Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
161 results about "Fibrous ring" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
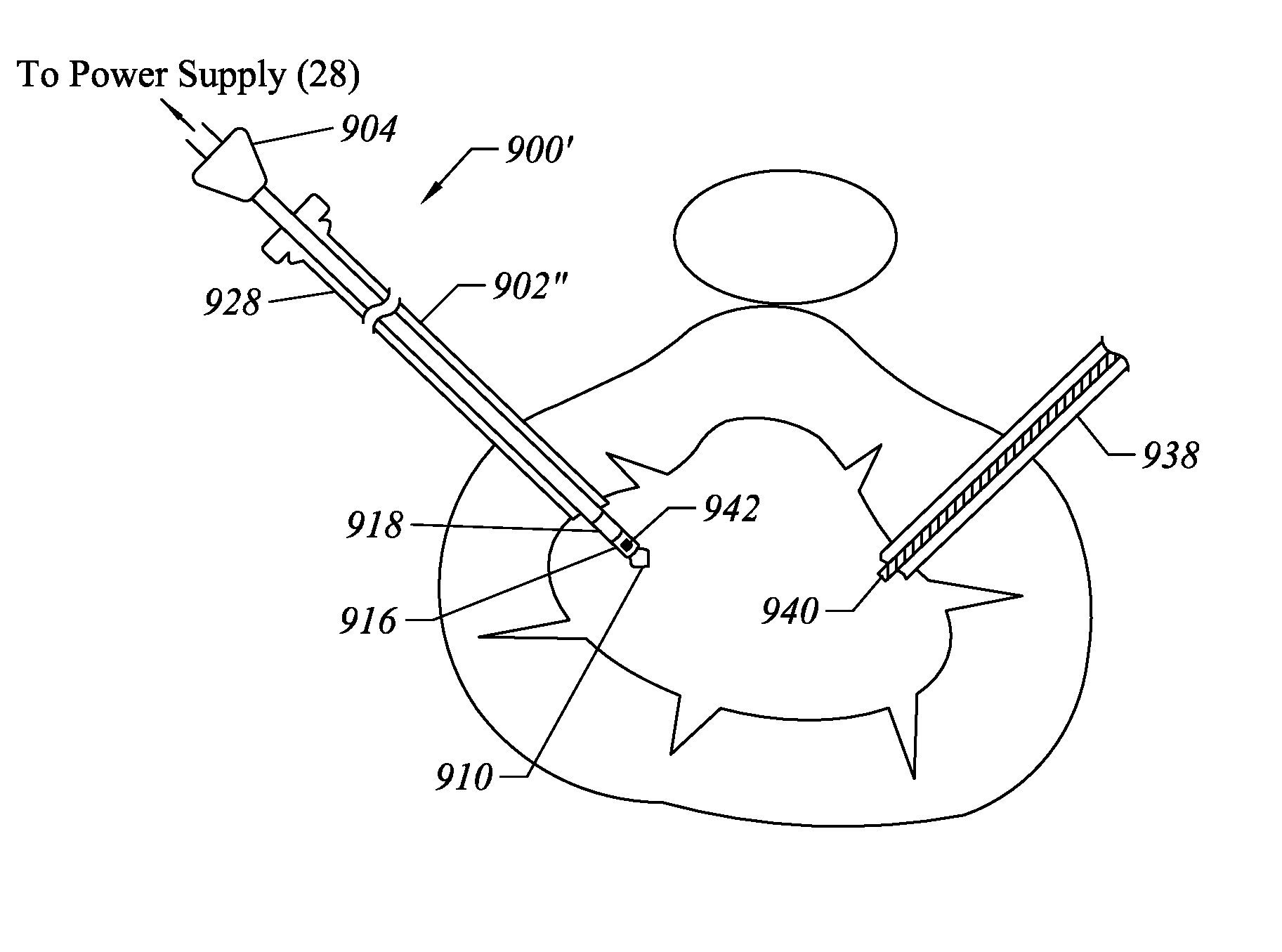
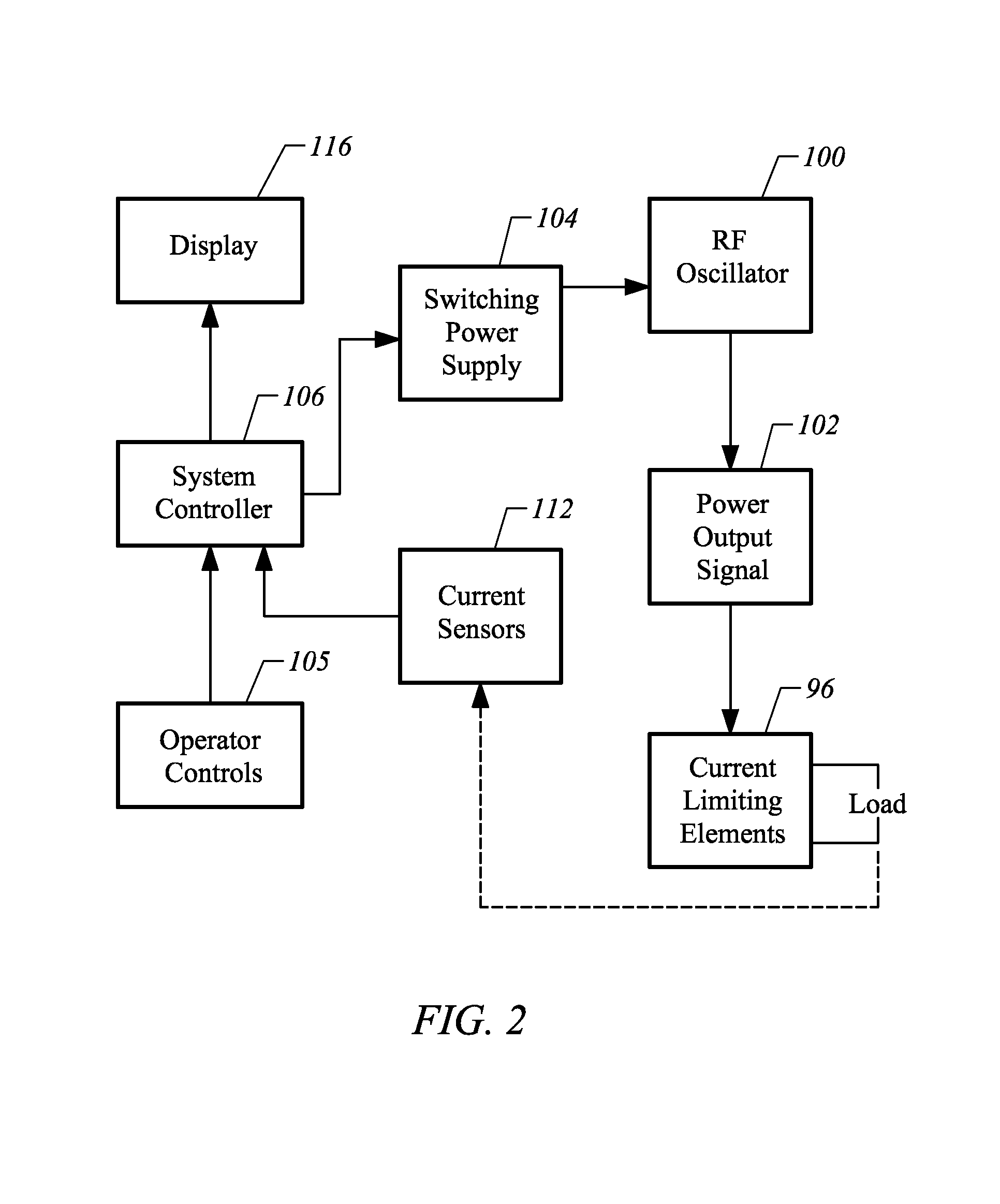
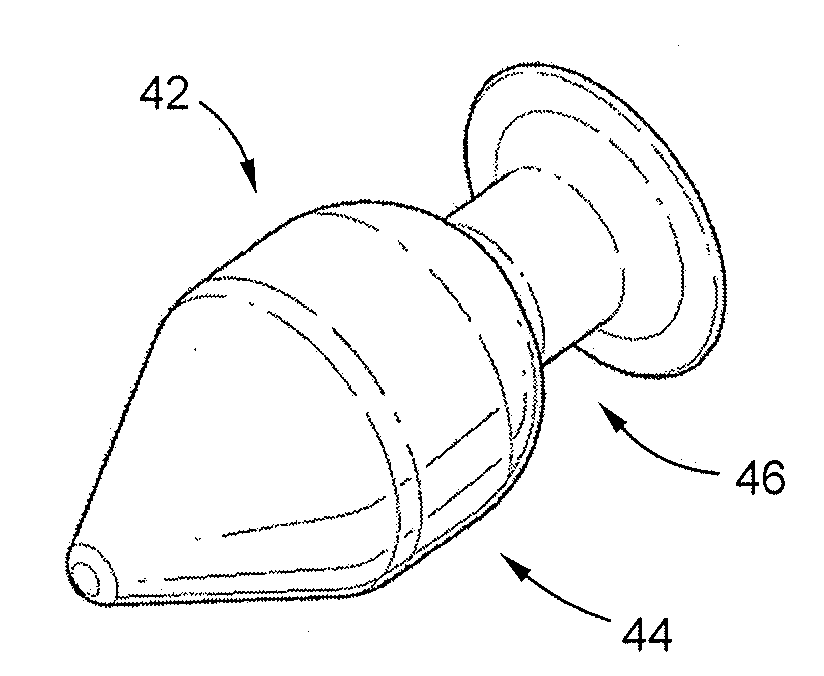
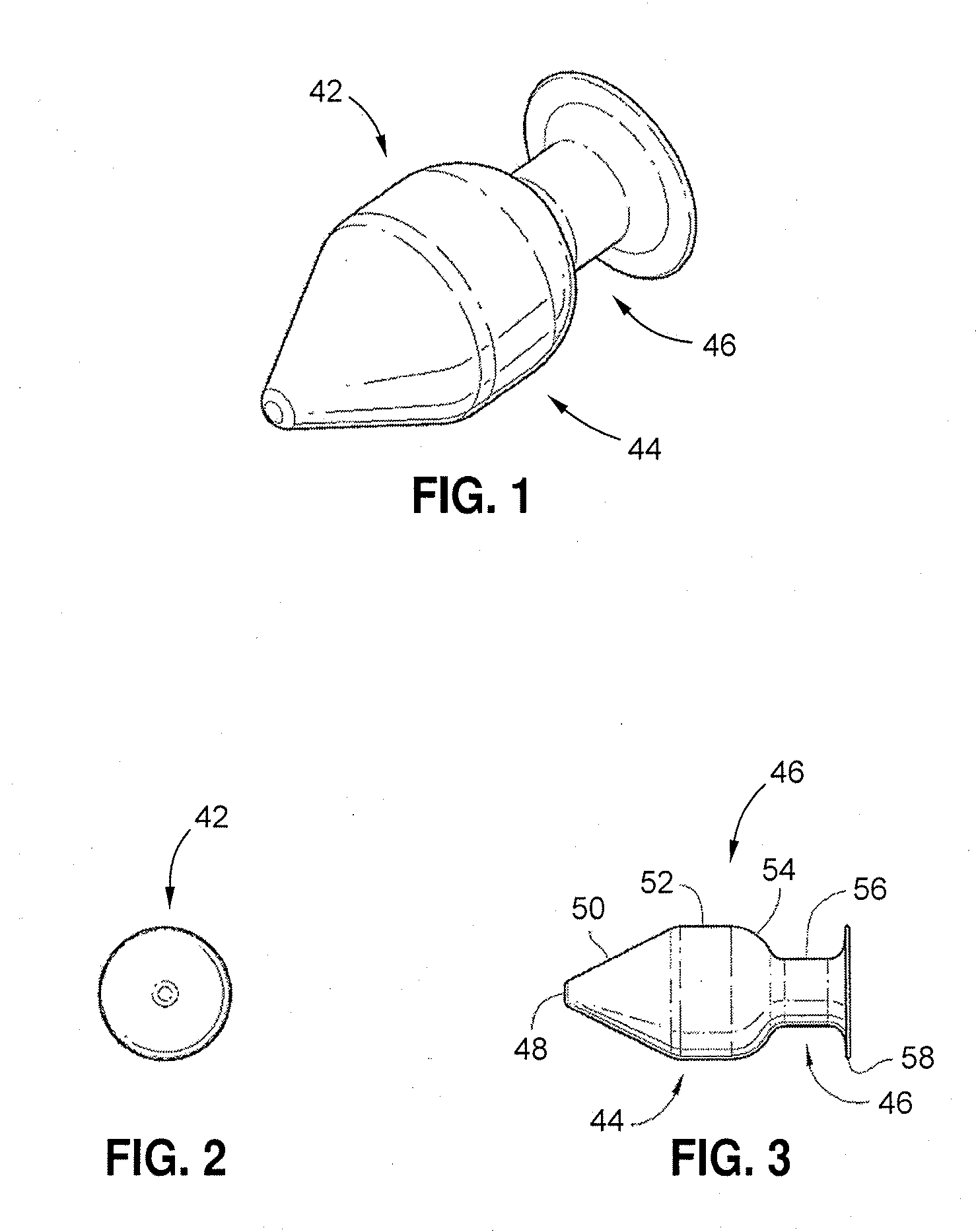
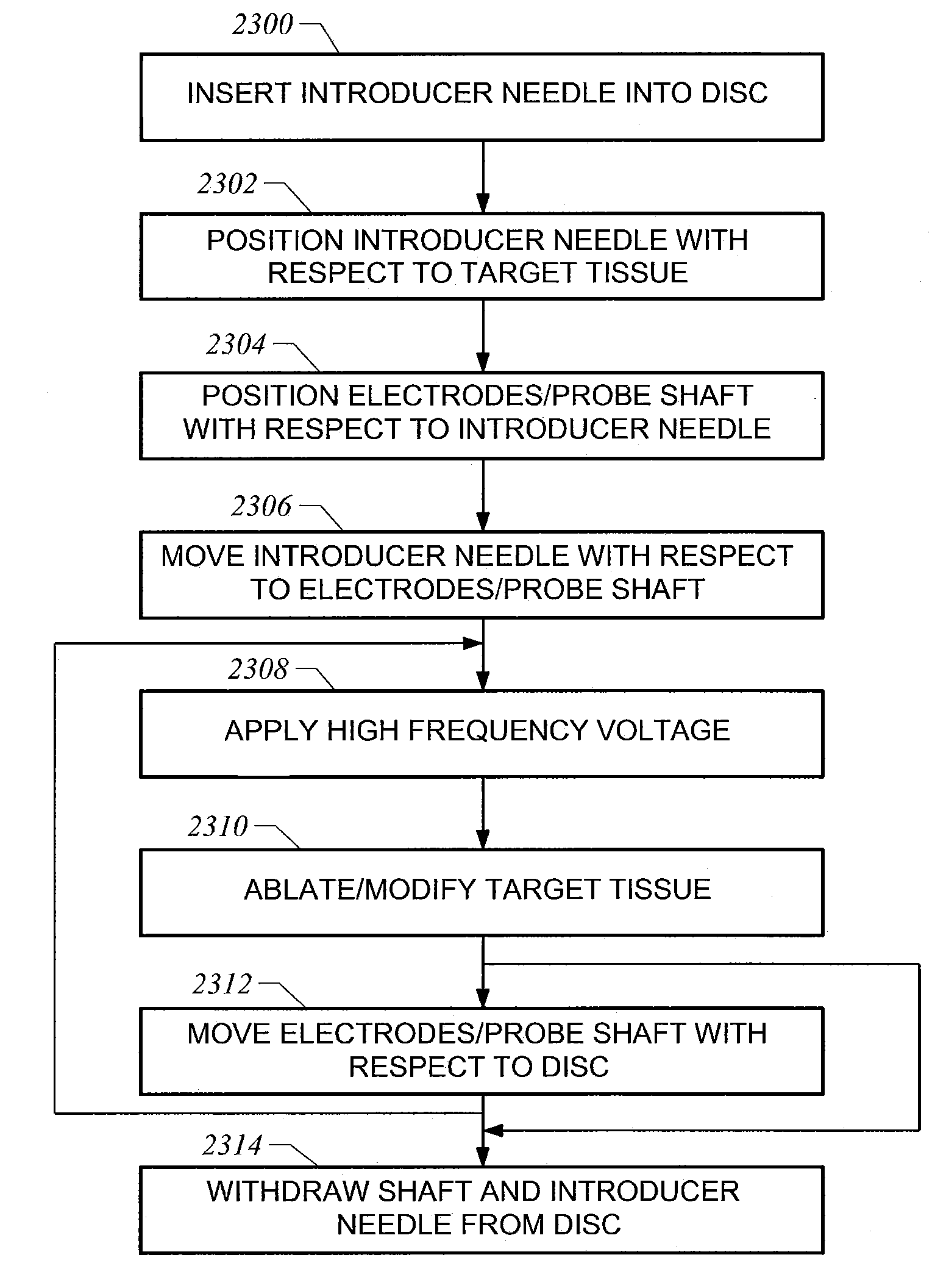
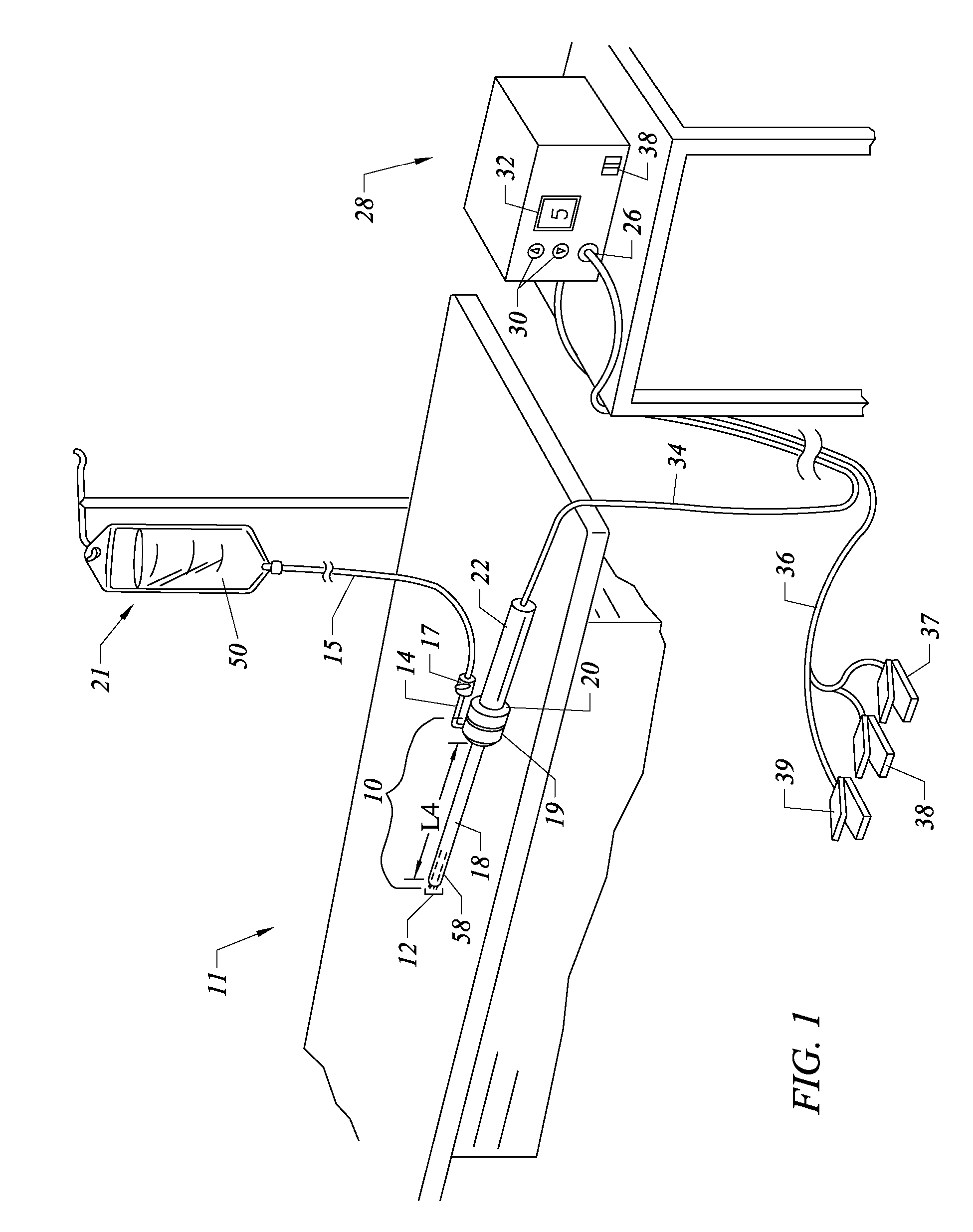
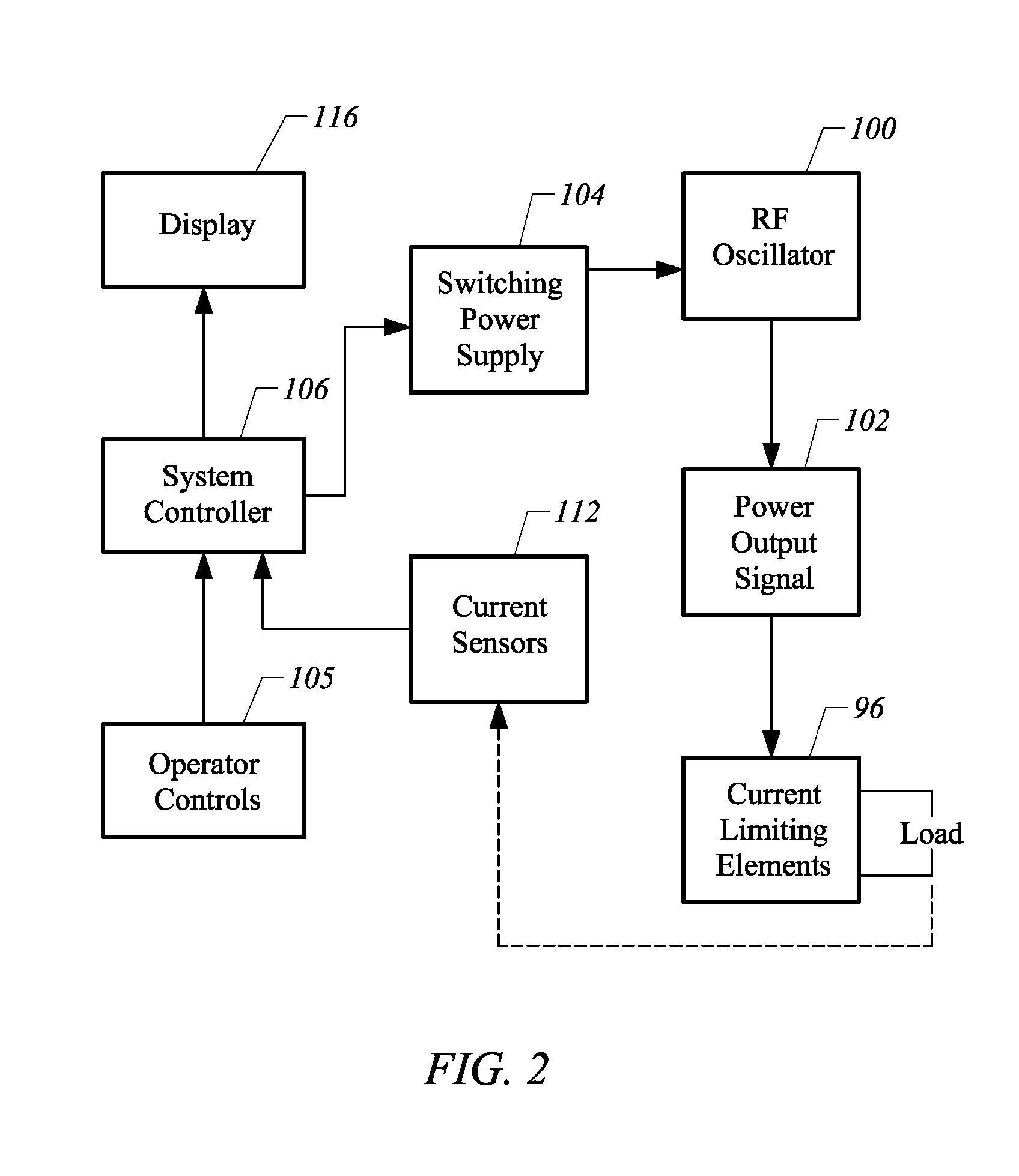
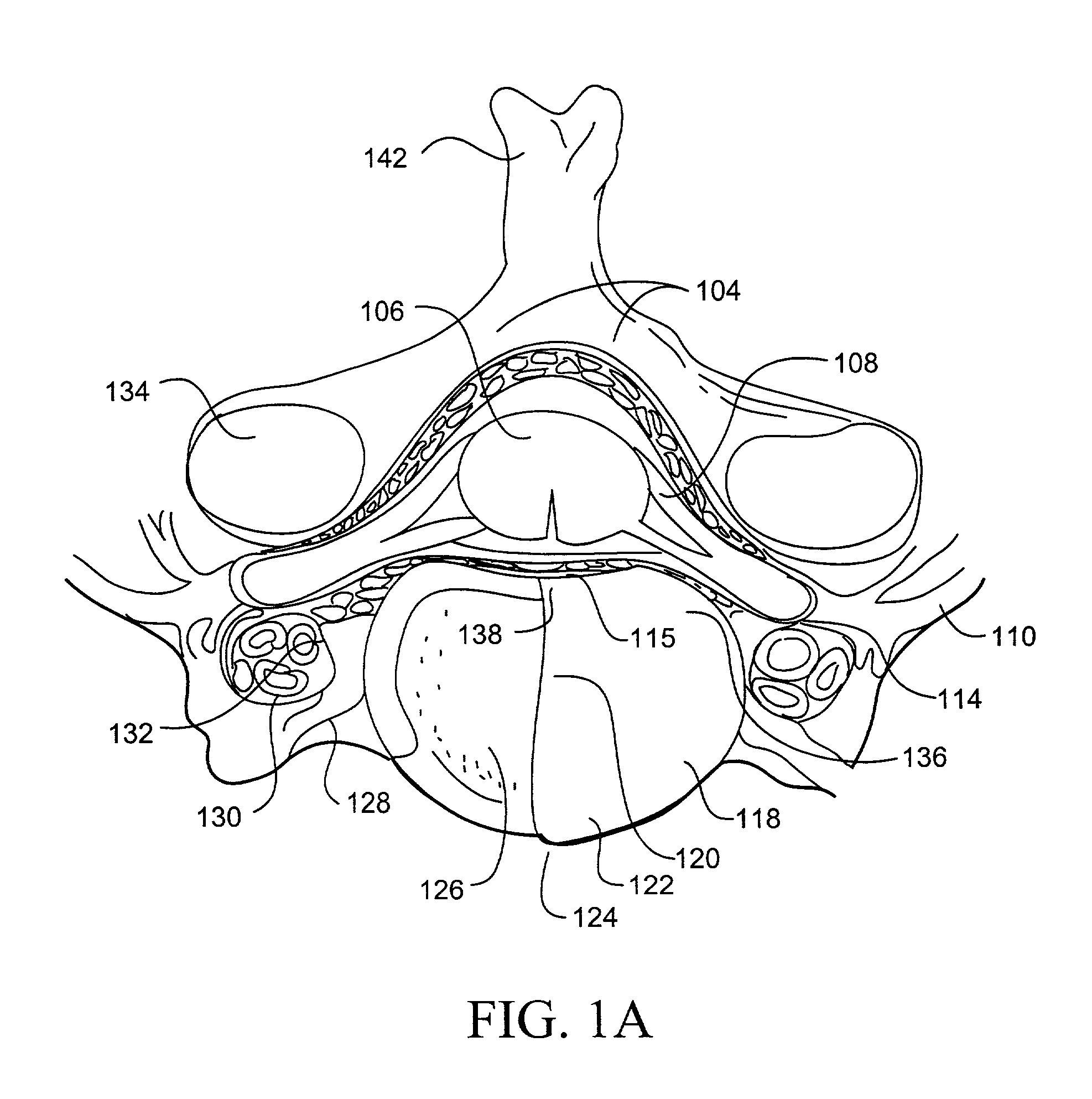
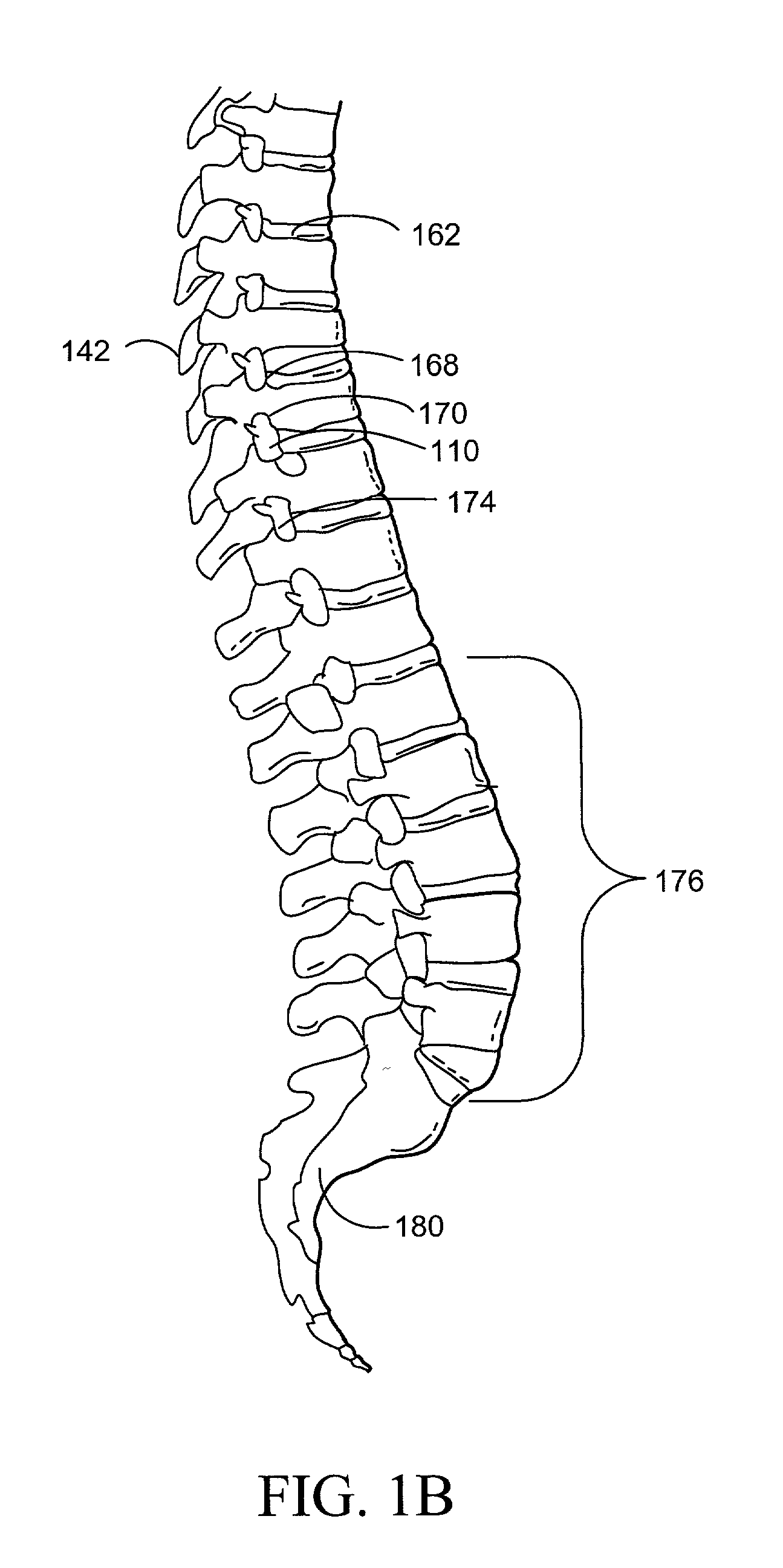
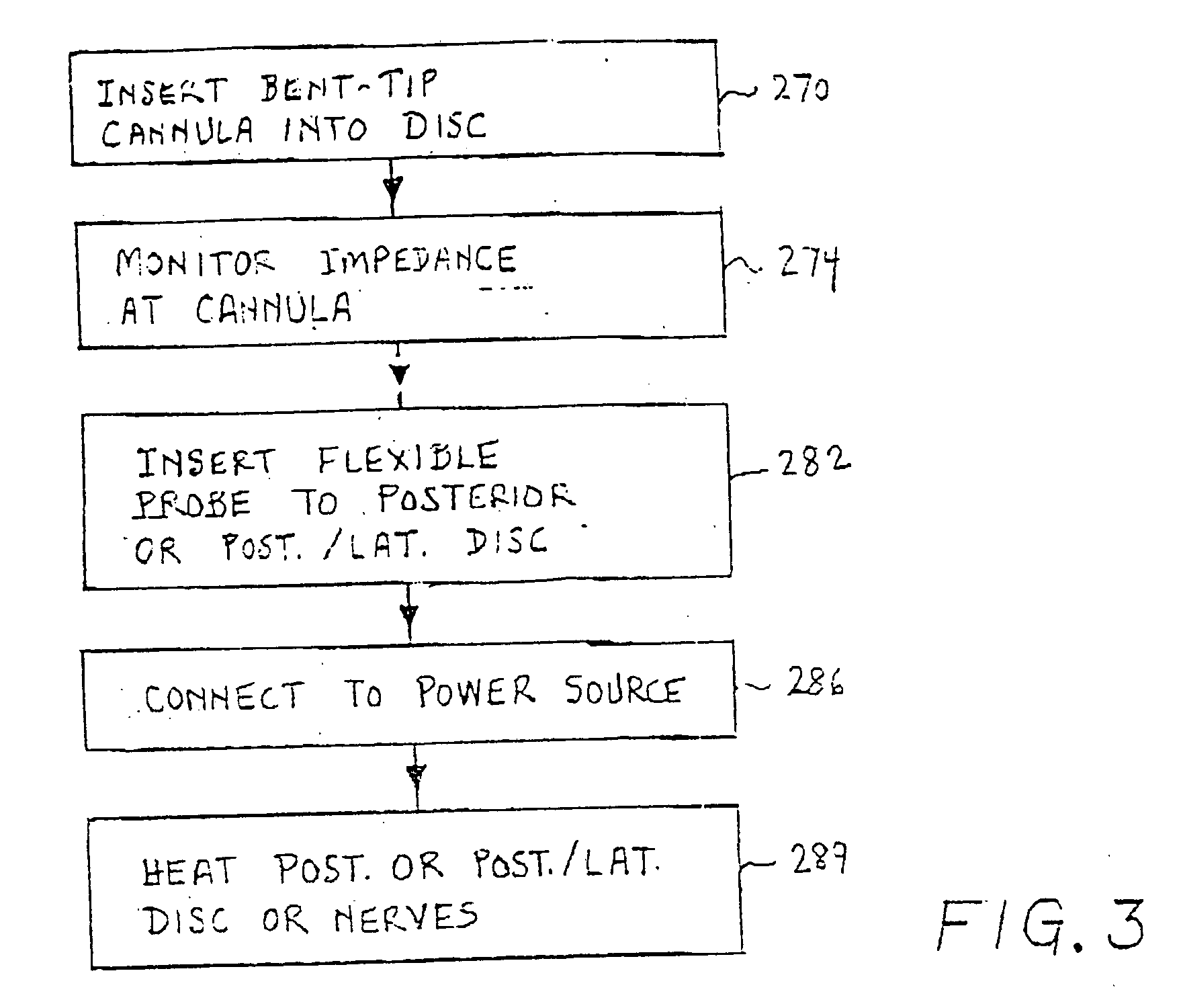
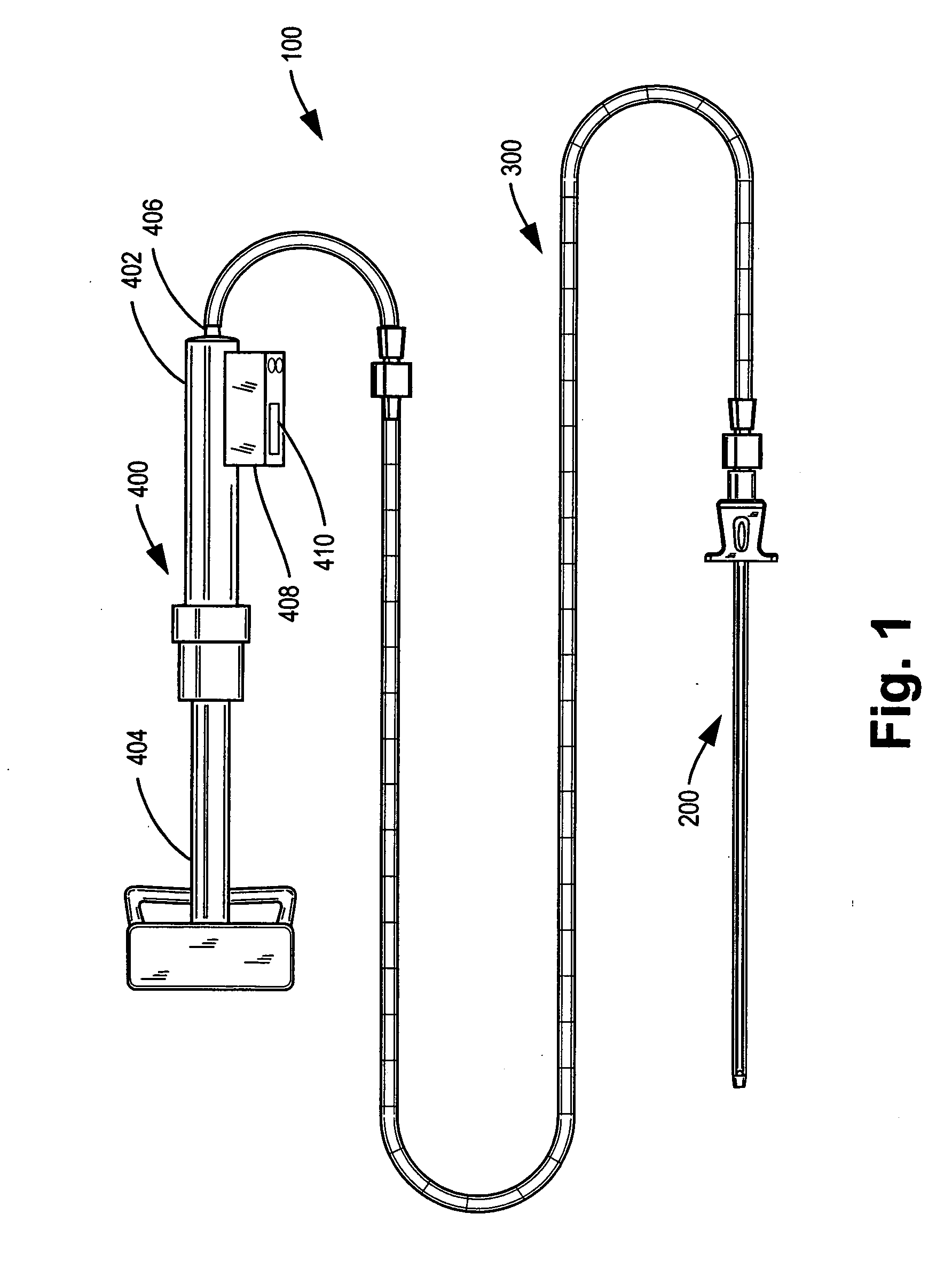
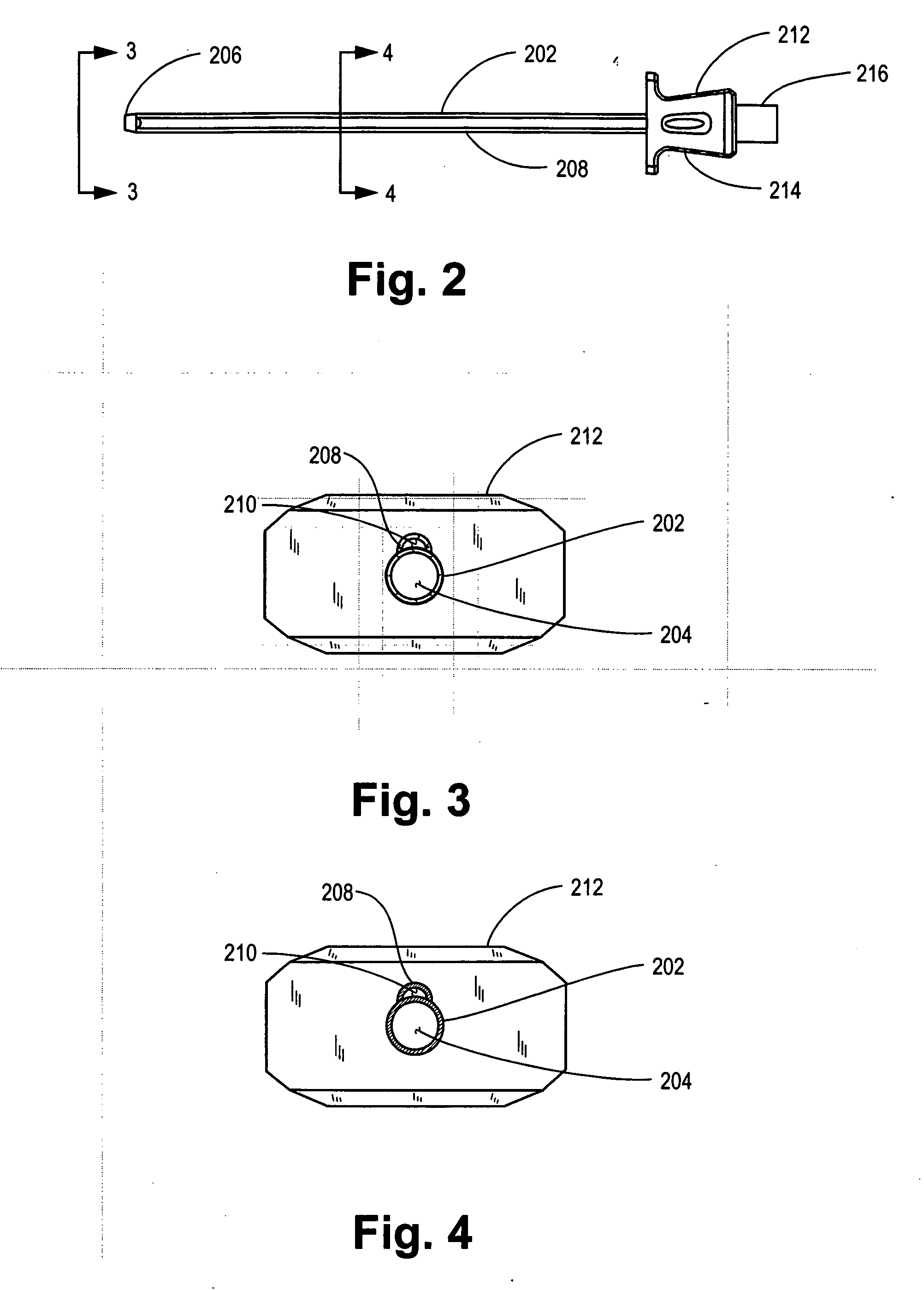
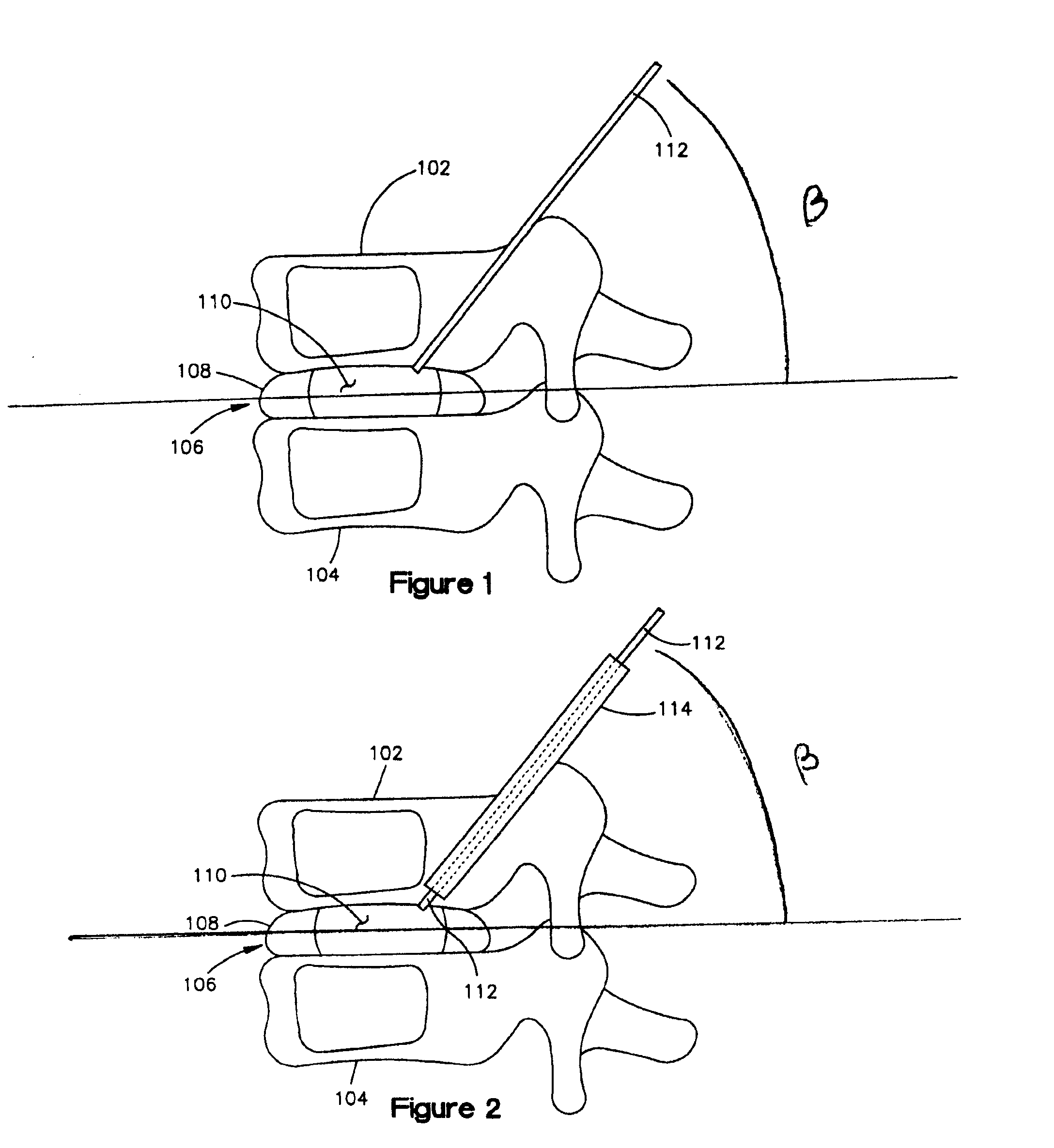
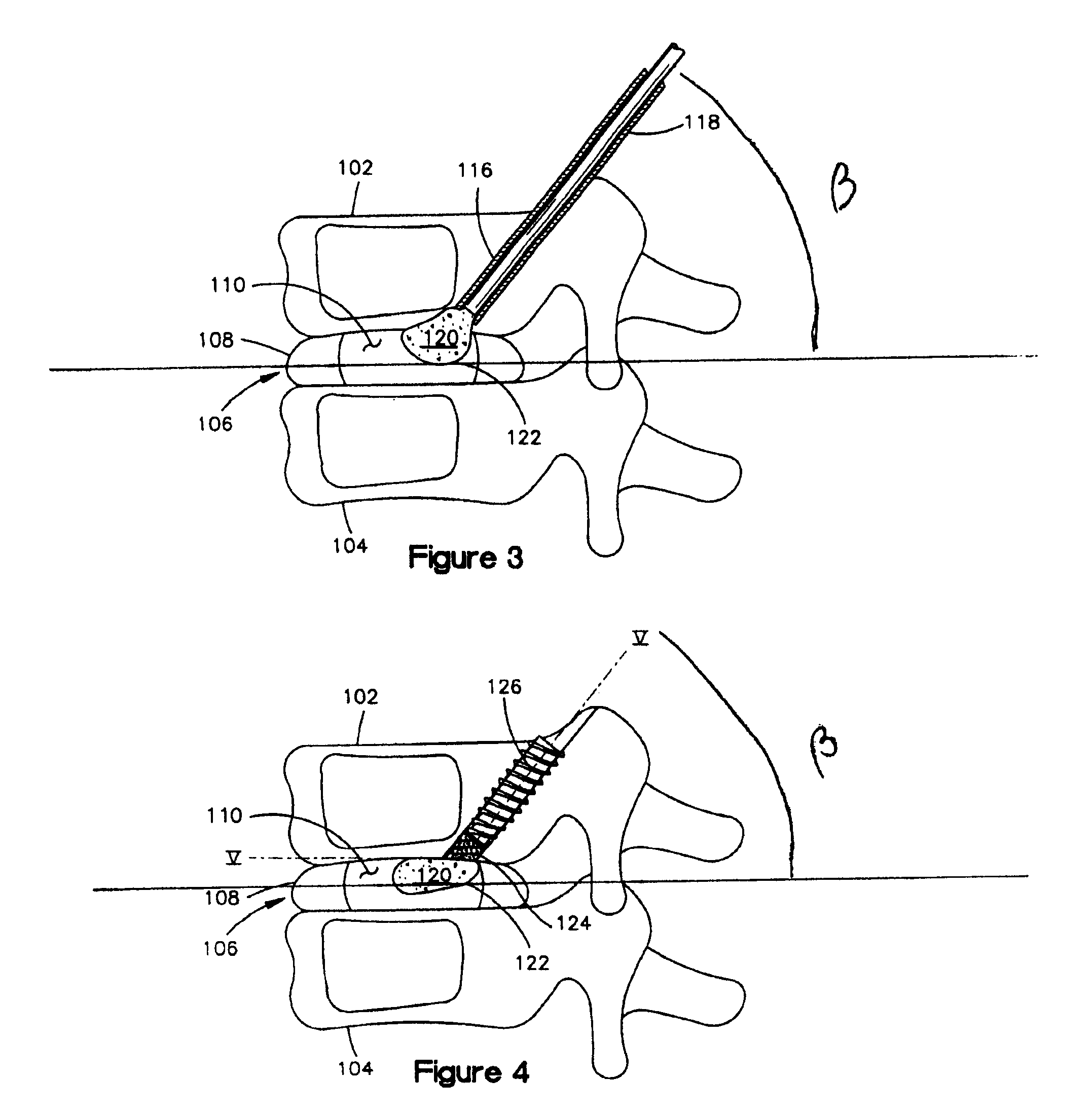
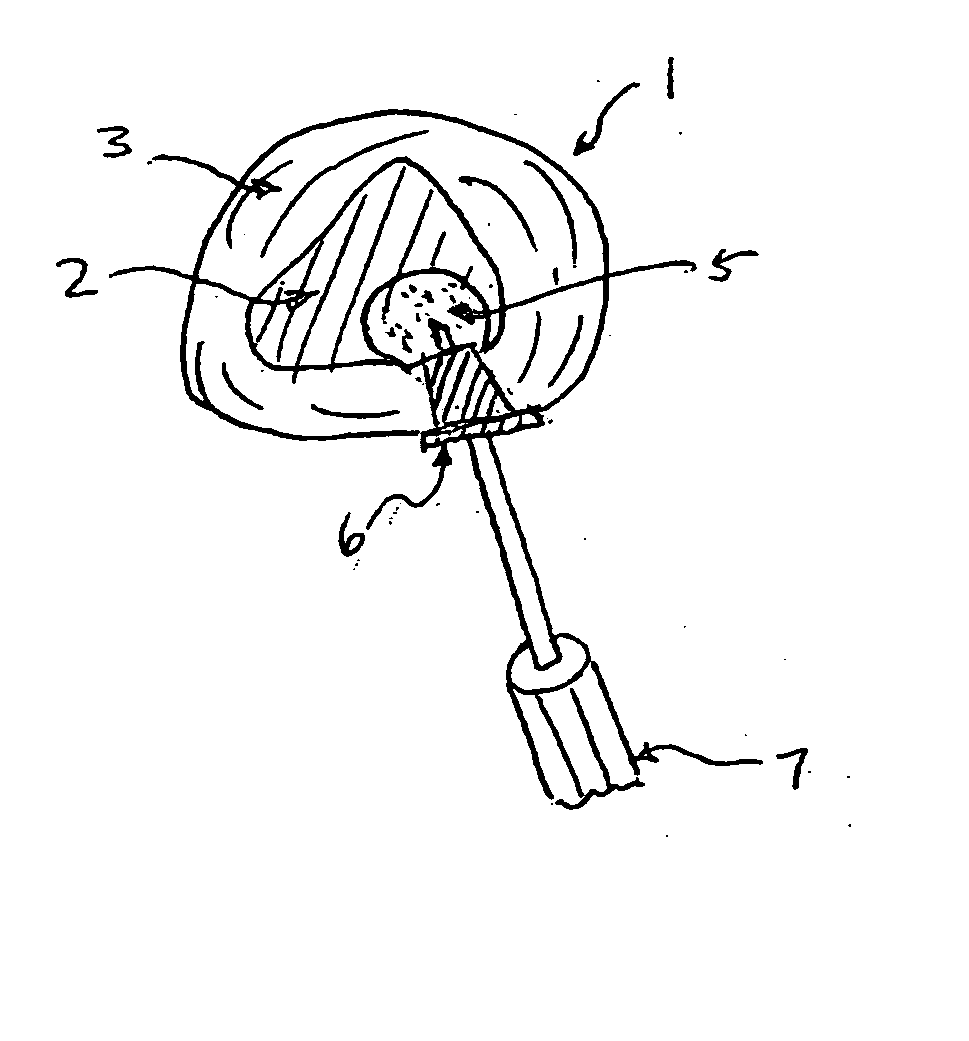
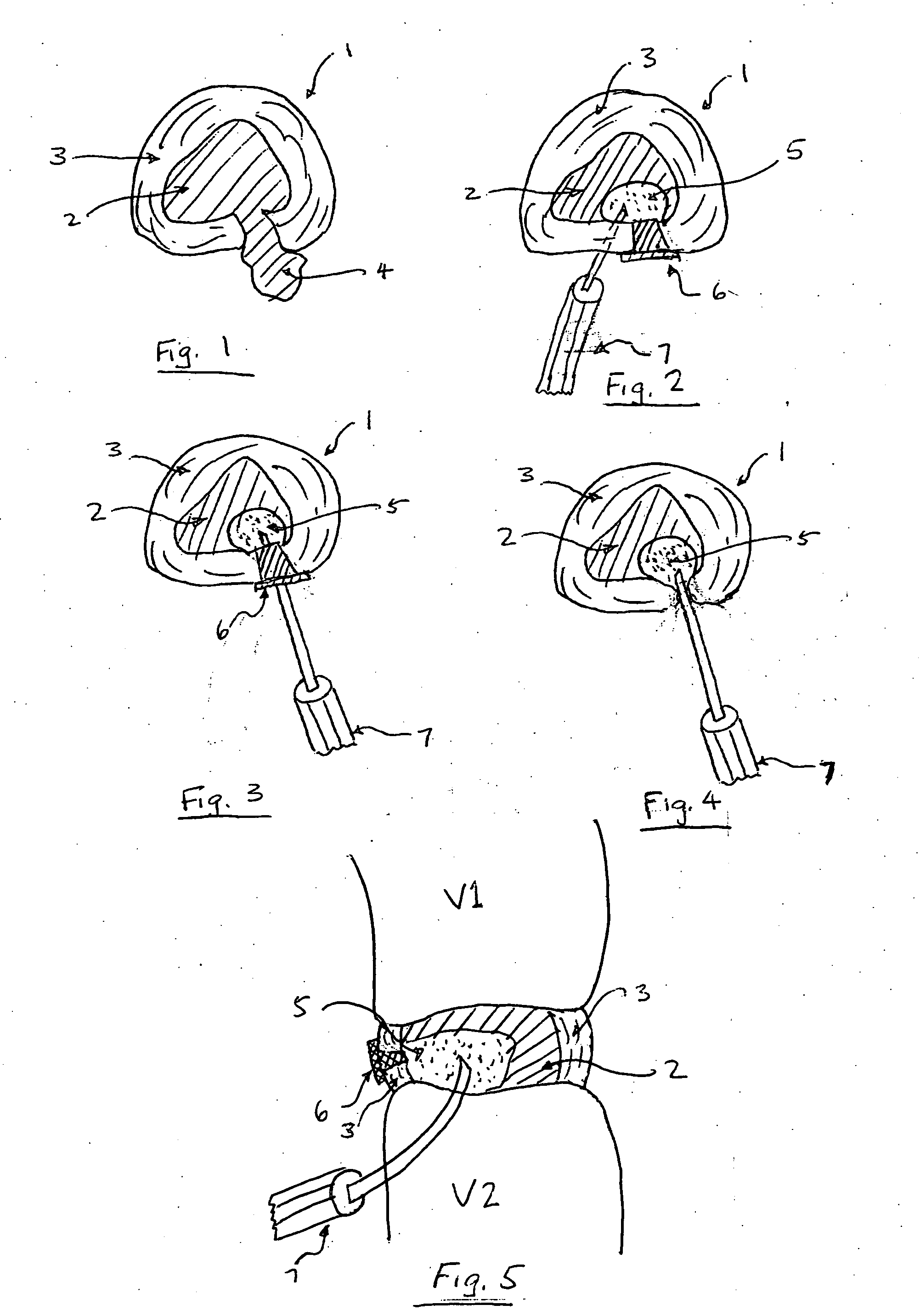
Methods for targeted electrosurgery on contained herniated discs
InactiveUS7179255B2Reduce pressureReduced neckingEnemata/irrigatorsHeart valvesFibrous ringCorneal ablation
