Compositions and formulations for the treatment of thyroid eye disease
A pharmaceutical preparation, technology of use, applied in the field of compositions and preparations for the treatment of thyroid eye disease, capable of solving problems such as loss of vision and diplopia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
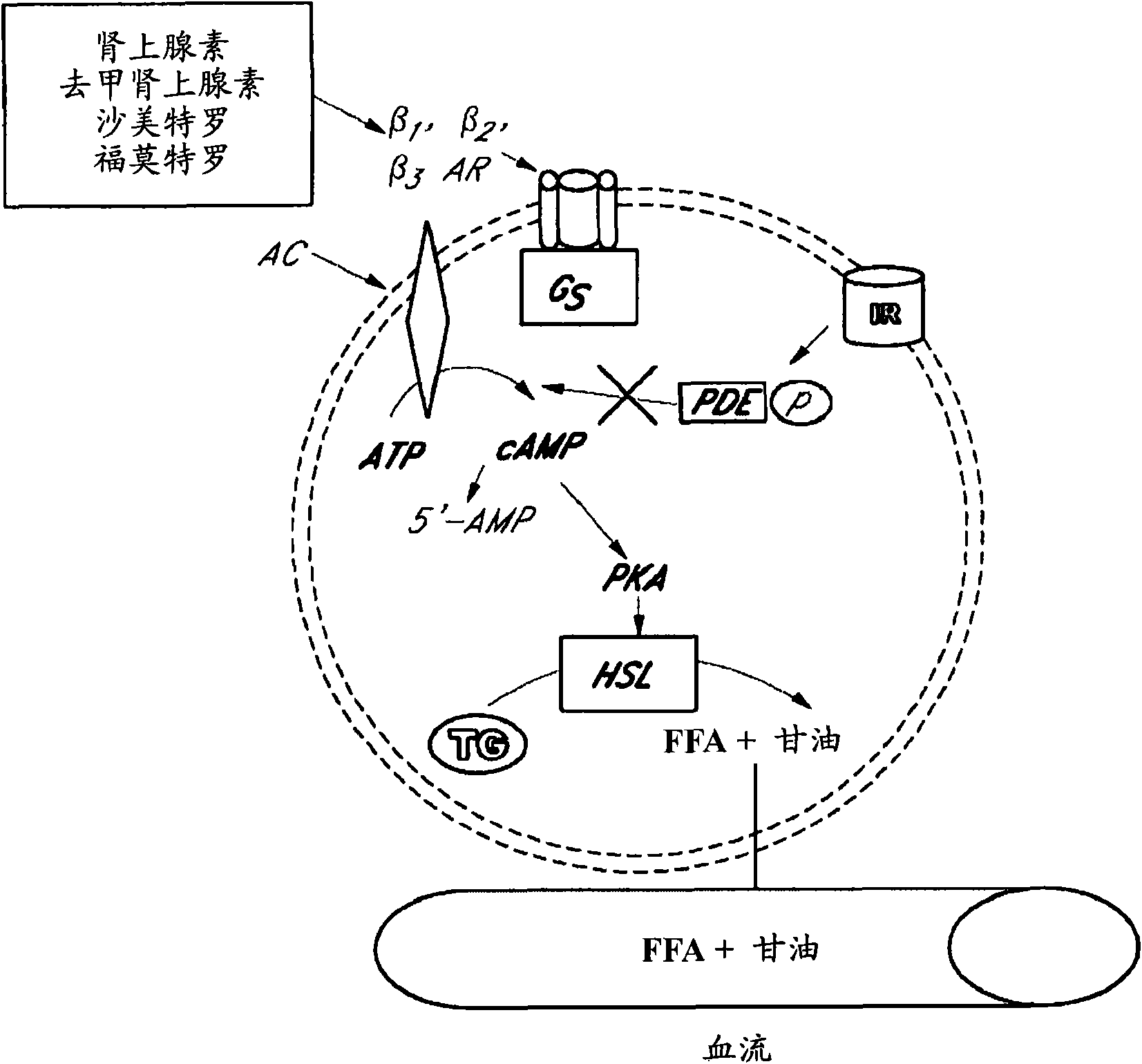
[0110] Example 1: In vitro lipolysis experiment of rat adipocytes with β agonists and glucocorticoids
[0111] Glycerol was detected spectrophotometrically in cell culture media following chemical oxidation with hydrogen peroxide in bulk in vitro lipolysis experiments. Glycerol was assayed within 3 hours. Lipolysis levels were measured in cultured human adipocytes following one or more pre-incubation periods of exposure to beta agonists alone, glucocorticosteroids alone, or a combination of both, see below for more details . Preadipocytes are isolated and differentiated into adipocytes:
[0112] Human subcutaneous adipocytes were used in lipolysis experiments. Adipose tissue was collected by liposuction or lipectomy and preadipocytes were isolated as follows. Briefly, adipose tissue was minced and placed in a shaking chamber enriched with oxygen (5% CO 2 ; 75 times / min), incubate at 37°C for 1 hour in Krebs-Ringer bicarbonate buffer containing 1% bovine serum albumin an...
