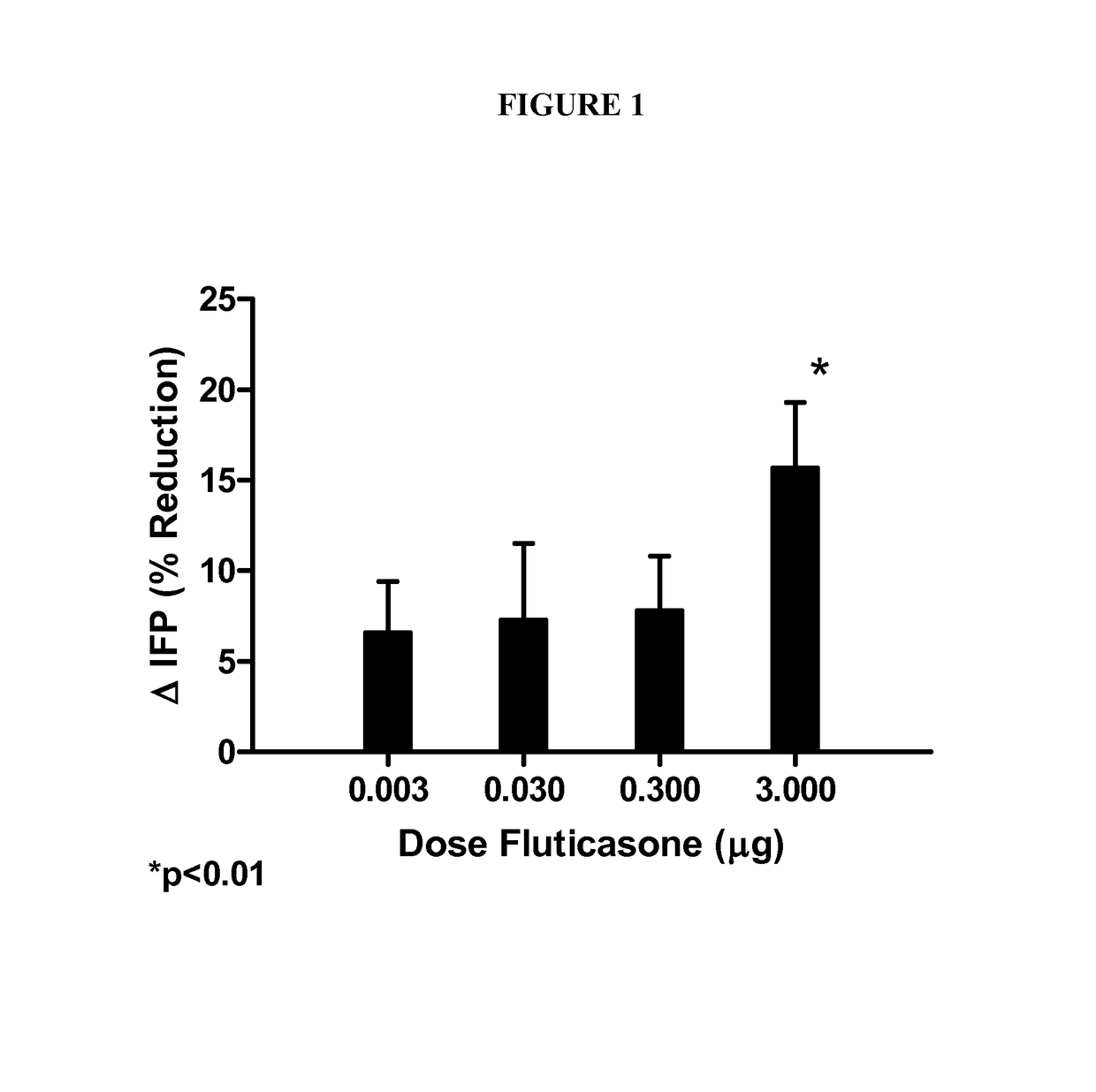
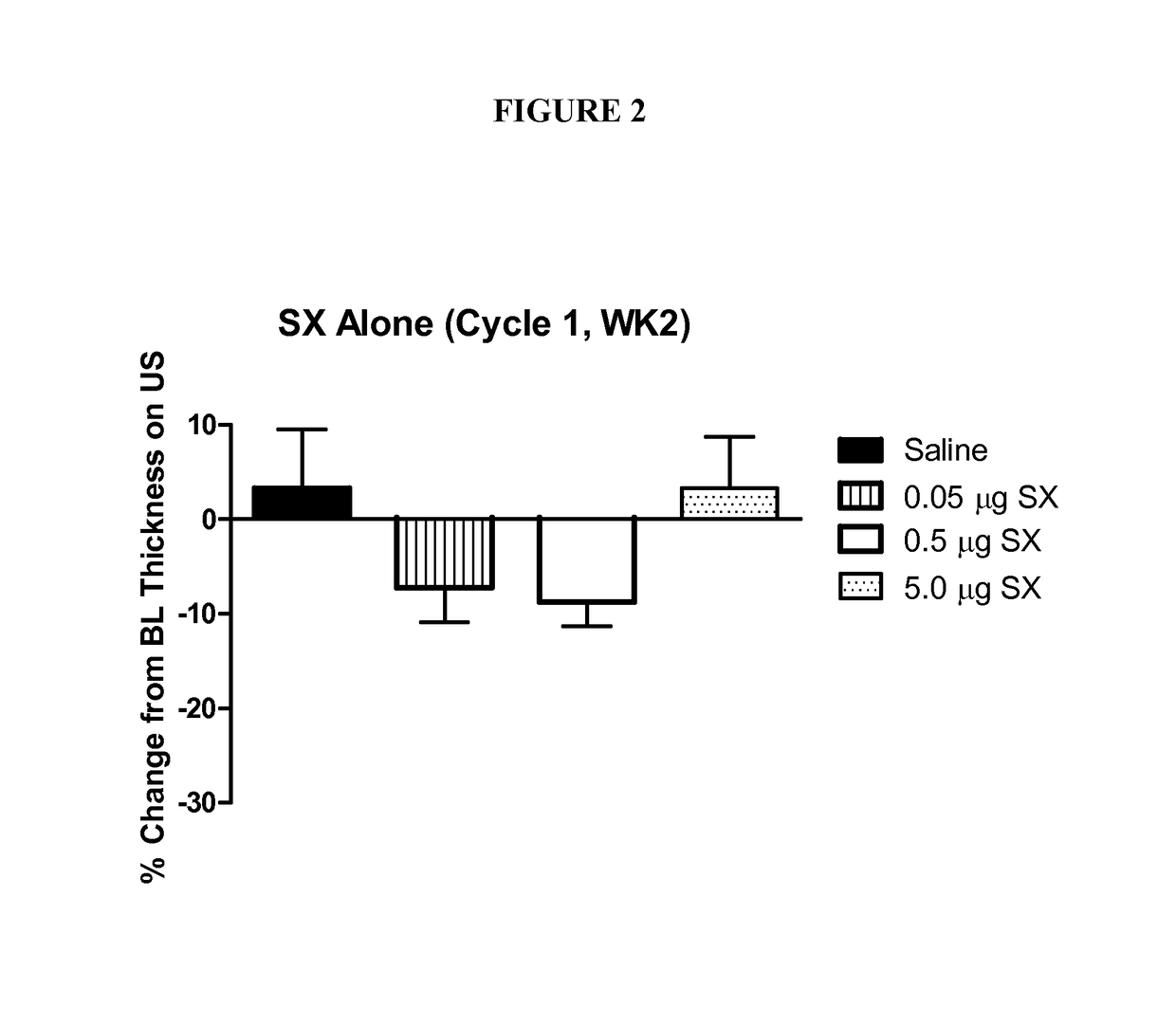
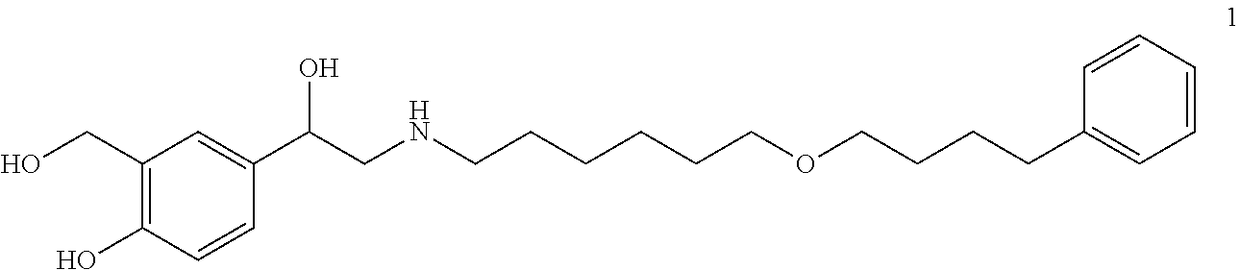
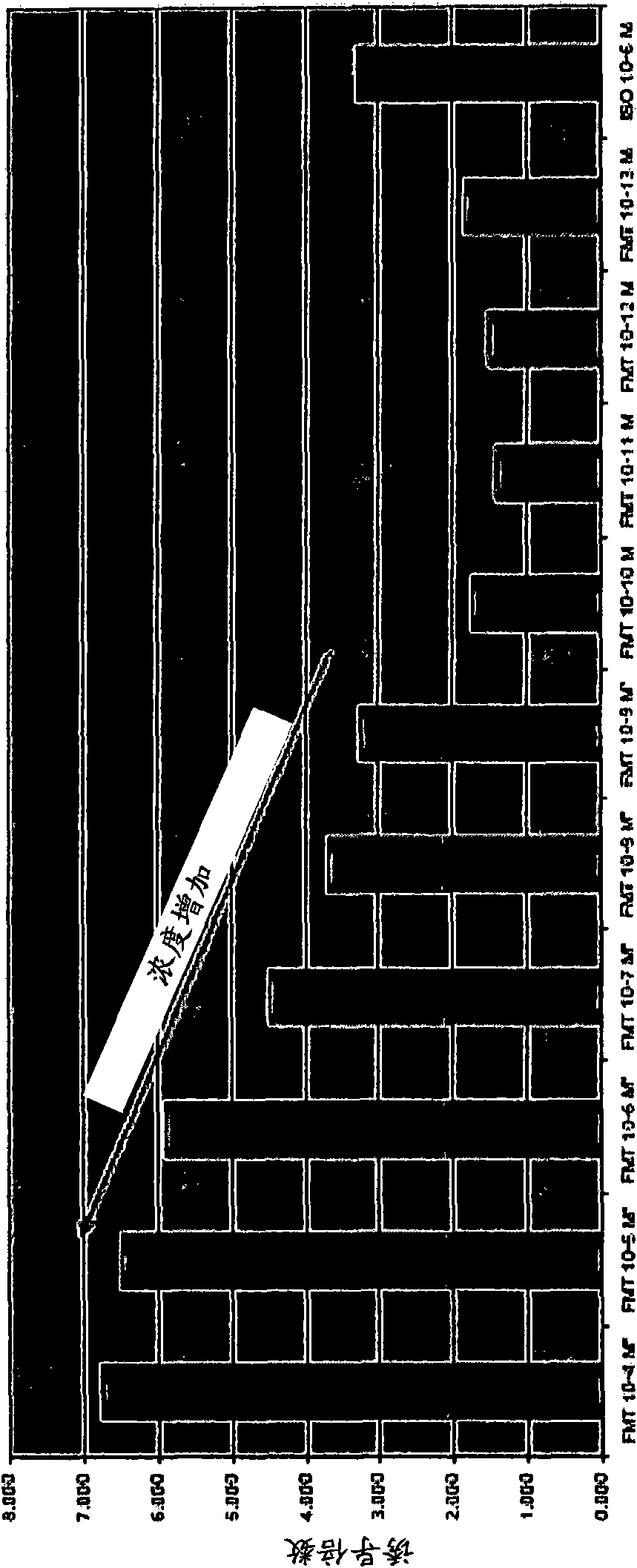
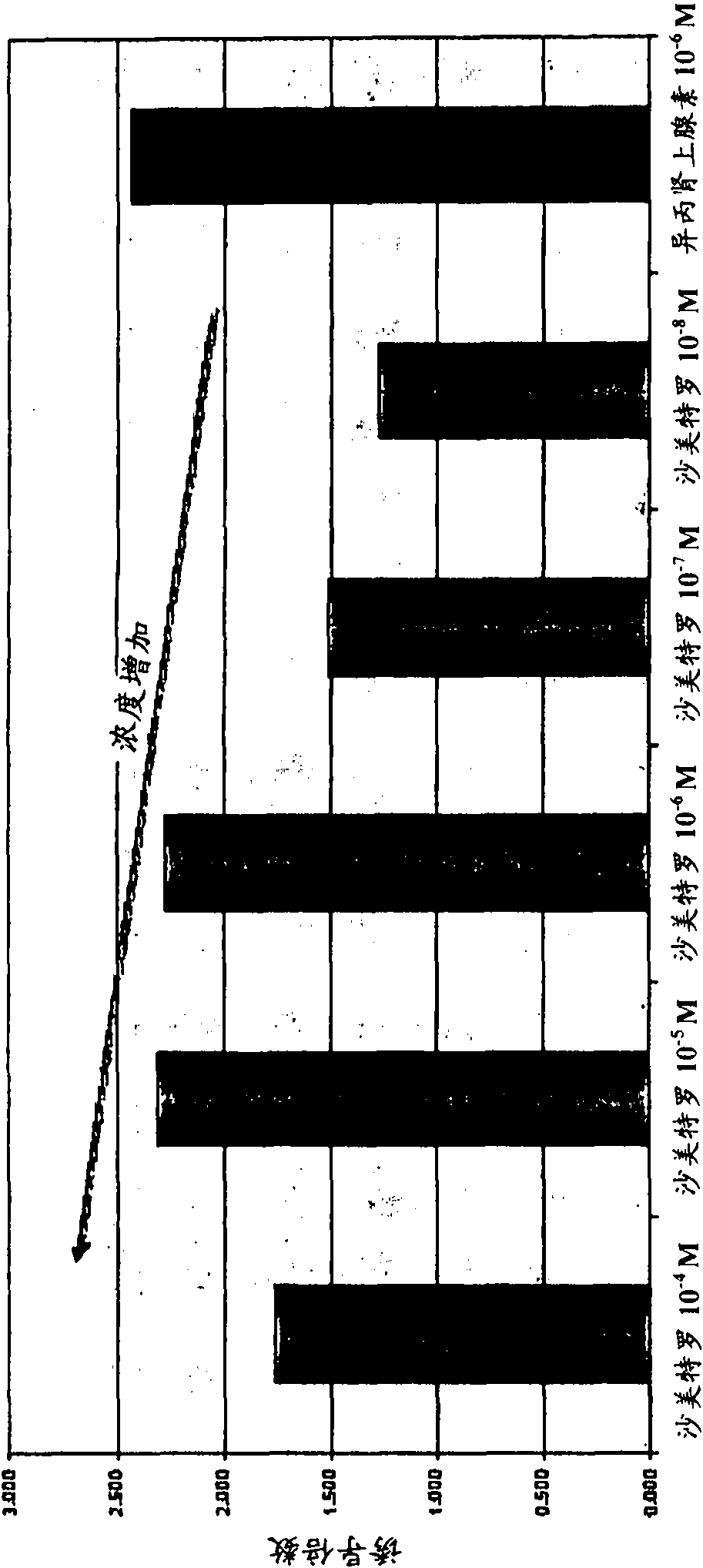
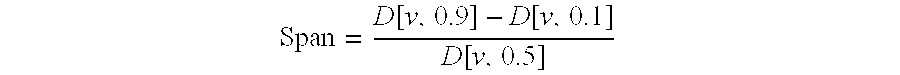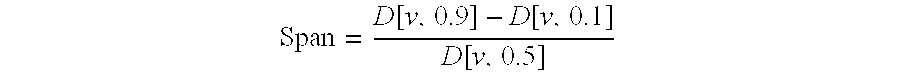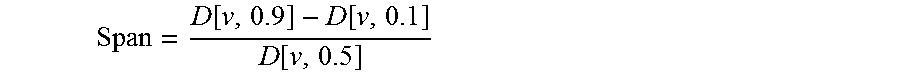Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Long acting beta" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