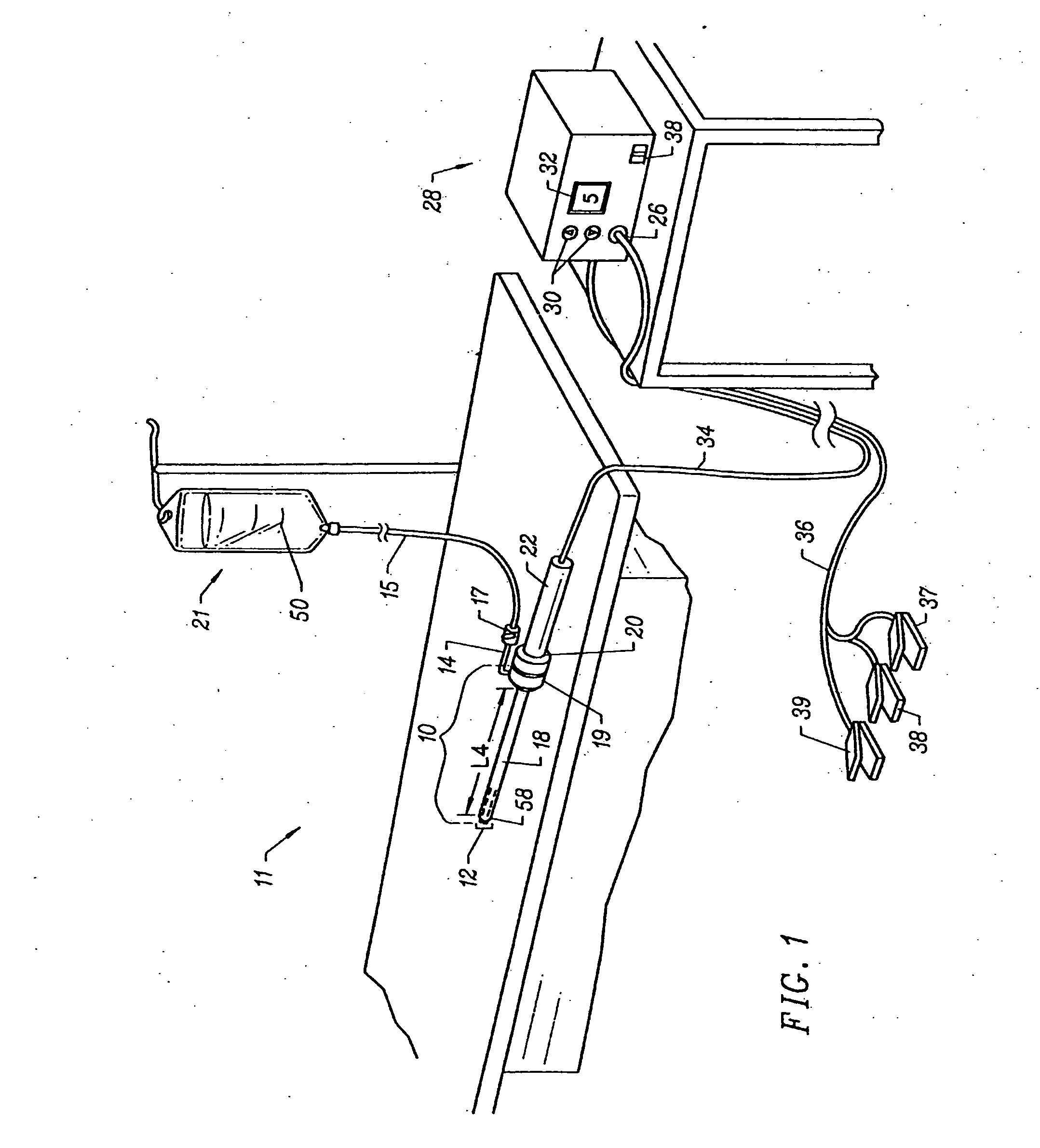
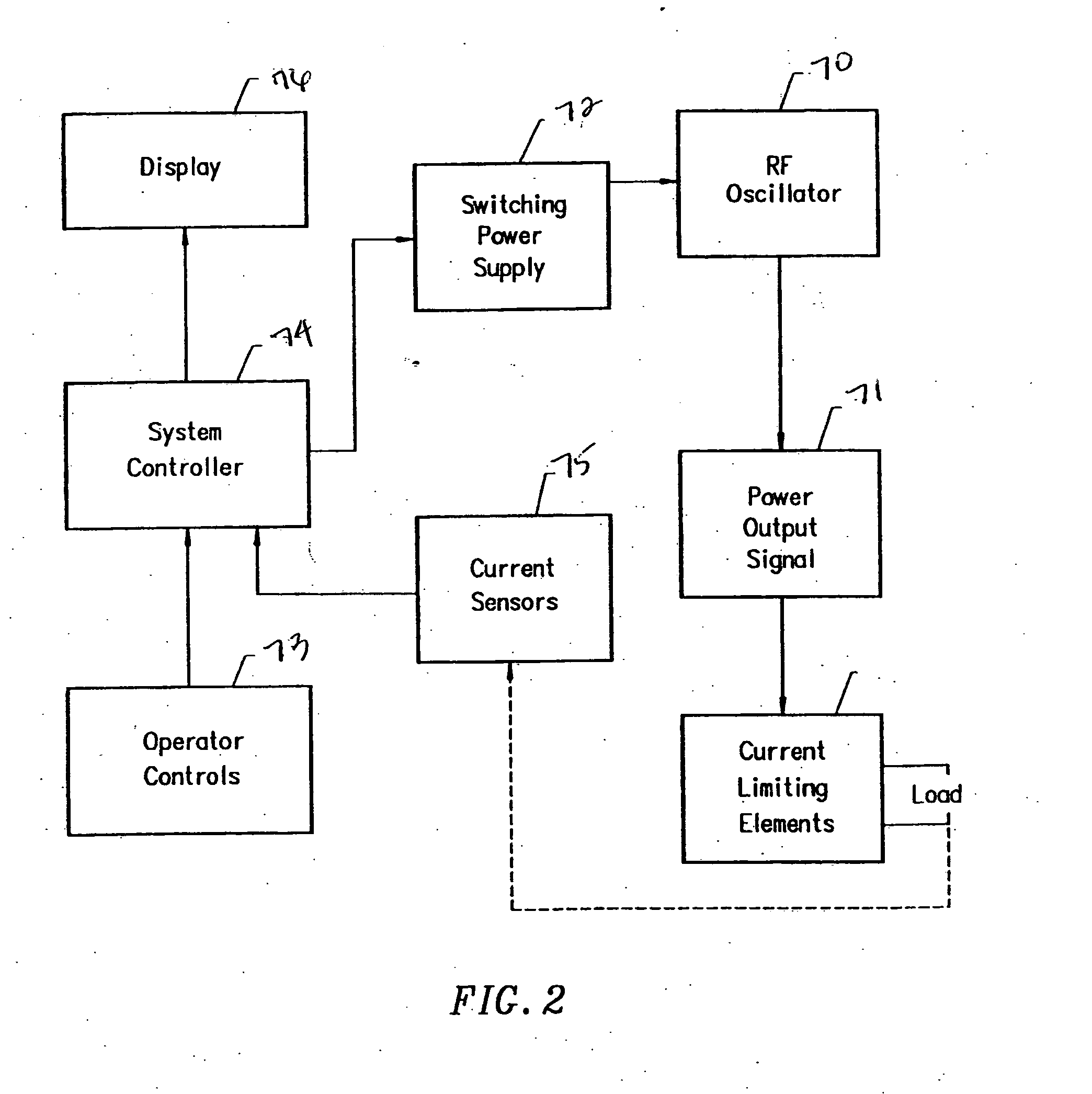
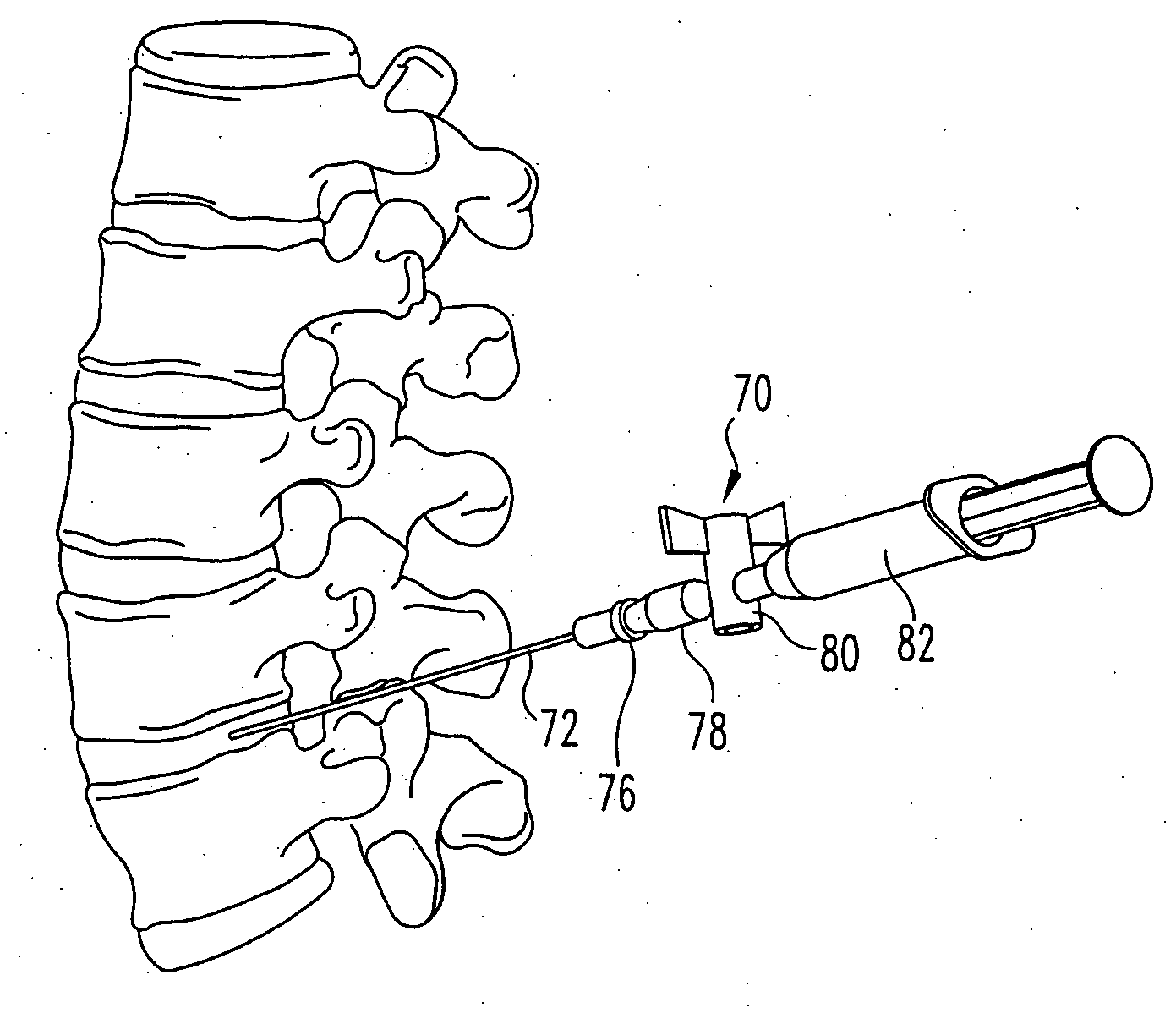
Apparatus and methods for treating an intervertebral disc by ablation of disc tissue. A method of the invention includes positioning at least one active electrode within the intervertebral disc, and applying at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s), wherein the volume of the nucleus pulposus is decreased, pressure exerted by the nucleus pulposus on the annulus fibrosus is reduced, and discogenic pain of a patient is alleviated. In other embodiments, a curved or steerable probe is guided to a specific target site within a disc to be treated, and the disc tissue at the target site is ablated by application of at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s). A method of making an electrosurgical probe is also disclosed.
