Preparation method of inhalation dry powder composition
A technology of composition and dry powder, which is applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve problems such as hydrolysis instability, high industrial production costs, and reduced interparticle forces , to achieve the effects of excellent inhalation characteristics, strong stability of formulation characteristics, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of micronized tiotropium bromide
[0033] Tiotropium bromide monohydrate and tiotropium bromide anhydrate are micronized respectively in a jet mill, and the pulverization pressure is 0.6Mpa, and the resulting product particle diameter is D 50 Reach below 5μm.
Embodiment 2
[0034] Embodiment 2: Preparation of micronized formoterol fumarate
[0035] Formoterol fumarate dihydrate and formoterol fumarate were micronized in a jet mill respectively, the crushing pressure was 0.6Mpa, and the particle size of the obtained product was D 50 Reach below 5μm.
Embodiment 3
[0036] Embodiment 3: take lactose monohydrate as pharmaceutical carrier, magnesium stearate as lubricant, tiotropium bromide formol for inhalation Tero dry powder preparation
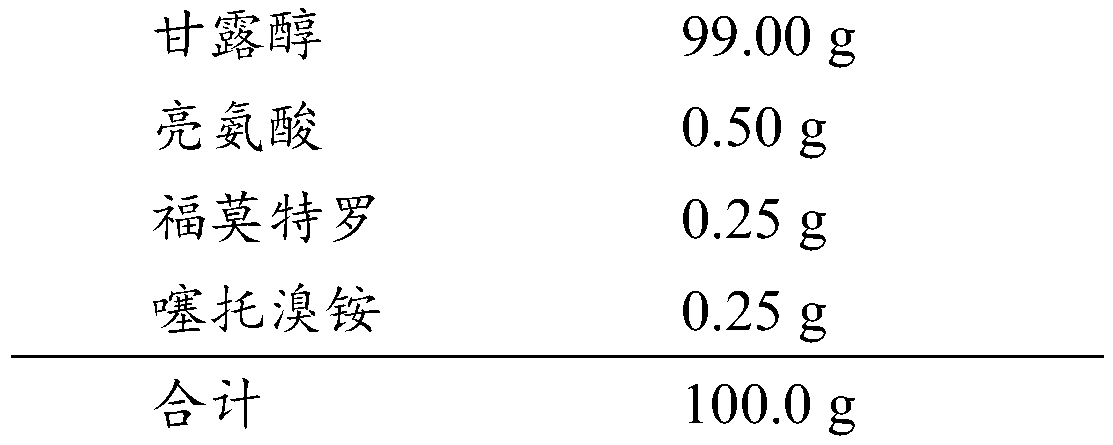
[0037] Weigh lactose monohydrate and magnesium stearate (D 50= 3.01 μm) mixed in a three-dimensional mixer for 10min, transferred to a high-speed mixer, mixed for 10min with a cutter linear speed of 10m / s, and left to stand properly to prepare a carrier compound, and then added the micronized thiamine prepared in Example 1 Trotropium bromide monohydrate and the formoterol fumarate dihydrate prepared in Example 2 were mixed in a high-speed mixer for 10 minutes at a cutter speed of 10 m / s, and finally mixed in a three-dimensional mixer for 20 minutes.
[0038]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| D50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com