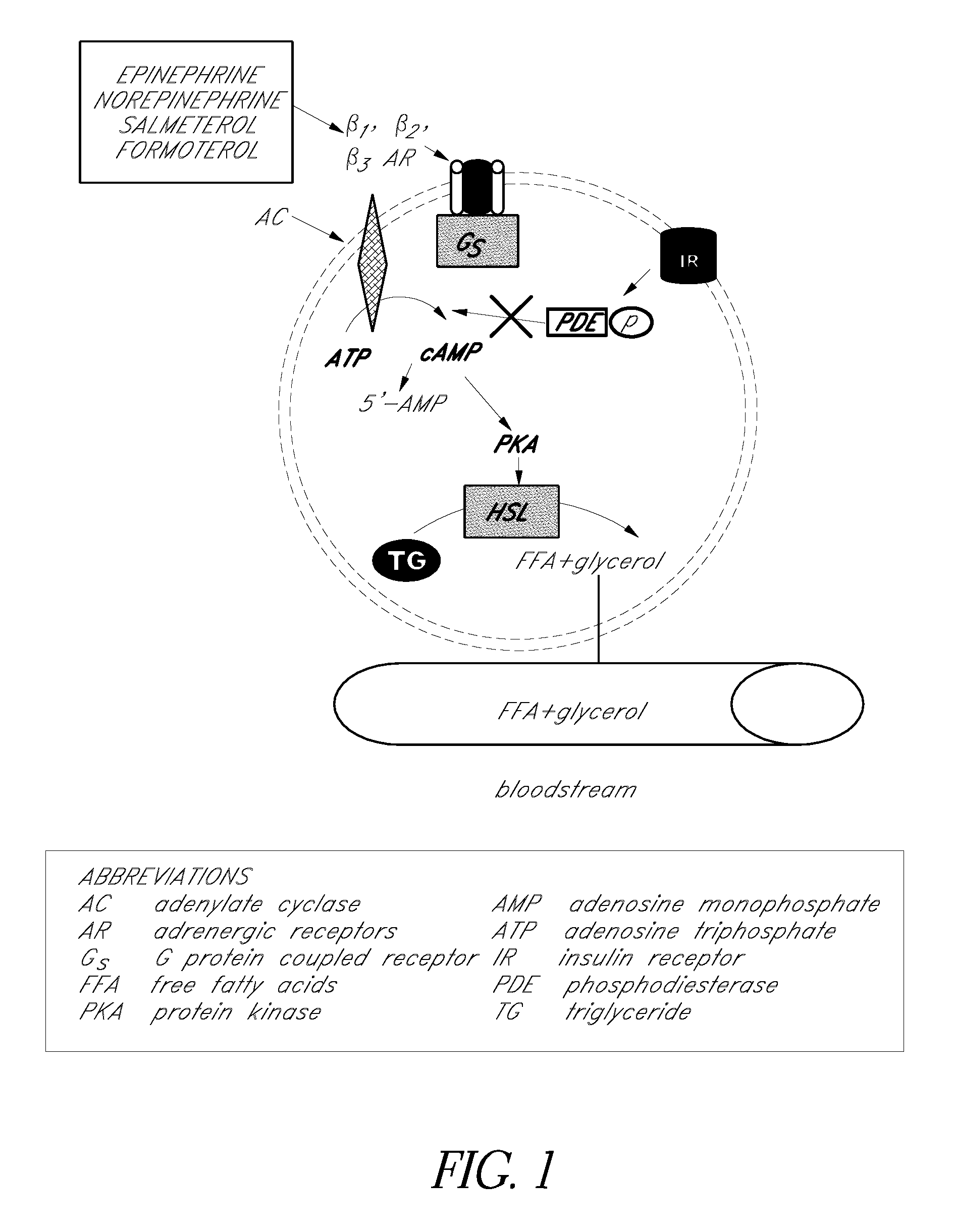
Long-Acting Beta Agonist (LABA) Information. Long-Acting Beta Agonists (LABAs) are inhaled medications that are used in the treatment of asthma and chronic obstuctive pulmonary disease (COPD). To report any serious adverse events associated with the use of these drugs, please contact the FDA MedWatch program using the contact information at the bottom of this page.