Owner:ARTHROCARE
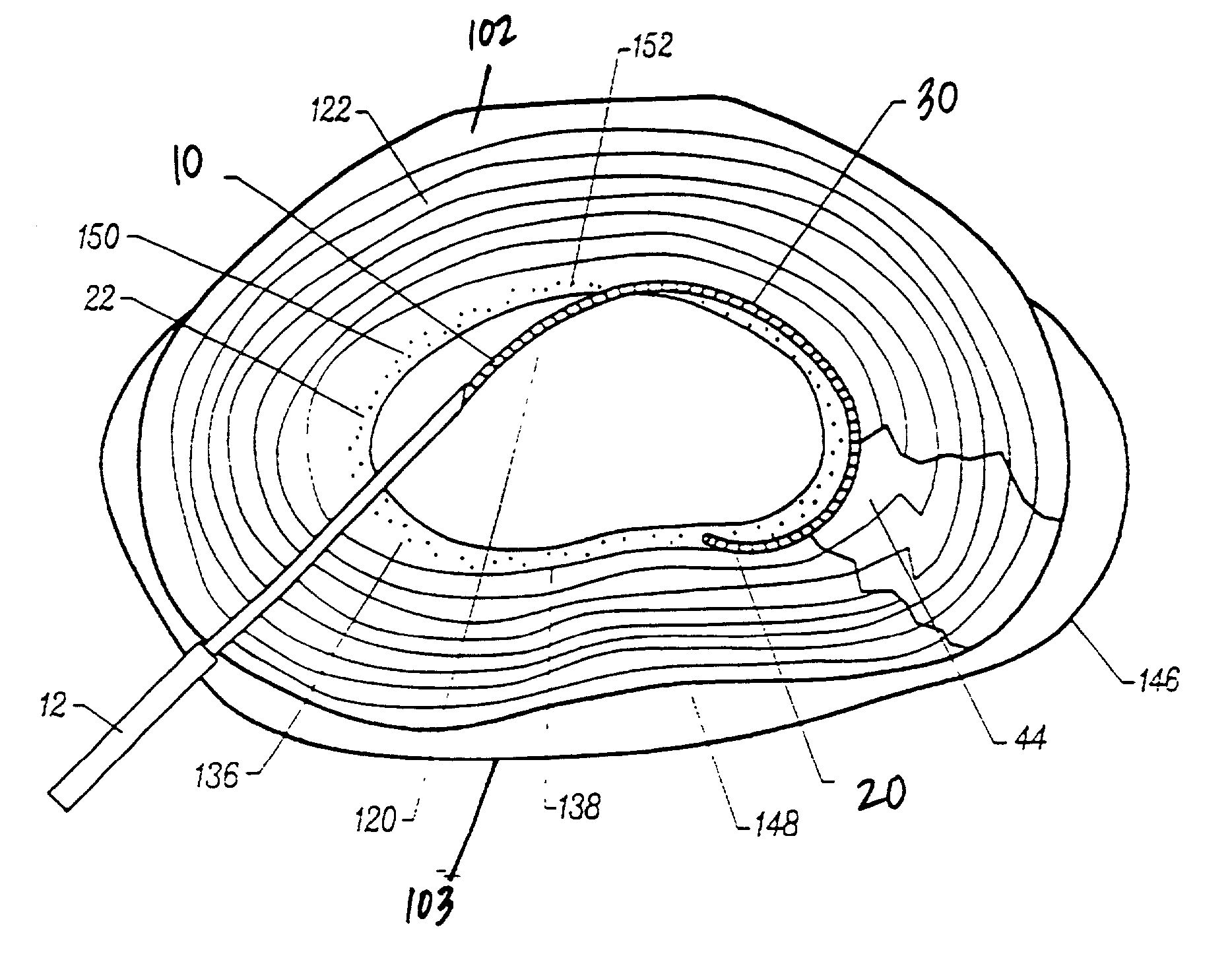
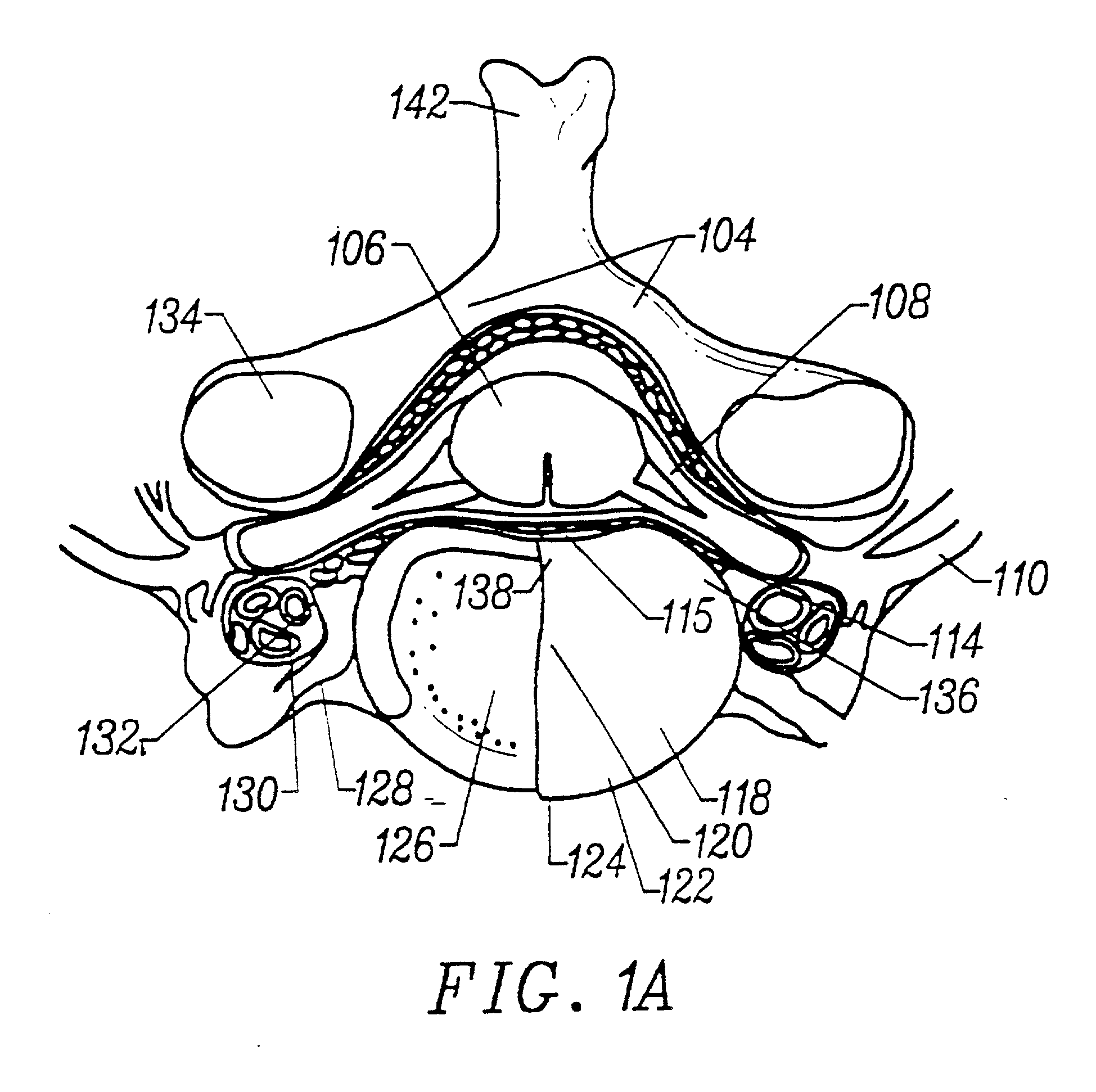
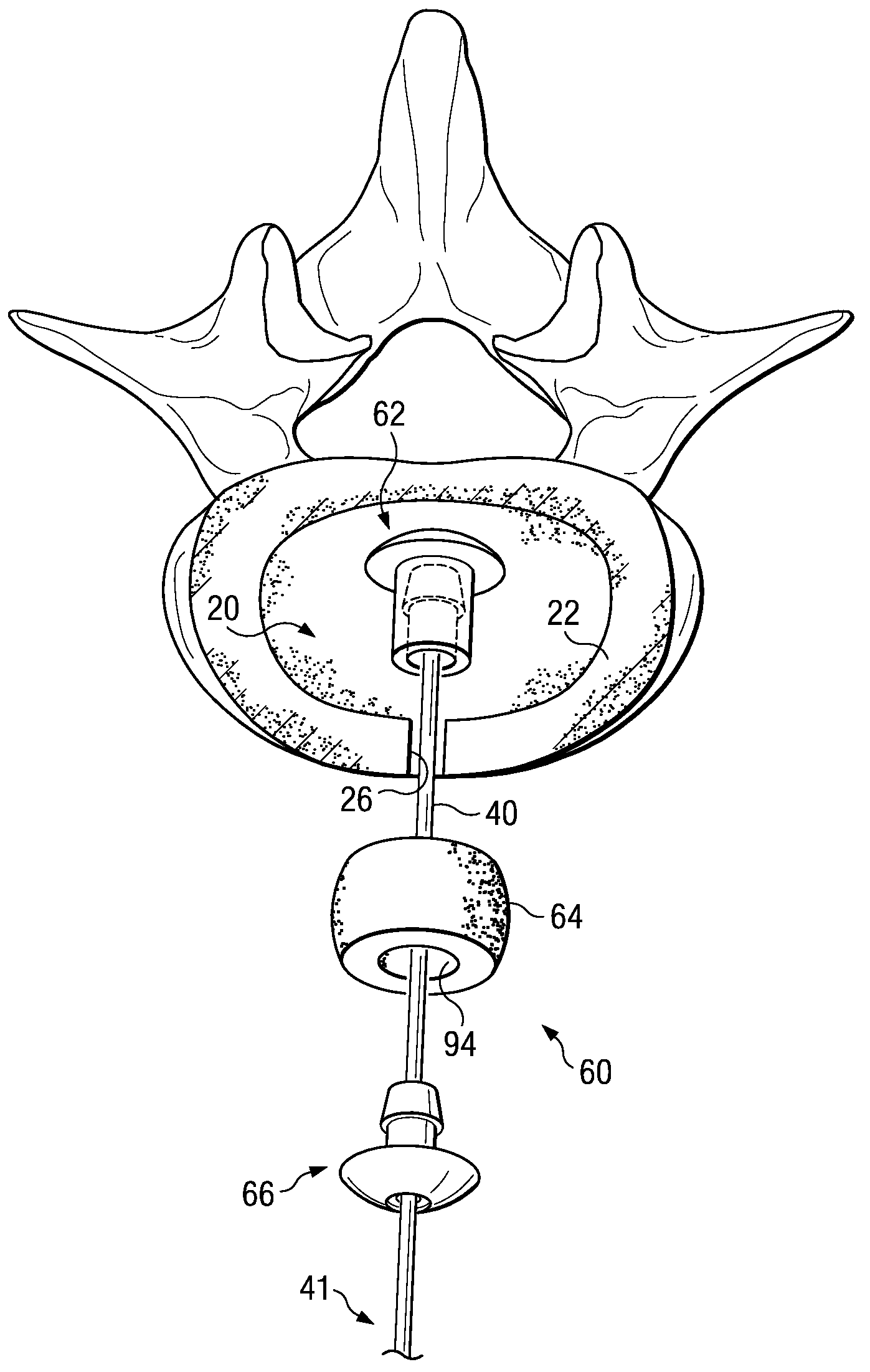
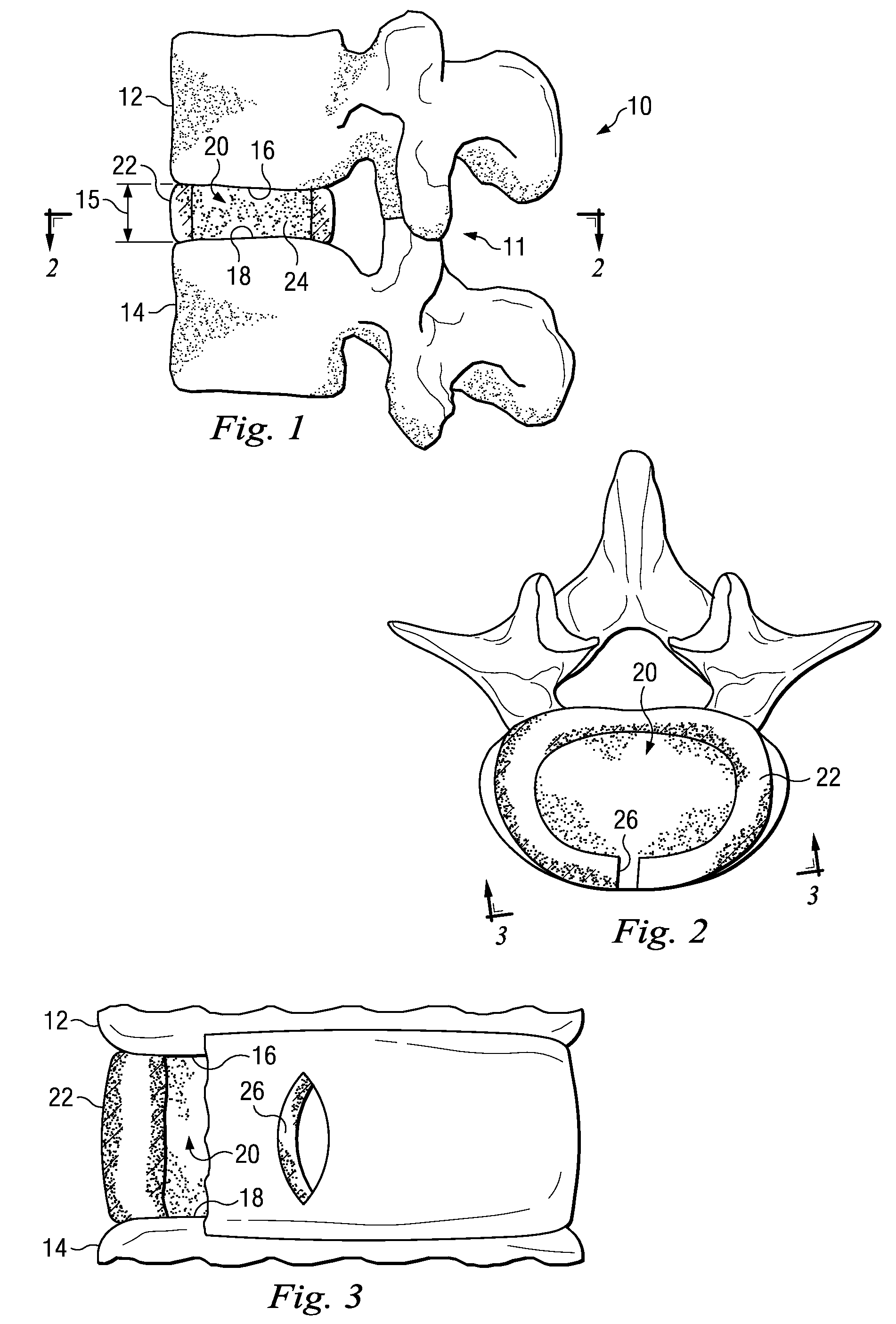
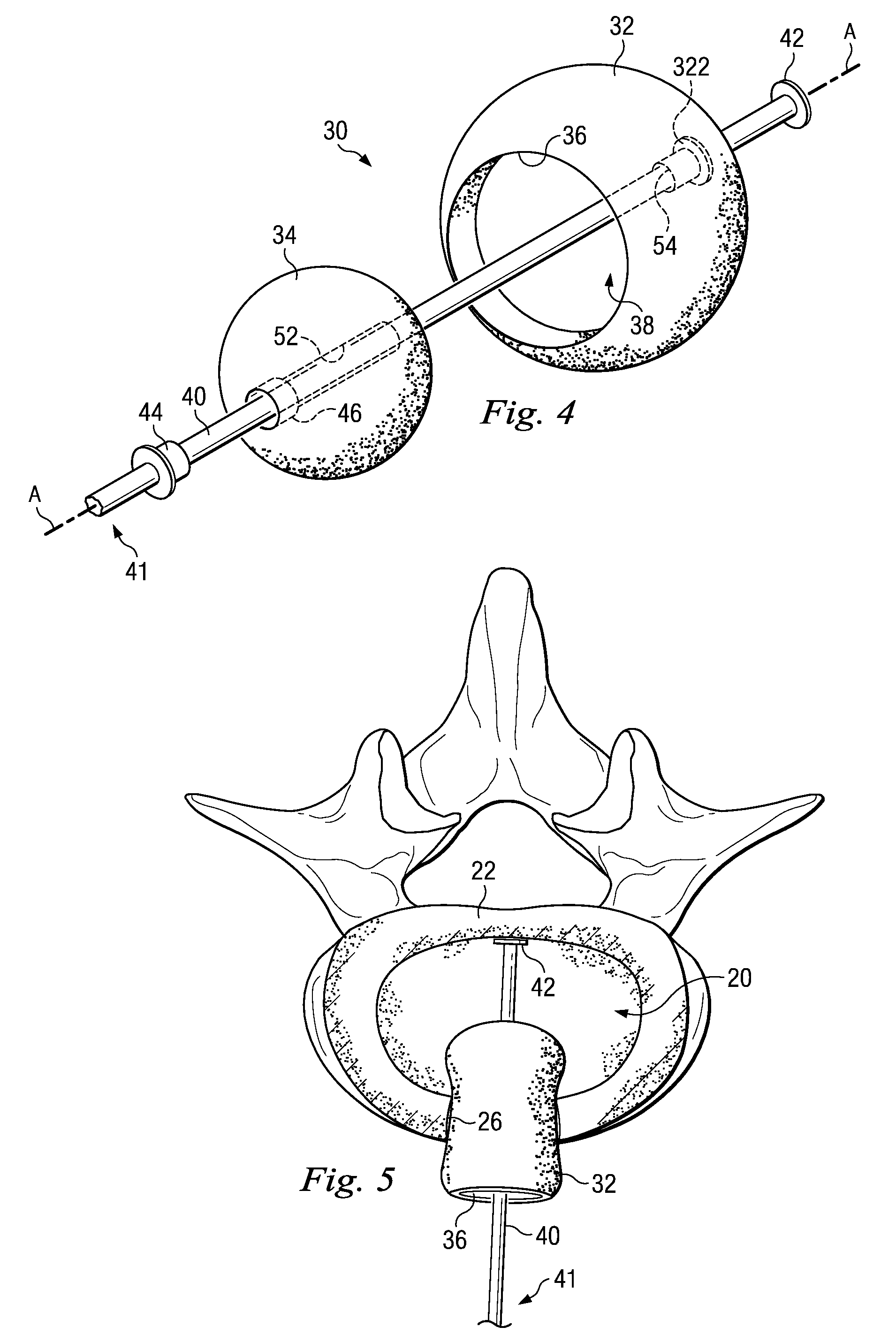
Annulus fibrosis augmentation methods and apparatus
A device and method are used in fortifying an intervertebral disc having an annulus fibrosis with an inner wall. According to the method, a hole is formed through the annulus fibrosis, and a collapsed bag is inserted into the disc through the hole. The bag is inflated, or allowed to expand within the disc space, then filling with one or more biocompatible materials. The hole in the annulus fibrosis is then closed. In one preferred embodiment, the bag includes an inflatable bladder or balloon which is filled with a gas or liquid to expand the bag. In an alternative preferred embodiment, the bag includes a self-expanding