Dry powder composition for inhalation and preparation method thereof
A composition and dry powder technology, which is applied in the directions of pharmaceutical combinations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. Avoid stability degradation, extend shelf life, and have good chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of micronized tiotropium bromide
[0035] Tiotropium bromide monohydrate and tiotropium bromide anhydrate are micronized respectively in a jet mill, and the pulverization pressure is 0.6Mpa, and the resulting product particle diameter is D 50 Reach below 5μm.
Embodiment 2
[0036] Embodiment 2: Preparation of micronized formoterol fumarate
[0037] Formoterol fumarate dihydrate and formoterol fumarate were micronized in a jet mill respectively, the crushing pressure was 0.6Mpa, and the particle size of the obtained product was D 50 Reach below 5μm.
Embodiment 3
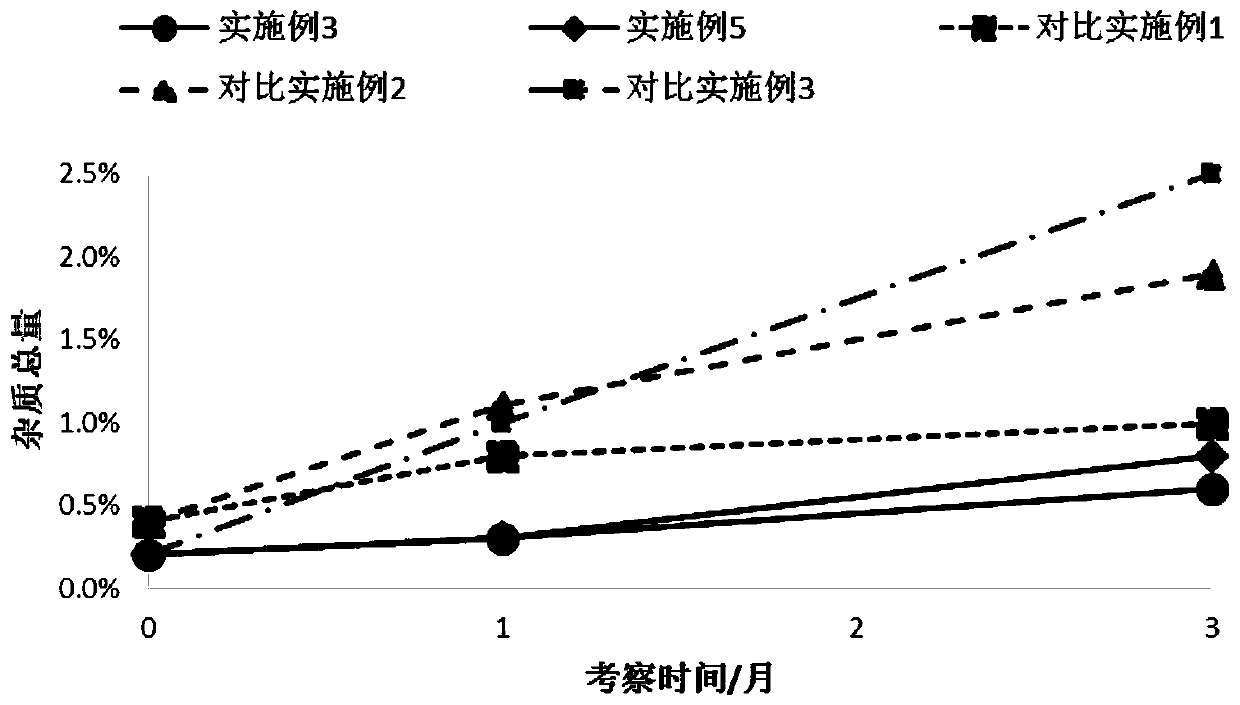
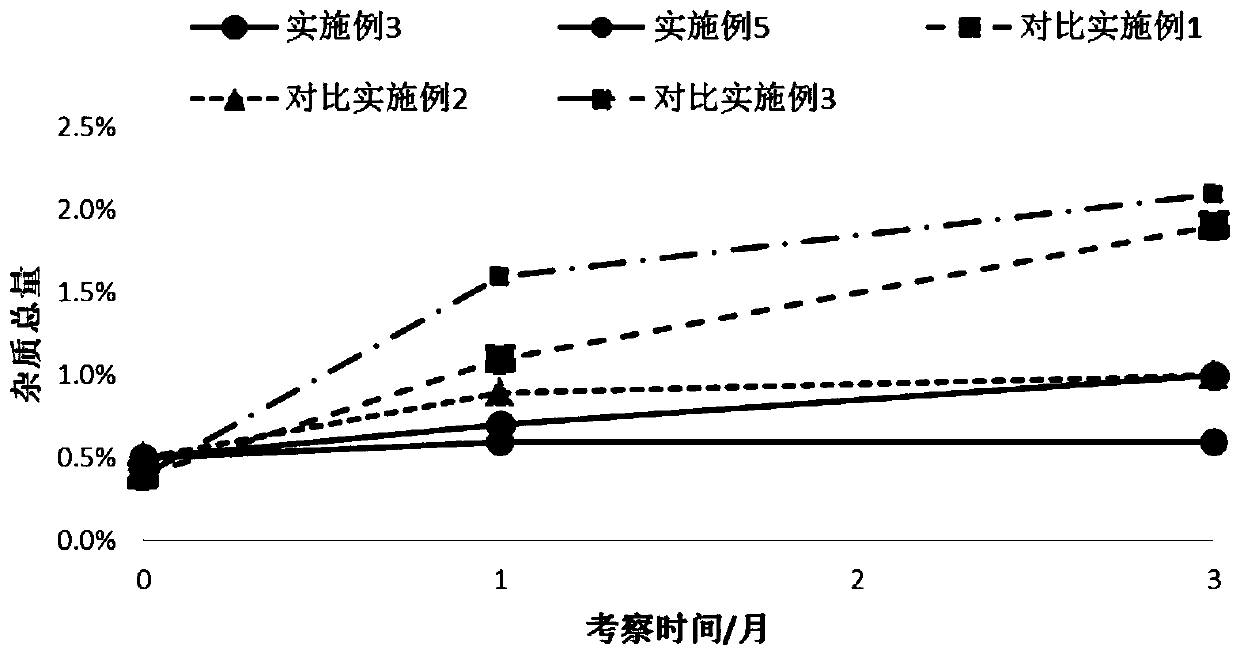
[0038] Embodiment 3: take lactose monohydrate as carrier, magnesium stearate as stabilizer, tiotropium bromide formoterol for inhalation Dry powder preparation
[0039] Take by weighing the magnesium stearate of recipe quantity 1 / 10 and the micronized formoterol dihydrate that makes in the embodiment 2 and stir and mix 2min according to the table, transfer in the planetary ball mill, set the sun gear rotating speed 500r / min mixed for 10min, allowed to stand properly, and prepared powder compound (D 50 =2.23 μm), then add the micronized tiotropium bromide monohydrate (D 50 = 2.65 μm), lactose monohydrate and the remaining magnesium stearate of the prescription amount were mixed in a high-speed mixer for 2 min, and the linear speed of the stirring paddle was 10 m / s.
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com