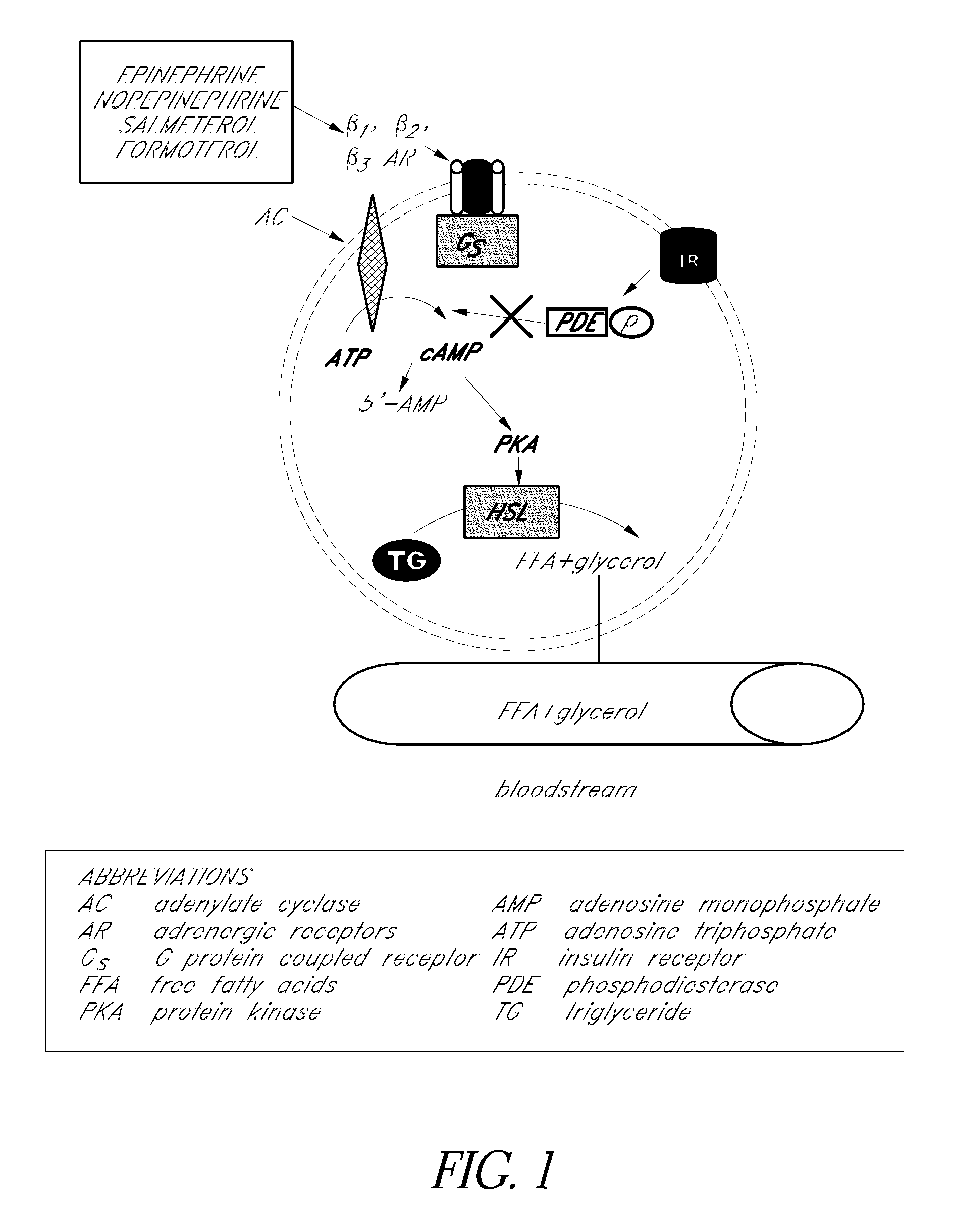
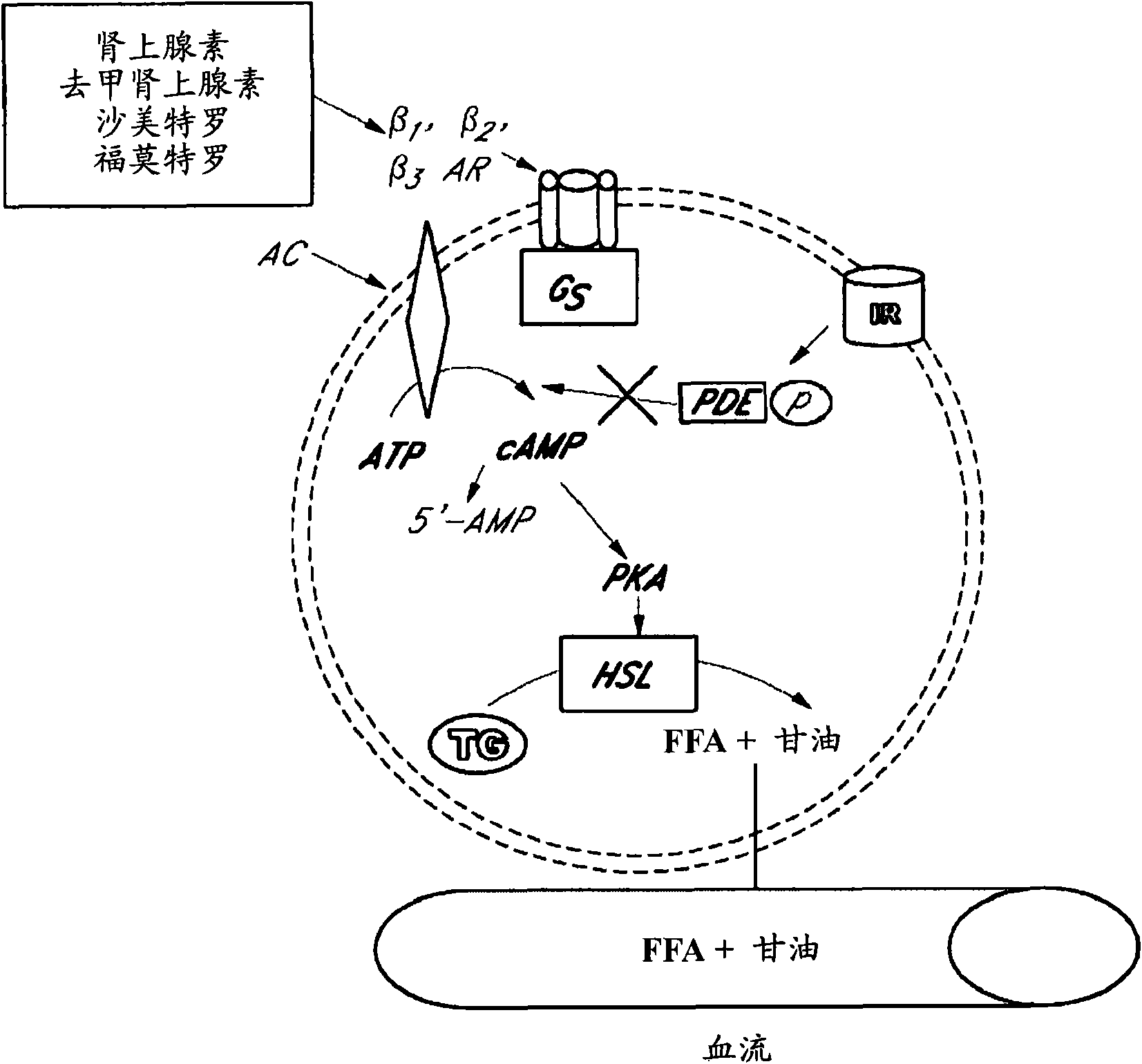
Sustained release enhanced lipolytic formulation for regional adipose tissue treatment
InactiveUS20070014843A1Reducing regional fat accumulationGood lookingBiocideMetabolism disorderAdrenergicAdrenergic receptor agonists
Compositions, formulations, methods, and systems for treating regional fat deposits comprise contacting a targeted fat deposit with a composition comprising long acting beta-2 adrenergic receptor agonist and a compound that reduces desensitization of the target tissue to the long acting beta-2 adrenergic receptor agonist, for example, glucocorticosteroids and / or ketotifen. Embodiments of the composition are administered, for example, by injection, and / or transdermally.
Owner:LITHERA