frame that assumes a collapsed state for introduction into the disc space and an expanded state once inserted through the hole in the annulus. The self-expanding frame is composed of a shape-memory material, for example. The bag preferably features a wall which is porous to allow for the diffusion of body fluids therethrough, and the bag and / or frame may be fastened to the inner wall of the annulus at one or more points. The biocompatible material may include autograft nucleus pulposis, allograft nucleus pulposis or xenograft nucleus pulposis. In the preferred embodiment, the biocompatible material includes morselized nucleus or annulus from the same disc.
Owner:ANOVA
Method and apparatus for treating annular fissures in intervertebral discs
InactiveUS6997941B2Sufficient energyReduce pressureElectrotherapyInternal osteosythesisSufficient timeMedicine
A device is described that may be positioned at a location in an intervertebral disc for diagnosis or treatment of the disc. Treatment may include, for example, applying energy or removing material, and may decrease intradiscal pressure. Radiofrequency energy may be applied. A percutaneous method of repairing a fissure in the annulus pulposus comprises placing an energy source adjacent to the fissure and providing sufficient energy to the fissure to raise the temperature to at least about 45-70° C. and for a sufficient time to cause the collagen to weld. An intervertebral fissure also can be treated by placing a catheter with a lumen adjacent to the fissure and injecting sealant into the fissure via the catheter, thereby sealing the fissure. An intervertebral fissure additionally can be treated by providing a catheter having a distal end, a proximal end, a longitudinal axis, and an intradiscal section at the catheter's distal end on which there is at least