Embodiment 2
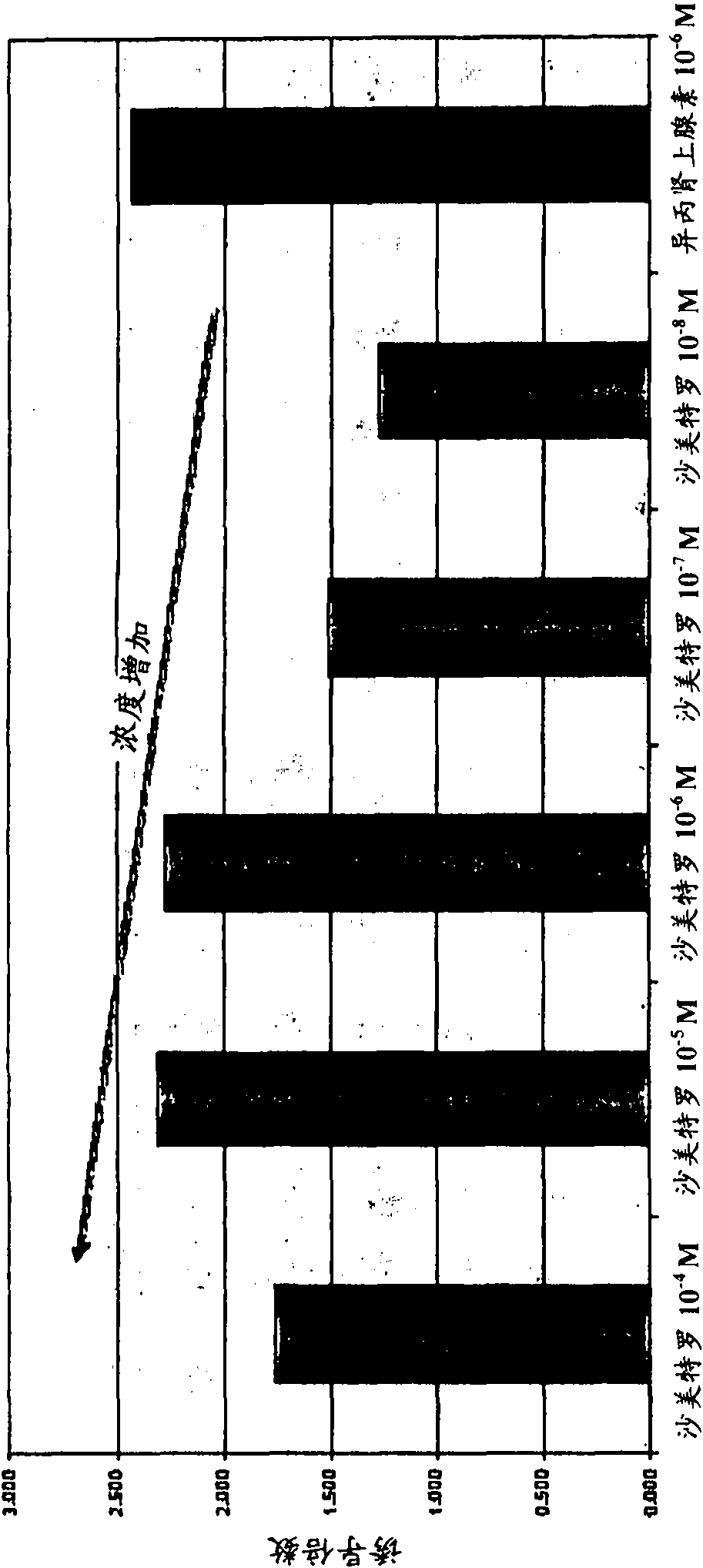
[0120] Example 2: Inhibition of lipogenesis by beta agonists and glucocorticoids
[0121] Non-limiting examples of such adipogenesis inhibition are as follows: Cell culture: 3T3-L1 preadipocyte cell line (ATCC, Manassas, VA) was divided into 4×10 5 Cells / T75ml flasks were inoculated into Dulbecco's Modified Eagle Medium (DMEM) containing 10% normal bovine serum and 1% penicillin / streptomycin antibiotics. Cells were incubated at 37°C, 5% CO 2 incubate. After 3 days, the cells were detached with trypsin, counted, and resuspended in a medium containing 6 × 10 5 Cells / well in 2 ml of medium in a 24-well plate. After 1-2 days, the cells are close to confluence and ready to initiate adipogenesis. Lipogenetic material: lipogenesis priming medium : DMEM / 10% fetal bovine serum / 0.5mM IBMX / 1μM dexamethasone adipogenesis medium : DMEM / 10% fetal bovine serum / 10μg / ml insulin lipogenesis maintenance medium : DMEM / 10% fetal bovine serum Negative Control Medium : DMEM / 10% normal ...