Treatment and diagnostic methods for hypersensitive disorders
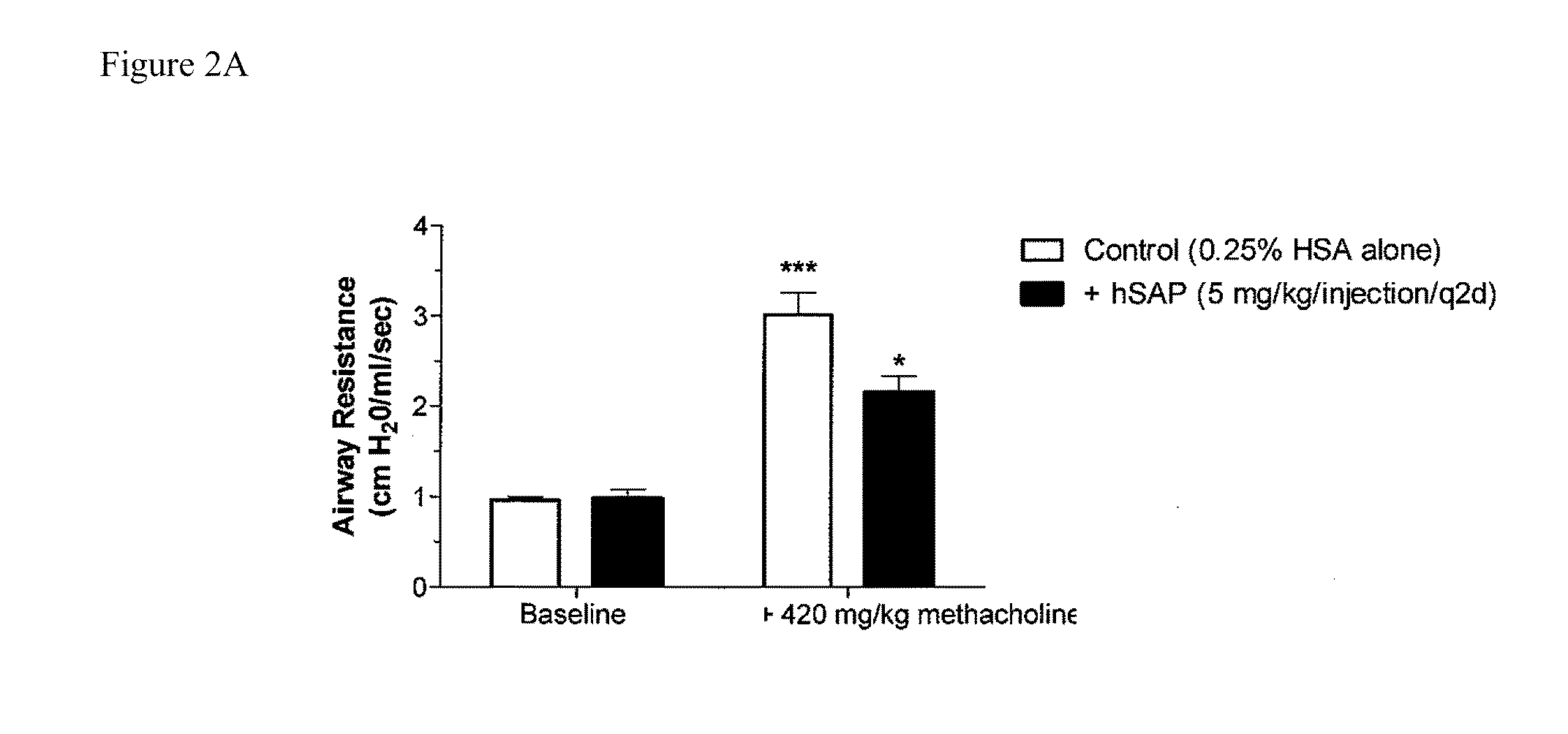
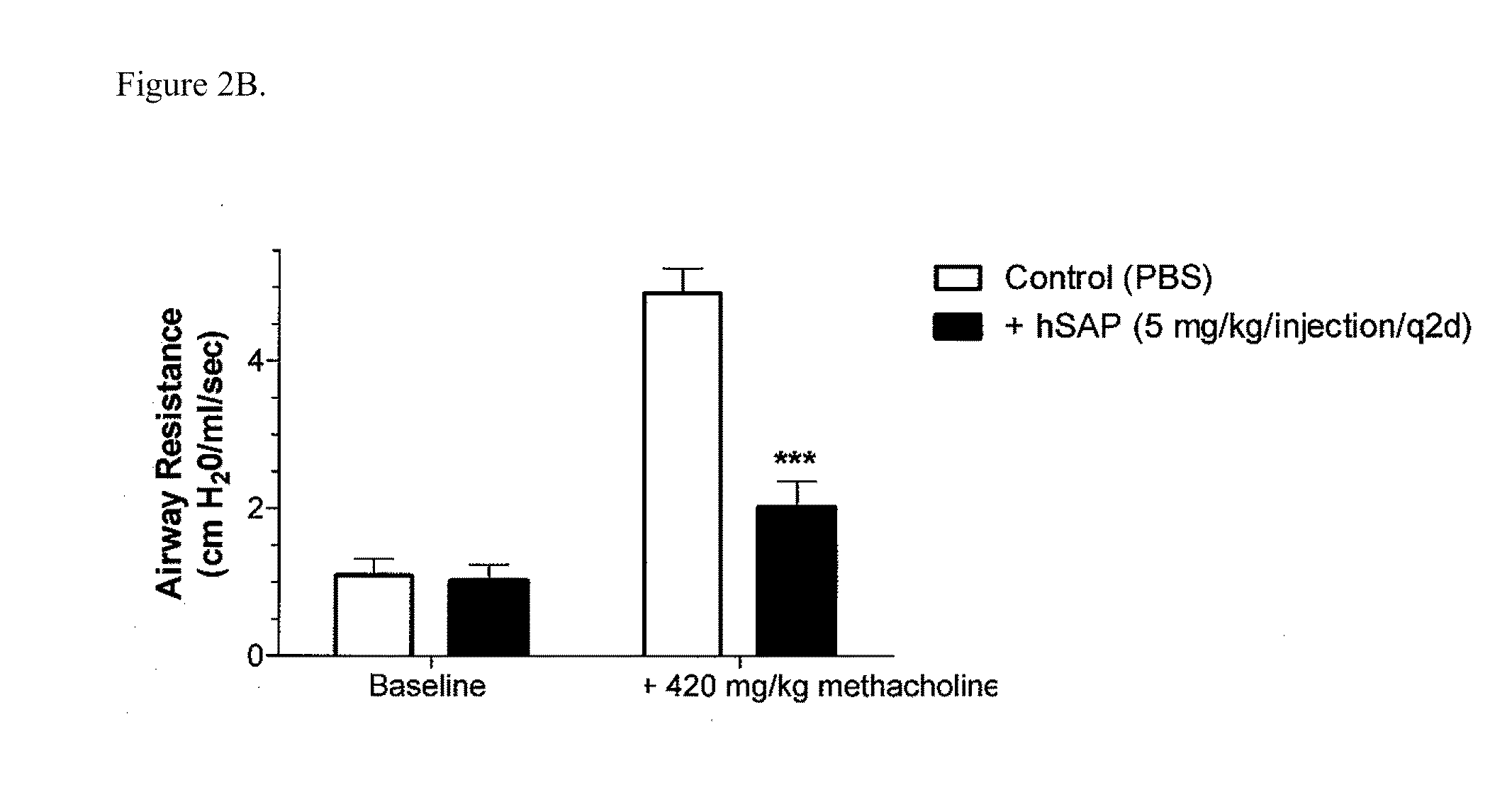
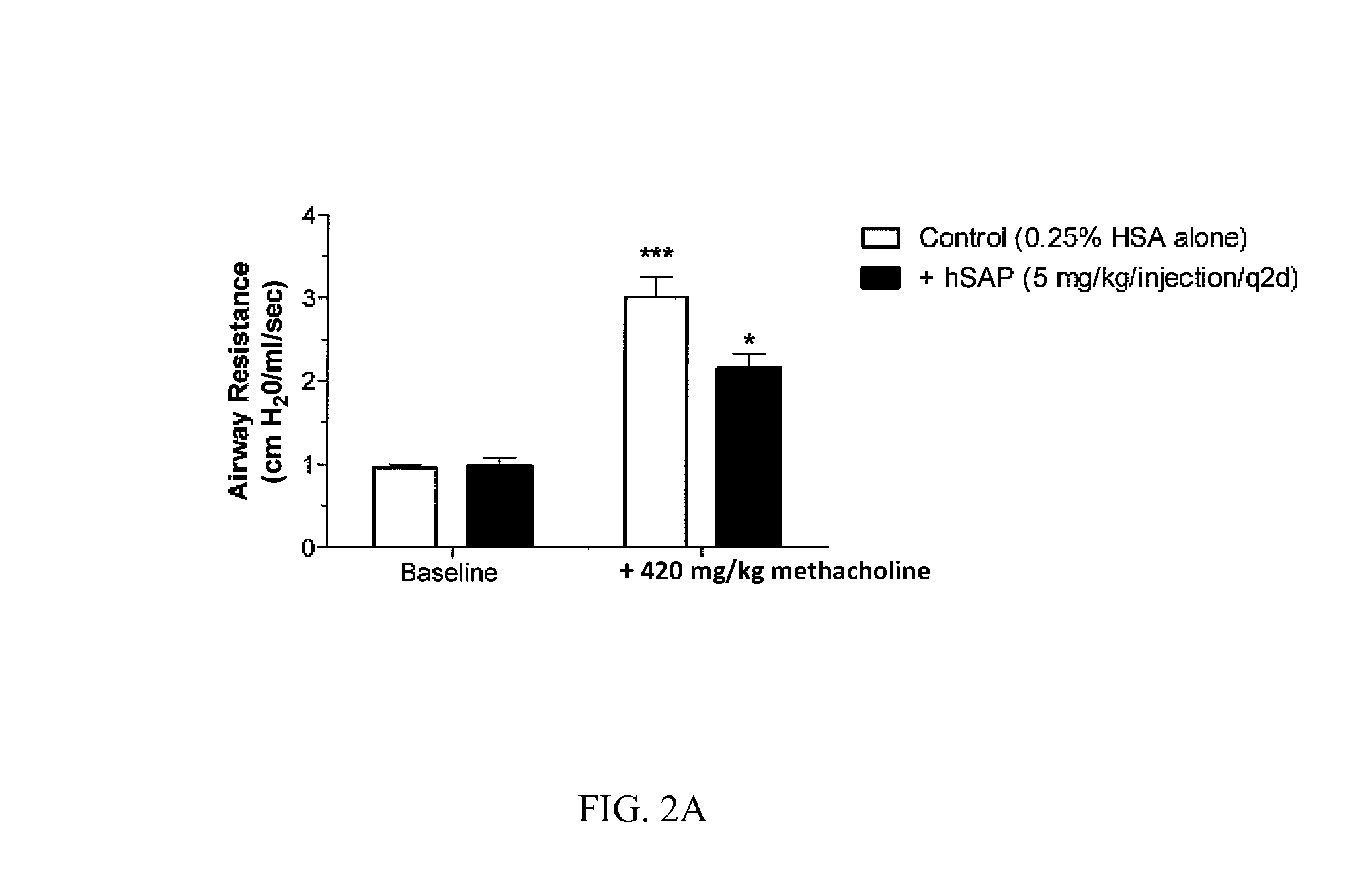
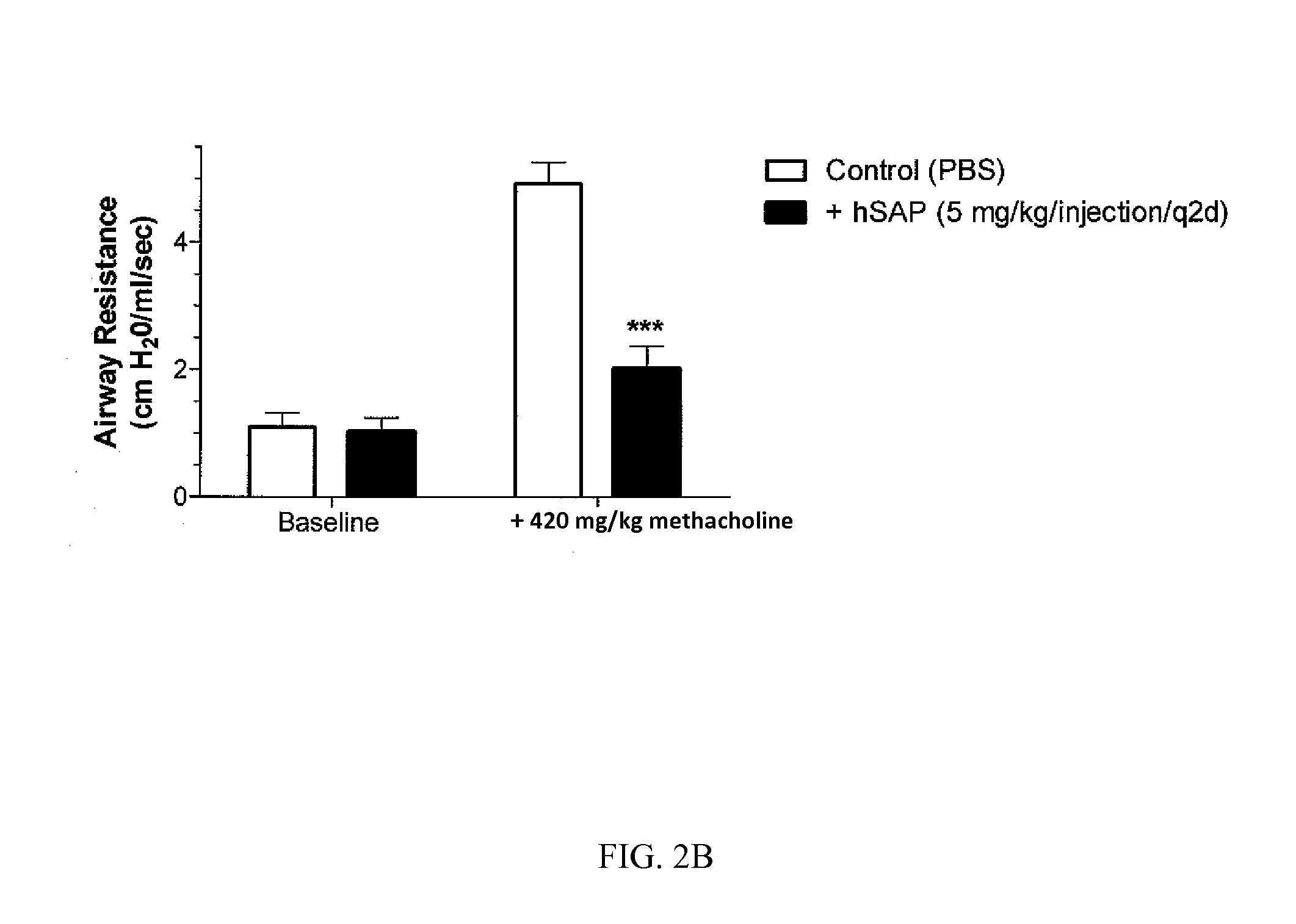
The current standard of care for the treatment of allergic airway diseases include short and long acting beta-agonists, and inhaled or systemic corticosteroids, cromolyn and xanthines that all have the potential of detrimental side-effects. The present invention describes a new mechanistic protein-based therapeutic approach for the treatment of allergic airway disease and diseases associated with excessive Th2 pathology. The present invention relates to the surprising discovery that serum amyloid P (SAP) demonstrates a therapeutic affect in the treatment of hypersensitive disorders.