one functional element. The next step is applying a force longitudinally to the proximal of the catheter which is sufficient to advance the intradiscal section through the nucleus pulposus and around an inner wall of an annulus fibrosus, but which force is insufficient to puncture the annulus fibrosus. Next the functional element is positioned at a selected location of the disc by advancing or retracting the catheter and optionally twisting the proximal end of the catheter. Then the functional unit treats the annular fissure. Optionally, there is an additional step of adding a substance to seal the fissure. An externally guidable intervertebral disc apparatus also is disclosed.
Owner:NEUROTHERM
Supplementation or replacement of a nucleus pulposus of an intervertebral disc
InactiveUS20060122704A1Avoid damageFunction increaseBone implantJoint implantsMedicineIntervertebral disc
A degenerated nucleus pulposus located in a central core region of an intervertebral disc within the annulus fibrosus is supplemented or replaced by a method wherein an amount of a biocompatible material is introduced into the central core region by a process including the steps of 1) forming a channel through a vertebral body adjacent to said intervertebral disc, extending from an exterior surface of the vertebral body to the central core region of the annulus fibrosus; 2) introducing an amount of a biocompatible material through the channel into the central core region of the annulus fibrosus; 3) pressurizing the biocompatible material through the channel to a postsurgical pressure sufficient to alleviate symptoms caused by the degenerated nucleus pulposus; and 4) sealing the channel while maintaining the sufficient postsurgical pressure. After sealing the channel, a vertebroplasty may optionally be performed in the vertebra.