Embodiment 3
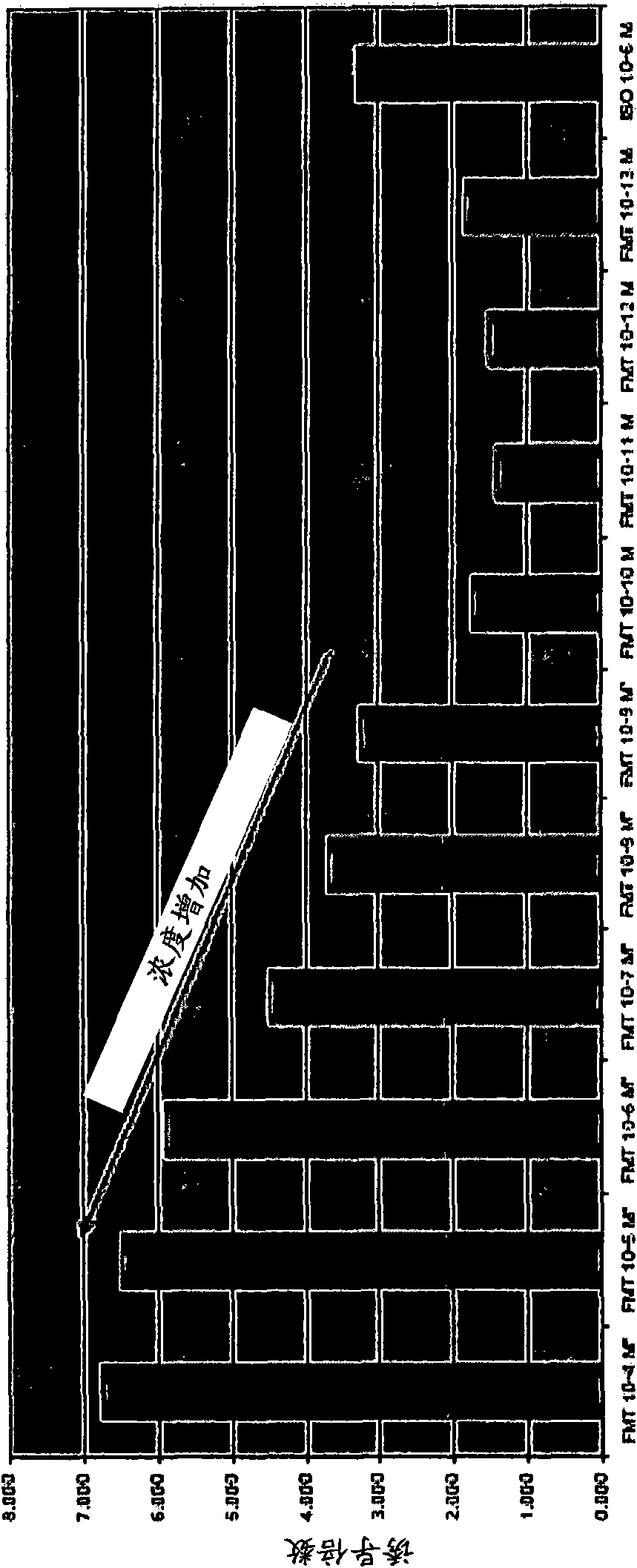
[0122] Example 3: Beta-2 Agonist Combination with Glucocorticosteroids Reduces Epididymal Fat Pad Mass
[0123] We sought to determine whether glucocorticosteroids reduce fat in vivo in a manner consistent with the in vitro lipolysis data described in Example 1. To this end, we measured epididymal fat pad mass in rats treated with the long-acting β-2 adrenergic agonist formoterol alone and in combination with budesonide.
[0124] Male Sprague Dawley rats (-500 g) were anesthetized in 4% isoflurane using a Matrx 3000 vaporizer. Then, inject 0.4 ml of vehicle (2% PEG) at the front 5 mm of the rear end of the fat pad to the animals listed in Table 2 below; formoterol in vehicle (3.48 μg / ml; dose=1.39 μg); or Formoterol (3.48 μg / ml) plus budesonide (10 μg / ml; dose = 1.39 μg formoterol, 4 μg budesonide). Each animal received drug treatment on one side and vehicle (2% PEG) on the opposite side; groups were balanced for drug and vehicle (see Table 2).
[0125] Injections were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com