Owner:PROMEDIOR
Compositions comprising an antimuscarinic and a long-acting beta-agonist
InactiveUS20090181935A1Substantial therapeutic benefitQuick effectBiocideAnimal repellantsDermatologyAirways disease
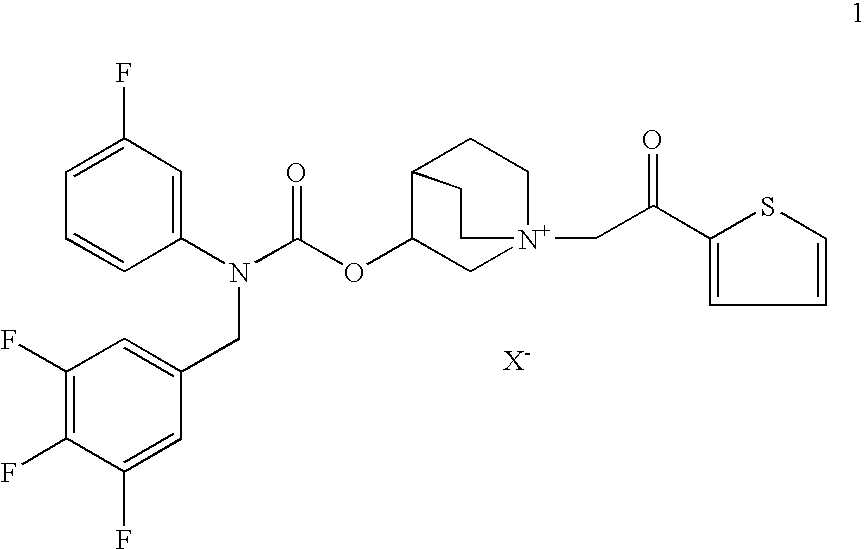
Compositions which comprise a combination of a salt of 3-[[[(3-fluorophenyl)[(3,4,5-trifluoro phenyl)methyl]amino]carbonyl]oxy]-1-[2-oxo-2-(2-thienyl)ethyl]-1-azoniabicyclo [2.2.2]octane, and a long-acting phenylalkylamino beta2-agonist are effective for the prevention and treatment of inflammatory or obstructive airways diseases.
Owner:CHIESI FARM SPA
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
InactiveUS20070293460A1Adequate levelShortness of breathRespiratorsBiocideObstructive Pulmonary DiseasesCombination therapy
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and an aqueous solution comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium. A pharmaceutical composition is also described for the treatment of respiratory conditions and diseases comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer.
Owner:CMPD LICENSING
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and a fluid comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic in a pharmaceutically acceptable vehicle, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium in a an aqueous solution, suspension or emulsion suitable for administration with the nebulizer.
Owner:RICHIES PHARMACY & MEDICAL SUPPLY
Formulations for treatment of adipose tissue, cutaneous tissue and disorders, and muscular tissue
Compositions, formulations, methods, and systems for treating regional fat deposits and fat-related conditions, dermal conditions, and muscular conditions. Methods comprise administering a composition comprising at least one compound that reduces desensitization of beta adrenergic receptors, for example a glucocorticosteroid, and / or at least one long-acting beta-2 adrenergic receptor agonist, for example, formoterol. Compositions to be administered include sustained release formulations comprising at least one long-acting beta-2 adrenergic receptor agonist, at least one compound that reduces desensitization of beta adrenergic receptors, or both in a sustained crystalline microparticle form.
Owner:LITHERA
Methods for administration and formulations for the treatment of regional adipose tissue
InactiveUS20110130373A1Reducing a regional fat depositReducing desensitizationPowder deliveryCosmetic preparationsAdditive ingredientAdrenergic receptor agonists
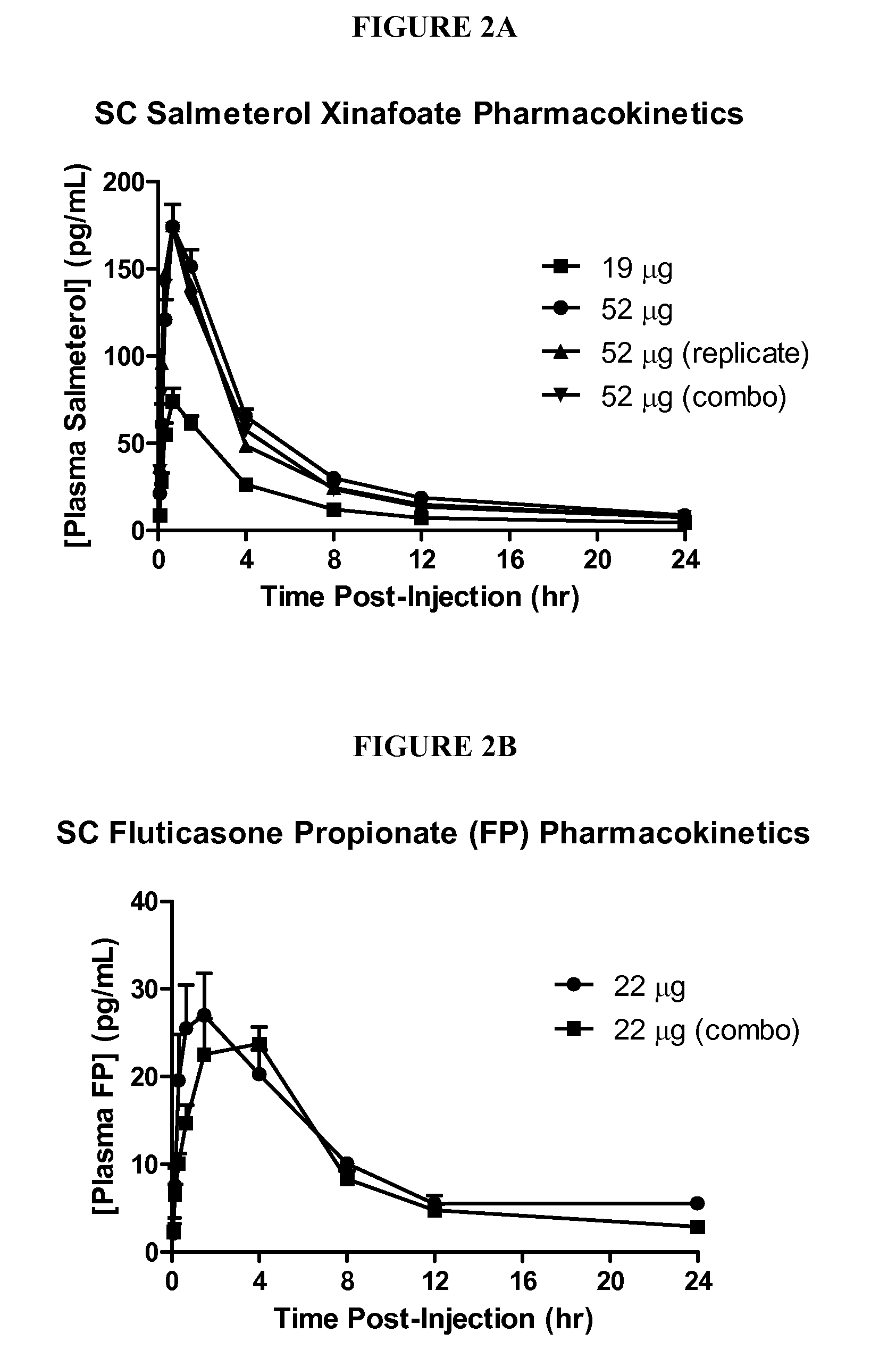
Provided herein are pharmaceutical formulations, methods, and systems for treating regional fat deposits and fat-related conditions and indications. Methods comprise administering a pharmaceutical formulation consisting essentially of a long-acting beta-2 adrenergic receptor agonist, for example, salmeterol, suitable for subcutaneous administration. Methods further comprise administering a pharmaceutical formulation that is suitable for subcutaneous injection comprising: (a) a lipophilic long-acting selective beta-2 adrenergic receptor agonist and / or glucocorticosteroid, or a salt, optical isomer, racemate, solvate, or polymorph thereof; and (b) at least one subcutaneously acceptable inactive ingredient.
Owner:LITHERA
Selective, Lipophilic, and Long-Acting Beta Agonist Monotherapeutic Formulations and Methods for the Cosmetic Treatment of Adiposity and Contour Bulging
InactiveUS20120178819A1Reduction of subcutaneous adiposityReducing regional fat depositCosmetic preparationsBiocideAdrenergicAdditive ingredient
Provided herein are pharmaceutical and cosmetic formulations and methods for regional adiposity reduction and treatment of body contour defects such as abdominal bulging; comprising an injectable formulation, said formulation comprising: an active ingredient that consists essentially of an adipose tissue-reducing amount of one or more lipophilic long-acting selective beta-2 adrenergic receptor agonists, or salts, solvates, or polymorphs thereof; and one or more subcutaneously acceptable inactive ingredients.
Owner:LITHERA
Methods for Administration and Formulations for the Treatment of Regional Adipose Tissue
InactiveUS20120046257A1Reducing a regional fat depositReducing desensitizationPowder deliveryCosmetic preparationsAdditive ingredientAdrenergic receptor agonists
Provided herein are pharmaceutical formulations, methods, and systems for treating regional fat deposits and fat-related conditions and indications. Methods comprise administering a pharmaceutical formulation consisting essentially of a long-acting beta-2 adrenergic receptor agonist, for example, salmeterol, suitable for subcutaneous administration. Methods further comprise administering a pharmaceutical formulation that is suitable for subcutaneous injection comprising: (a) a lipophilic long-acting selective beta-2 adrenergic receptor agonist and / or glucocorticosteroid, or a salt, optical isomer, racemate, solvate, or polymorph thereof; and (b) at least one subcutaneously acceptable inactive ingredient.
Owner:LITHERA
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
Sustained release enhanced lipolytic formulation for regional adipose tissue treatment
InactiveUS7829554B2Reducing desensitizationReduce fatBiocideMetabolism disorderAdrenergicAdrenergic receptor agonists
Compositions, formulations, methods, and systems for treating regional fat deposits comprise contacting a targeted fat deposit with a composition comprising long acting beta-2 adrenergic receptor agonist and a compound that reduces desensitization of the target tissue to the long acting beta-2 adrenergic receptor agonist, for example, glucocorticosteroids and / or ketotifen. Embodiments of the composition are administered, for example, by injection, and / or transdermally.
Owner:LITHERA
Dry powder inhalers comprising a carrier other than lactose
This invention relates to novel pharmaceutical compositions for inhalation comprising separately, sequentially or together, drugs having amine in the form of a dry powder in admixture with a pharmaceutically acceptable carrier, other than lactose, and its use in the treatment of respiratory condition selected from asthma and chronic obstructive pulmonary disease (COPD) and other obstructive airways diseases. In addition, the present invention relates to novel pharmaceutical composition for inhalation based on combinations of long acting muscarinic antagonists, long acting beta agonists, short acting beta-2 agonists, corticosteroids or a combination of two or more of them.
Owner:ARVEN ILAC SANAYI VE TICARET
Dry powder inhalers comprising a carrier other than lactose and a ternary component
This invention relates to novel pharmaceutical compositions for inhalation comprising separately, sequentially or together, drugs having amine in the form of a dry powder in admixture with a pharmaceutically acceptable carrier, other than lactose, and its use in the treatment of respiratory condition selected from asthma and chronic obstructive pulmonary disease (COPD) and other obstructive airways diseases. More particularly, the invention relates to pharmaceutical composition for inhalation further comprising a ternary component. In addition, the present invention relates to novel pharmaceutical composition for inhalation based on combinations of long acting muscarinic antagonists, long acting beta agonists, short acting beta-2 agonists, corticosteroids or a combination of two or more of them.
Owner:ARVEN ILAC SANAYI VE TICARET
Dry powder composition for inhalation and preparation method thereof
ActiveCN109745565AReduce exposureGood chemical stabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsLong acting β agonistsChemical stability
The invention relates to a dry powder composition for inhalation and a preparation method thereof. The composition comprises a long-acting cholinergic receptor blocker, a long-acting beta receptor agonist, a stabilizer and a pharmaceutically acceptable carrier, a portion of the stabilizer forms a coating with the long-acting beta receptor agonist, and the amount of the portion of the stabilizer isthe amount used for the purpose that the long-acting beta receptor agonist is completely coated. The dry powder composition has good chemical stability and can be targeted for pulmonary administration by a dry powder inhalation device.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD +1
Process for preparing a dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic
Dry powder formulations for inhalation containing a combination of an anti-cholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of an inflammatory and / or obstructive airways disease.
Owner:CHIESI FARM SPA
Aerosol containing muscarine receptor antagonist and beta 2 receptor stimulant, and preparation method thereof
InactiveCN106943350AImprove delivery efficiencyStable dose deliveredDispersion deliveryAerosol deliveryVasopressin AntagonistsStimulant
The invention discloses an aerosol containing muscarine receptor antagonist and beta 2 receptor stimulant, and a preparation method thereof. The aerosol contains a propulsive agent, long-acting beta 2 receptor stimulant particles, and long-acting muscarine antagonist particles; in LABA particles, by volume, the geometric particle size of 90% particles is smaller than 10<mu>m, and the geometric particle size of 50% particles is smaller than 4<mu>m or even smaller; the middle particle size of LAMA particles ranges from 500nm to 10<mu>m; the LAMA particles are particles composed of LAMA, calcium chloride, and a surfactant; or the LAMA particles are particles composed of LAMA and an excipient. 1 to 100<mu>g of the aerosol is sprayed in each time of pressing of a MDI (metered dose inhaler), and the amount of LABA or LAMA in the aerosol ranges from 0.02 to 2mg / mL. The advantages of the aerosol are that: delivery efficiency is relatively high, and the delivery dosage is stable in long term of storing.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Preparation method of inhalation dry powder composition
InactiveCN109745564AStrong stabilityHigh aerodynamic fine particle fractionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMedicineInhalation
The invention relates to a preparation method of an inhalation dry powder composition. The method comprises the following steps that 1, a lubricant and a medicinal carrier are subjected to first shearmixing to obtain a carrier compound; 2, the carrier compound in the step 1, a long-acting cholinergic receptor blocking agent and a long-acting beta receptor stimulant are subjected to second shear mixing, so that the inhalation dry powder composition is obtained.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
Inhalable aerosol, raw material composition thereof and preparation methods thereof
ActiveCN107243080ASingle dose adjustmentUniform particle distributionOrganic active ingredientsDispersion deliveryMedicineActive component
The invention discloses an inhalable aerosol, a raw material composition thereof and preparation methods thereof. The raw material composition of the inhalable aerosol, disclosed by the invention, contains a medicinal active component and a propellant, wherein the medicinal active component contains a long-acting beta-receptor agonist and glucocorticoid; the propellant comprises tetrafluoroethane. The inhalable aerosol disclosed by the invention contains the raw material composition, a pressure container and a valve system. The inhalable aerosol uses the tetrafluoroethane as the propellant; during preparation, medicine particles are prepared by a supercritical method and a spray drying method, so that the inhalable aerosol with uniform particle distribution can be obtained; in the preparation method, the loss is low; in addition, the single dose of the inhalable aerosol is adjusted, so that the greenhouse gas amount of a product is effectively reduced.
Owner:SHANGHAI SINE PHARMA LAB
Treatment methods for hypersensitive disorders
The current standard of care for the treatment of allergic airway diseases include short and long acting beta-agonists, and inhaled or systemic corticosteroids, cromolyn and xanthines that all have the potential of detrimental side-effects. The present invention describes a new mechanistic protein-based therapeutic approach for the treatment of allergic airway disease and diseases associated with excessive Th2 pathology. The present invention relates to the surprising discovery that serum amyloid P (SAP) demonstrates a therapeutic affect in the treatment of hypersensitive disorders.
Owner:PROMEDIOR
Process for preparing a dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic
Dry powder formulations for inhalation containing a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of an inflammatory and / or obstructive airways disease.
Owner:CHIESI FARM SPA
Process for preparing a dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic
ActiveUS10350164B2Powder deliveryPharmaceutical non-active ingredientsAdrenergicAnticholinergic agents
A process for preparing a dry powder formulation for inhalation comprising a combination of an anti-cholinergic, a long-acting beta2-adrenoceptor agonist, and, optionally, an inhaled corticosteroid, is provided.
Owner:CHIESI FARM SPA
Olodaterol hydrochloride crystalline form B and preparation method thereof
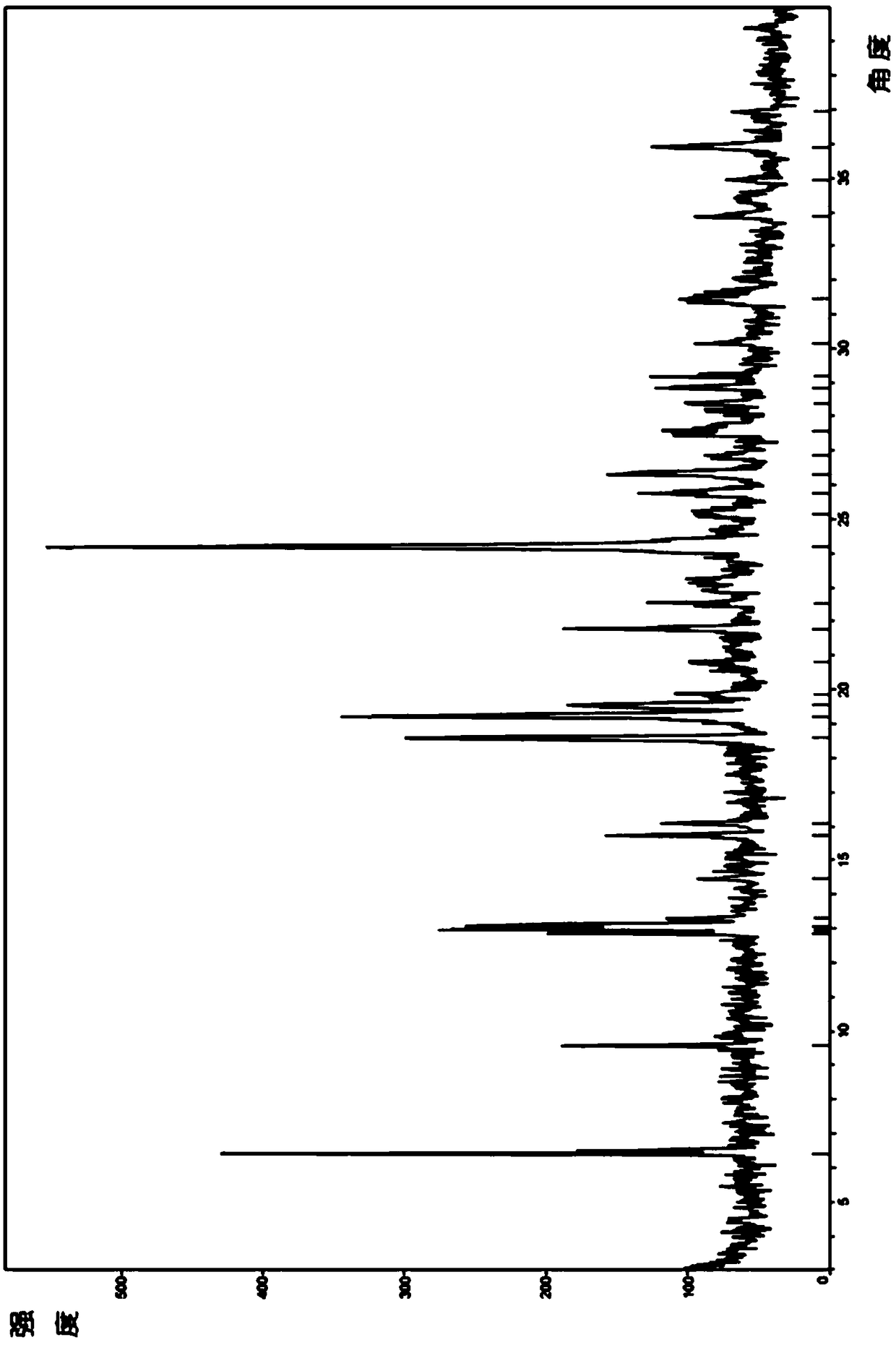
PendingCN108997248ANot easy to absorb moistureImprove stabilityOrganic active ingredientsOrganic chemistry methodsAdrenergic receptor agonistsAgonist drugs
The invention relates to a crystalline form B of a long-acting beta 2 adrenergic agonist drug olodaterol hydrochloride and a preparation method thereof, and a pharmaceutical composition containing thecrystalline form B, wherein the crystalline form is characterized by an X-ray diffraction pattern characteristic absorption peak thereof. Compared with the prior art, the crystalline form B of olodaterol hydrochloride provided by the invention is not easy to absorb moisture, remarkably improves stability and is convenient for product quality control; the preparation process is simple, which is beneficial to the cost control in industrial production and has high economic value.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
Dry powder inhalers comprising a carrier other than lactose and a ternary component
This invention relates to novel pharmaceutical compositions for inhalation comprising separately, sequentially or together, drugs having amine in the form of a dry powder in admixture with a pharmaceutically acceptable carrier, other than lactose, and its use in the treatment of respiratory condition selected from asthma and chronic obstructive pulmonary disease (COPD) and other obstructive airways diseases. More particulary, the invention relates to pharmaceutical composition for inhalation further comprising a ternary component. In addition, the present invention relates to novel pharmaceutical composition for inhalation based on combinations of long acting muscarinic antagonists, long acting beta agonists, short acting beta-2 agonists, corticosteroids or a combination of two or more of them.
Owner:ARVEN ILAC SANAYI VE TICARET ANONIM SIRKETI
Process for preparing a dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and, optionally, an inhaled corticosteroid are useful for the prevention and / or treatment of an inflammatory and / or obstructive airways disease.
Owner:CHIESI FARM SPA
Selective, lipophilic, and long-acting beta agonist monotherapeutic formulations and methods for the cosmetic treatment of adiposity and contour bulging
InactiveUS9597531B2Reduce obesityBiocideCosmetic preparationsLong acting β agonistsBULK ACTIVE INGREDIENT
Provided herein are pharmaceutical and cosmetic formulations and methods for regional adiposity reduction and treatment of body contour defects such as abdominal bulging; comprising an injectable formulation, said formulation comprising: an active ingredient that consists essentially of an adipose tissue-reducing amount of one or more lipophilic long-acting selective beta-2 adrenergic receptor agonists, or salts, solvates, or polymorphs thereof; and one or more subcutaneously acceptable inactive ingredients.
Owner:LITHERA
Process for preparing a dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic
ActiveUS20180325816A1Powder deliveryPharmaceutical non-active ingredientsAnticholinergic agentsAdrenergic
A process for preparing a dry powder formulation for inhalation comprising a combination of an anti-cholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid is provided.
Owner:CHIESI FARM SPA
Methods for Identifying a Patient as a Candidate for Treatment with a Long Acting Beta Agonist and for Predicting a Patient's Response to Long Acting Beta2 Agonist Therapy by Analysing Polymorphisms in the Beta2-Adrenergic Receptor Gene
InactiveUS20080176224A1Reduce reversibilityBetter assessmentSugar derivativesMicrobiological testing/measurementObstructive airway diseaseHaplotype
A method for identifying a patient as a candidate for treatment with a long acting beta agonist comprises isolating a biological sample from a patient and identifying the presence or absence of at least one haplotype C. The presence of at least one haplotype C in a patient sample indicates that the patient is a good candidate for treatment. For example, the patient may have a respiratory disease such as an obstructive airway disease.
Owner:ASTRAZENECA AB
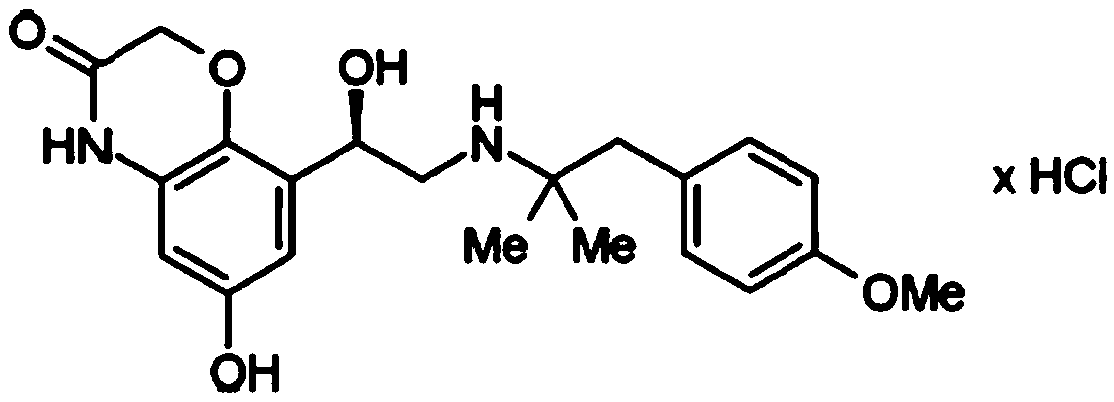
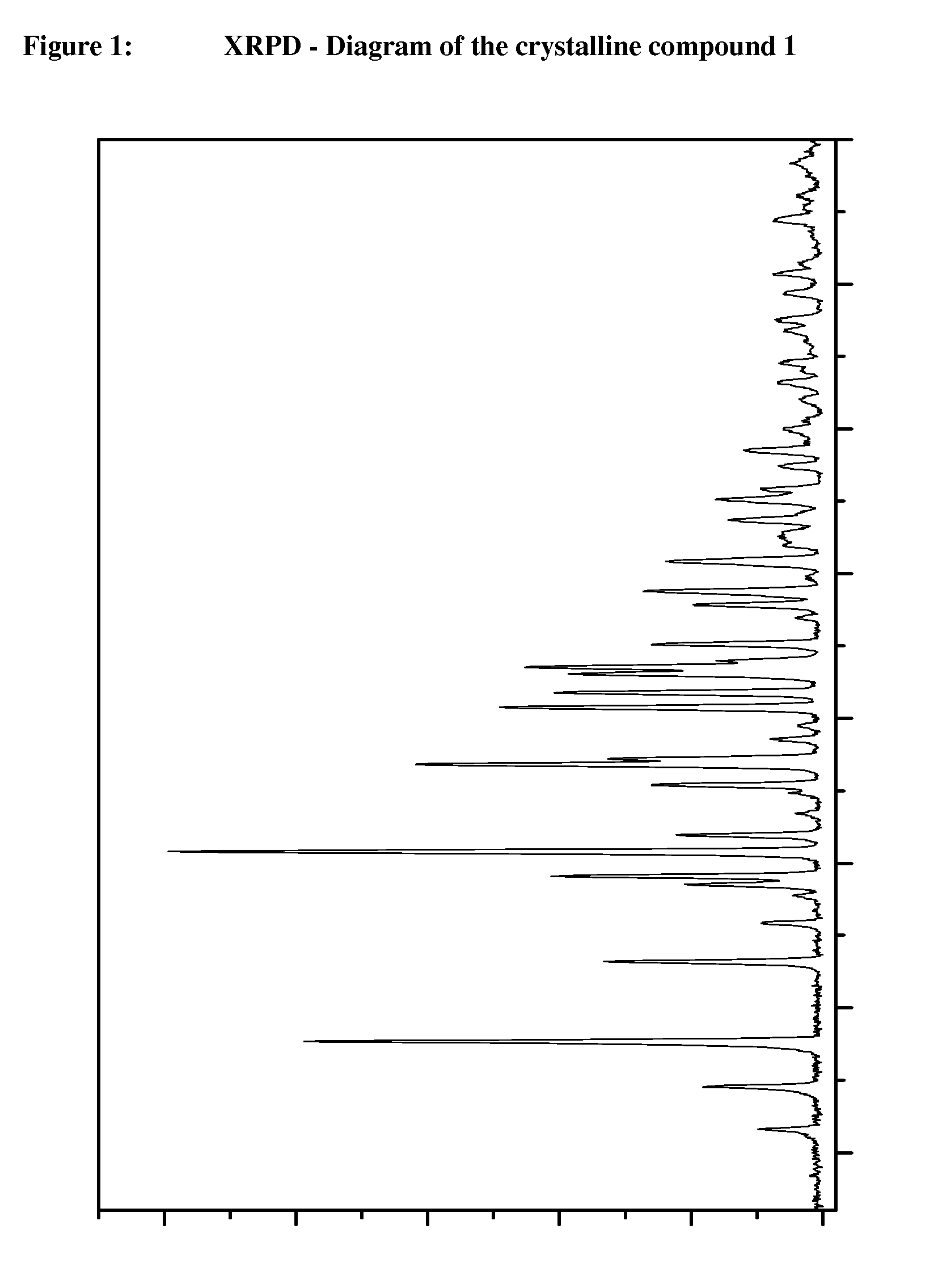
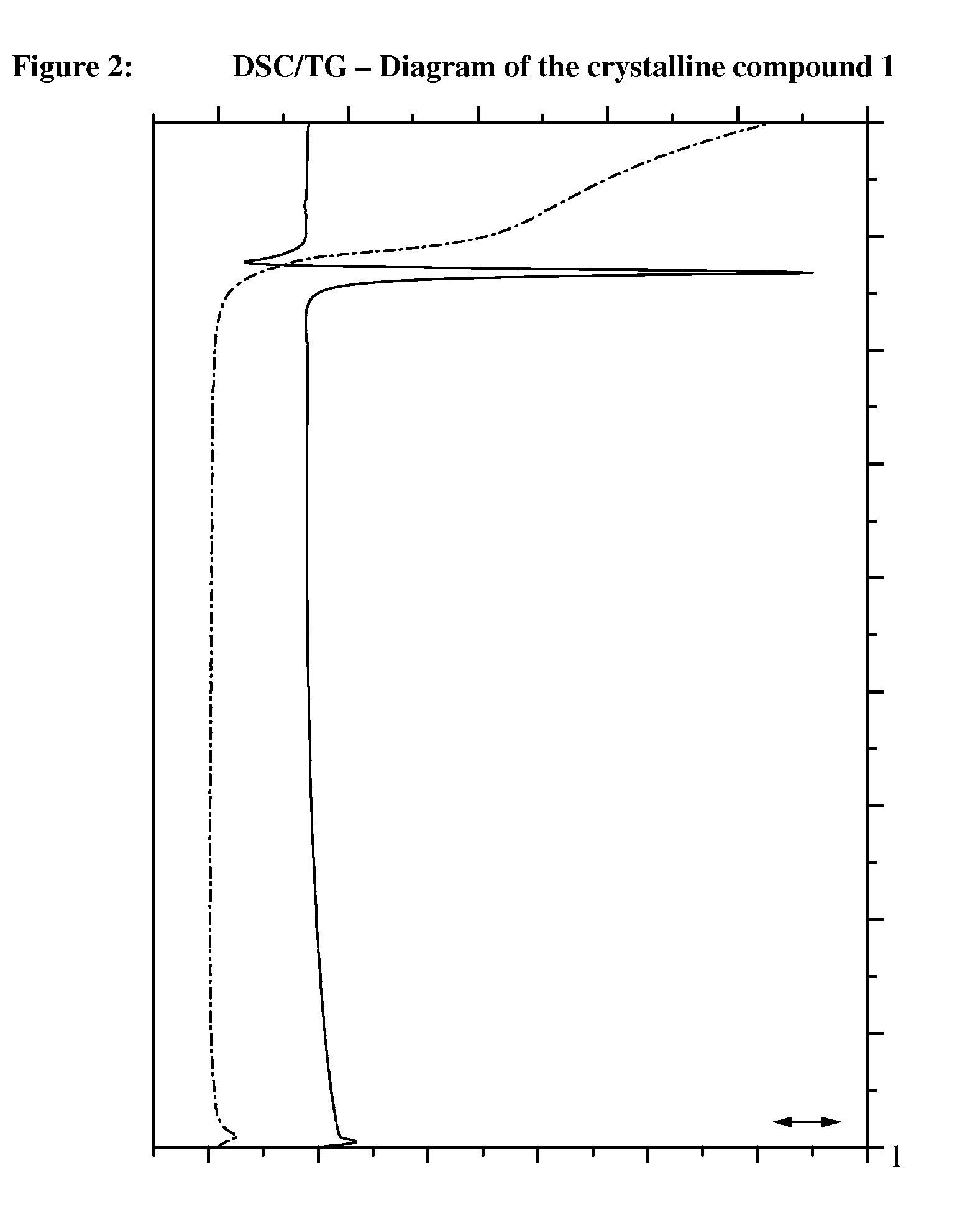
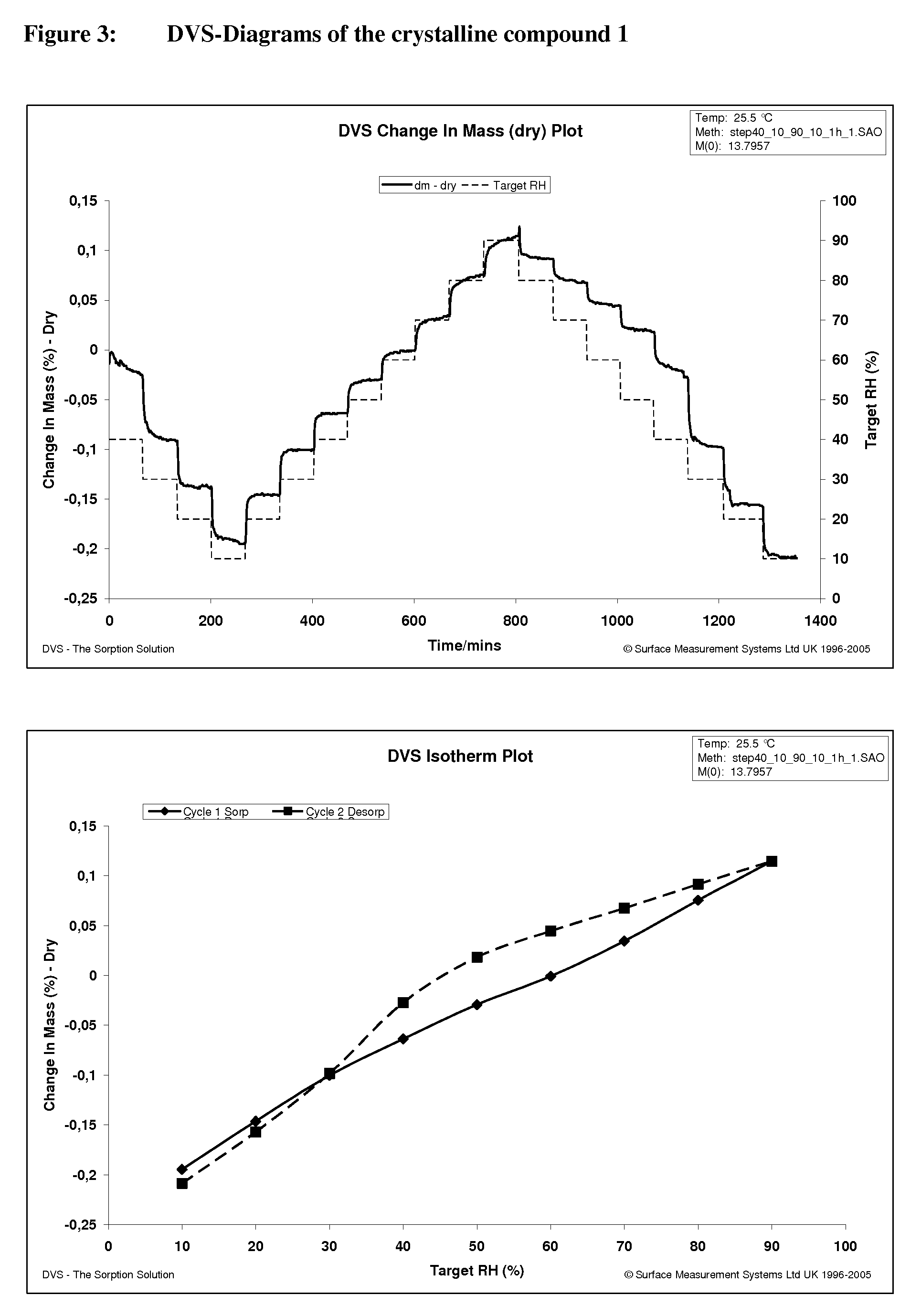
Crystalline, enantiomerically pure salt form of a beta-agonist, and the use thereof as a drug
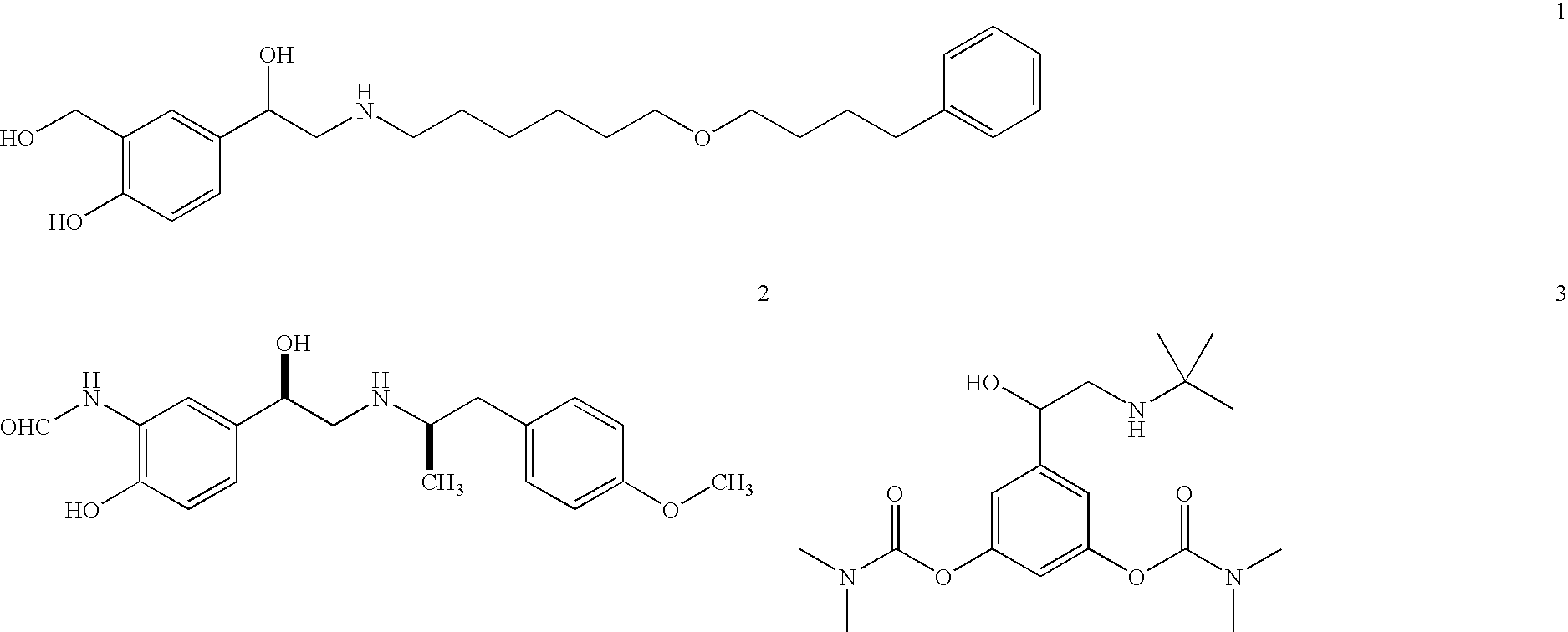
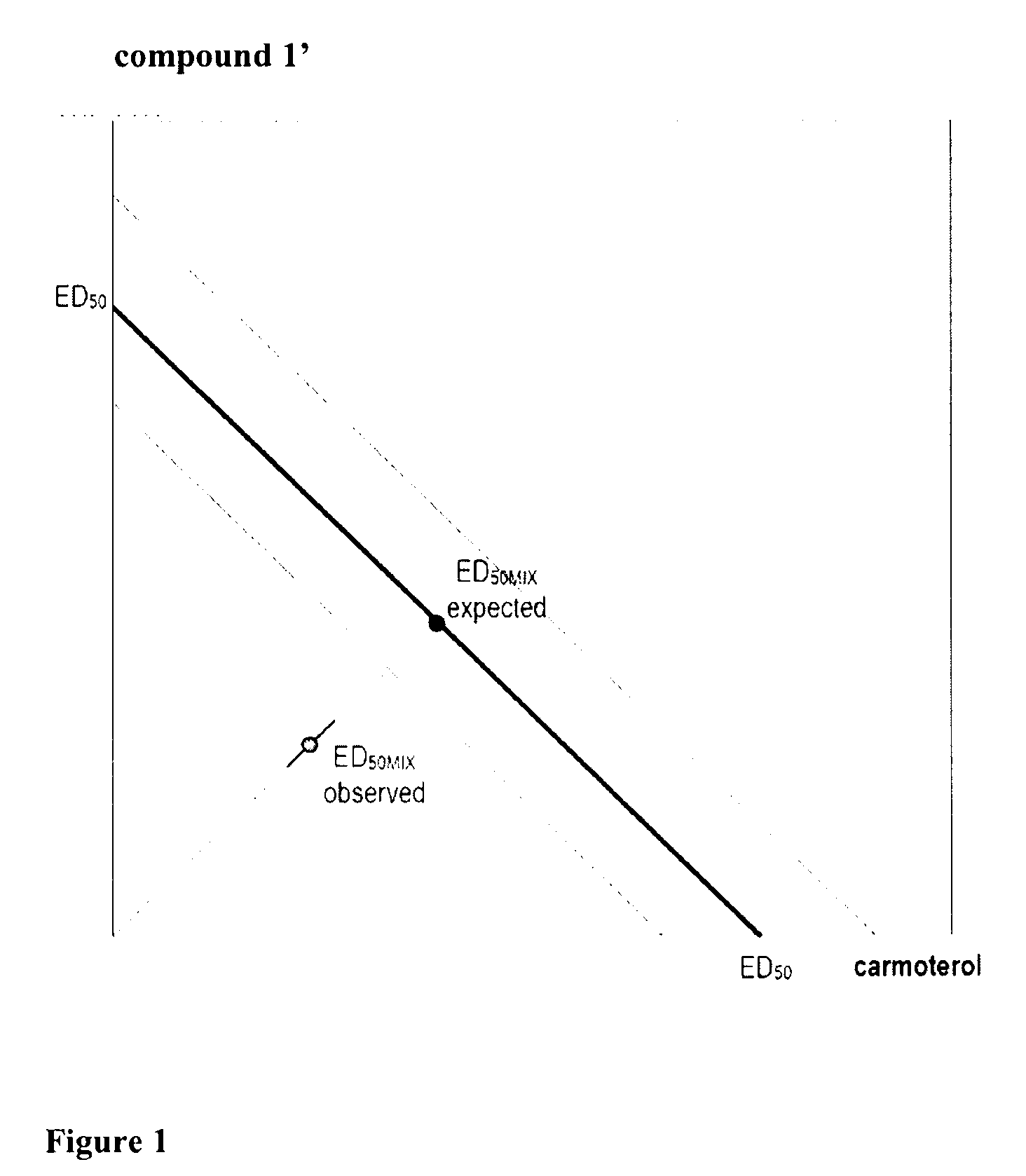
This invention concerns a crystalline, enantiopure hydrochloride salt of N-(5-{2-[3-(4,4-diethyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-1,1-dimethyl-propylamino-1-hydroxy-ethyl}-2-hydroxy-phenyl)-methanesulfonamide, preferably of N-(5-{(R)-2-[3-(4,4-diethyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-1,1-dimethyl-propylamino-1-hydroxy-ethyl}-2-hydroxy-phenyl)-methanesulfonamide and its action as a long acting beta-agonist, alone or in combination with one or multiple other active ingredients for the treatment of respiratory diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Compositions and formulations for the treatment of thyroid eye disease
InactiveCN101626759BOrganic active ingredientsMetabolism disorderDiseaseAdrenergic receptor agonists
Compositions, formulations, methods, and systems for treating regional fat deposits and fat-related conditions, dermal conditions, and muscular conditions. Methods comprise administering a composition comprising at least one compound that reduces desensitization of beta adrenergic receptors, for example a glucocorticosteroid, and / or at least one long-acting beta-2 adrenergic receptor agonist, for example, formoterol. Compositions to be administered include sustained release formulations comprising at least one long-acting beta-2 adrenergic receptor agonist, at least one compound that reduces desensitization of beta adrenergic receptors, or both in a sustained crystalline microparticle form.
Owner:尼奥特蒂克斯公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com