Owner:DEPUY SYNTHES PROD INC
Spinal implants and methods
InactiveUS20090149959A1Reduce relative motionSmooth motionDiagnosticsBone implantPosterior regionIntervertebral disk
Owner:MAGELLAN SPINE TECH
Apparatus and methods for treating cervical inter-vertebral discs
InactiveUS7393351B2Reduce the overall diameterAvoid arcingCannulasEnemata/irrigatorsMedicineActive electrode
Apparatus and methods for treating an inter-vertebral disc by ablation, coagulation, shrinking, stiffening, or other treatment of disc tissue. A method of the invention includes positioning at least one active electrode within the inter-vertebral disc, and applying at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s), wherein the volume of the nucleus pulposus is decreased, pressure exerted by the nucleus pulposus on the annulus fibrosus is reduced, and discogenic pain of a patient is alleviated. An apparatus of the invention includes an electrosurgical probe, an introducer needle adapted for passing the distal end of the probe therethrough, and a positioning unit for monitoring a position of the probe in relation to the introducer needle.
Owner:ARTHROCARE
Intervertebral disc augmentation and rehydration with superabsorbent polymers
ActiveUS20070150061A1Simple and fast and easy methodEasily reversibleSpinal implantsTissue regenerationMedicineFluid infusion
The embodiments provide a method for treating an intervertebral disc having a nucleus pulposus and an annulus fibrosis, using one or more superabsorbent polymers. Additionally, the embodiments provide a method for bulking up an intervertebral disc having a nucleus pulposus and an annulus fibrosis, using one or more superabsorbent polymers. The methods comprise introducing an amount of the superabsorbent polymers into the intervertebral disc space without removing nucleus pulposus or annulus fibrosis material.
Owner:WARSAW ORTHOPEDIC INC
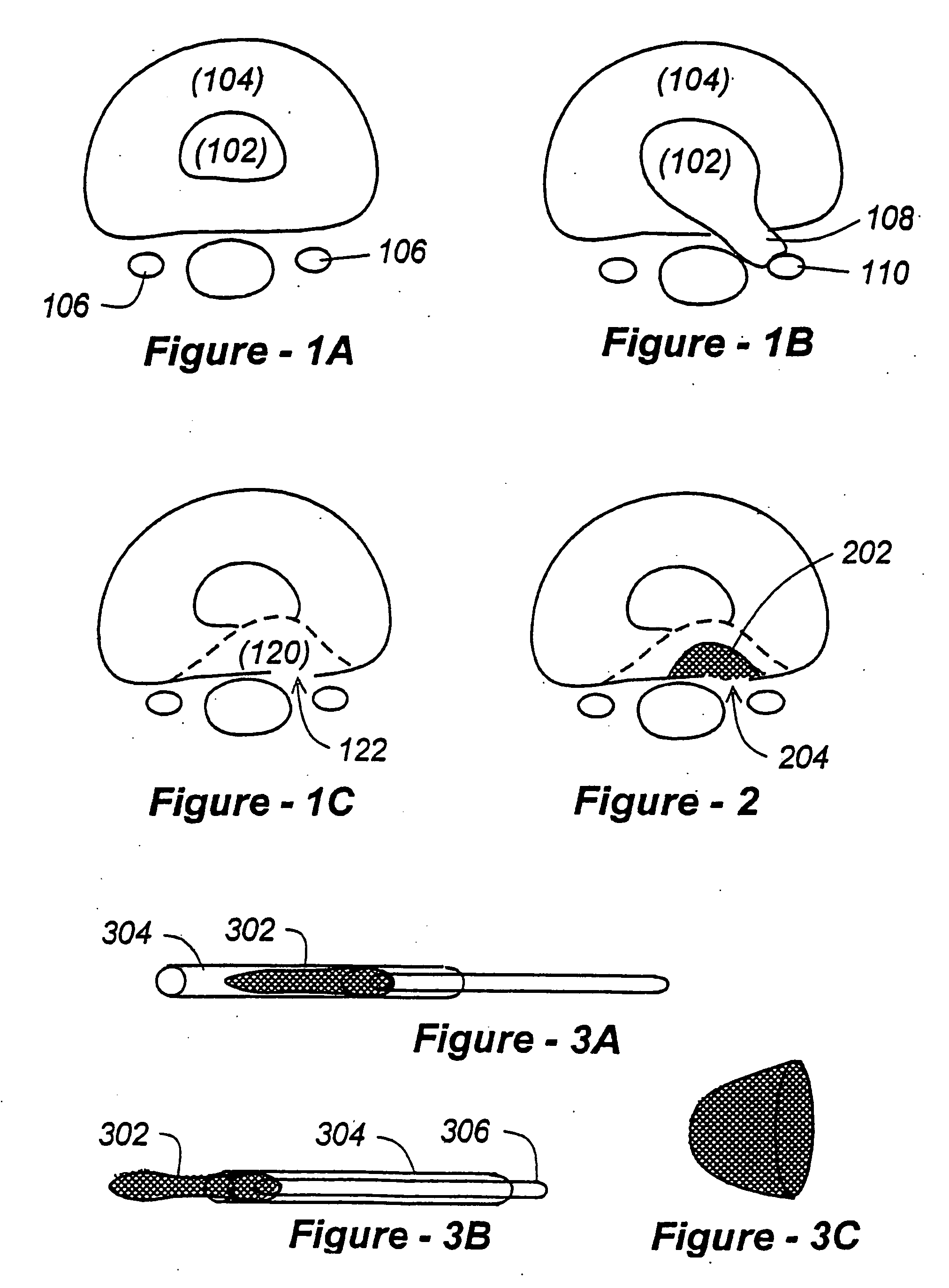
Annulus fibrosus stent
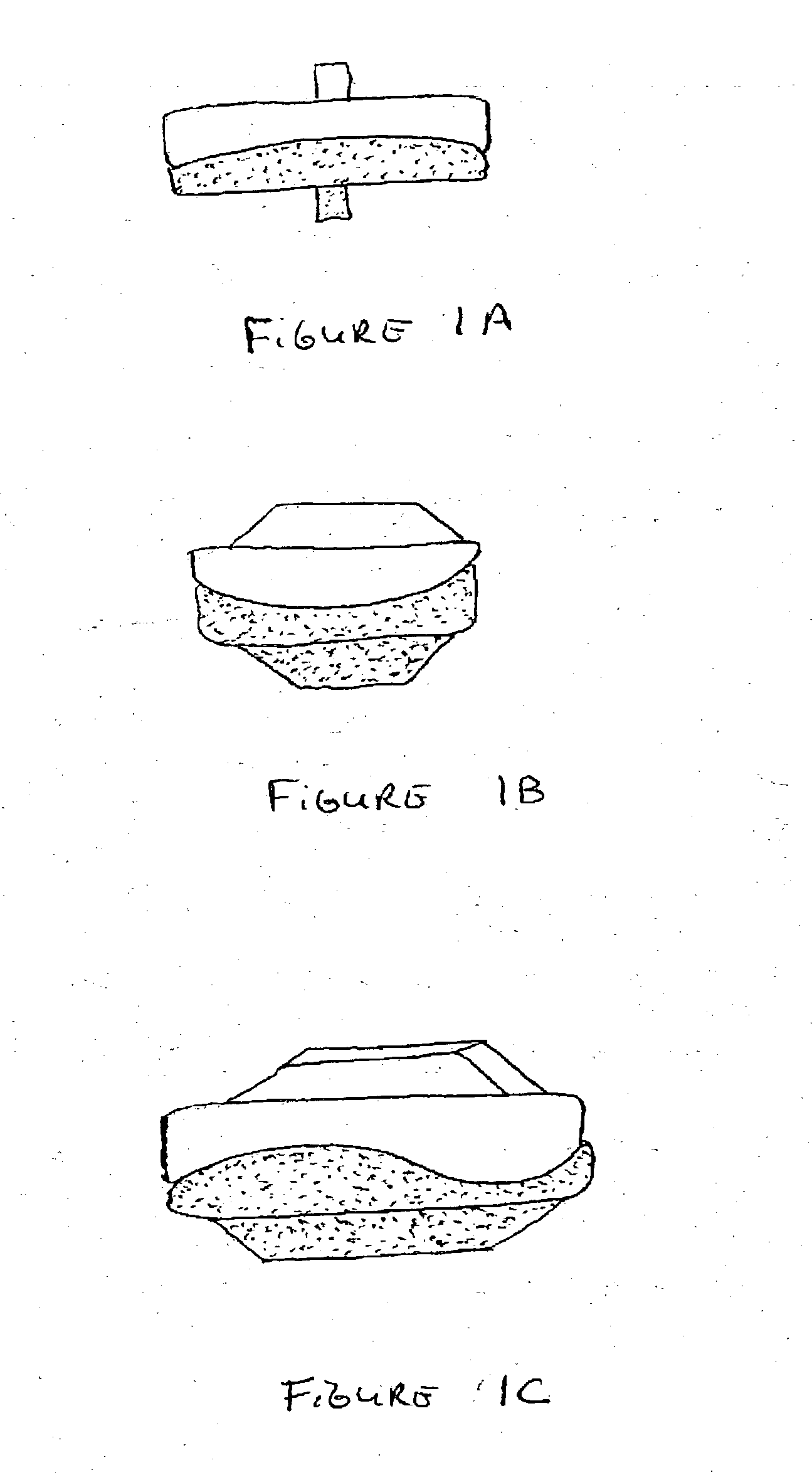
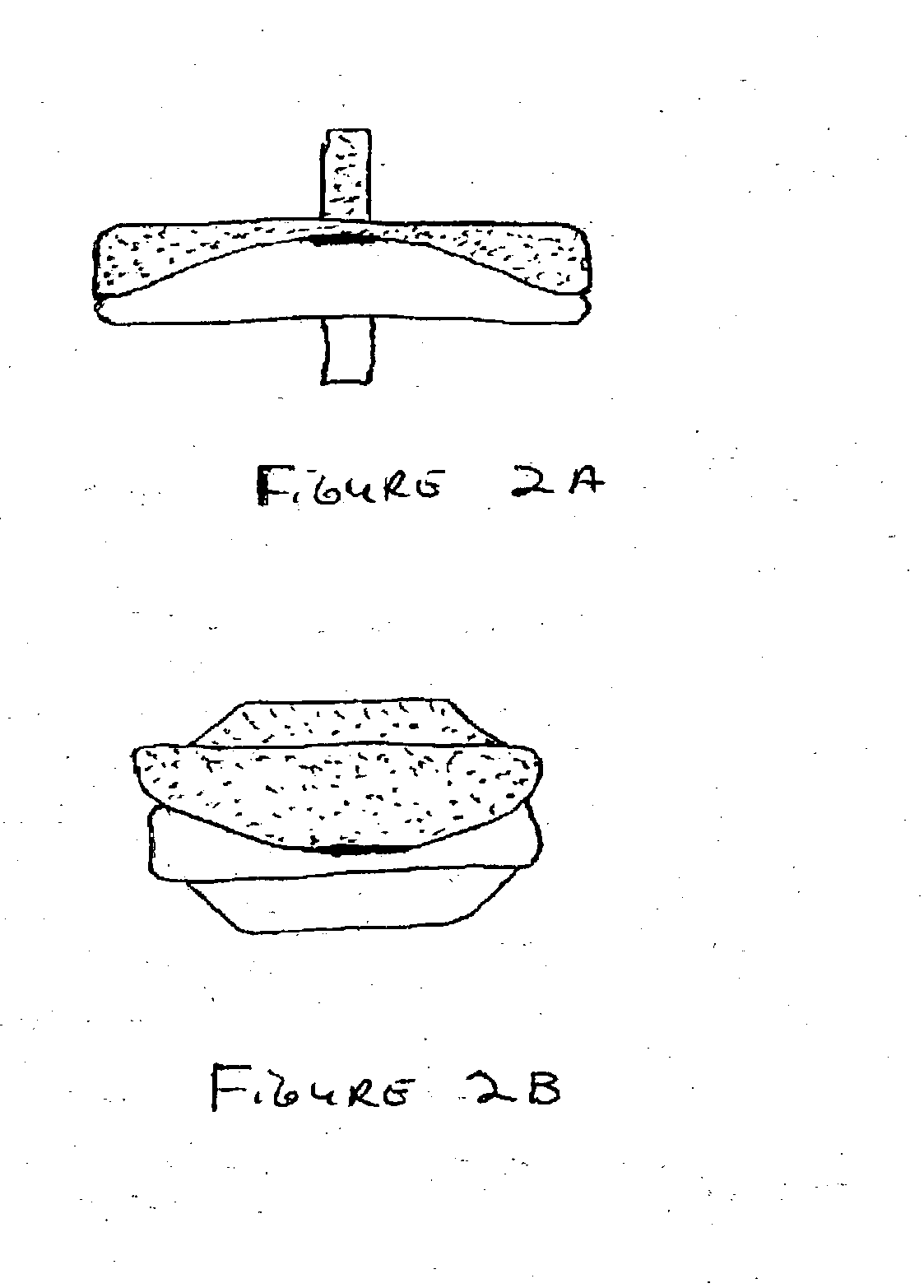
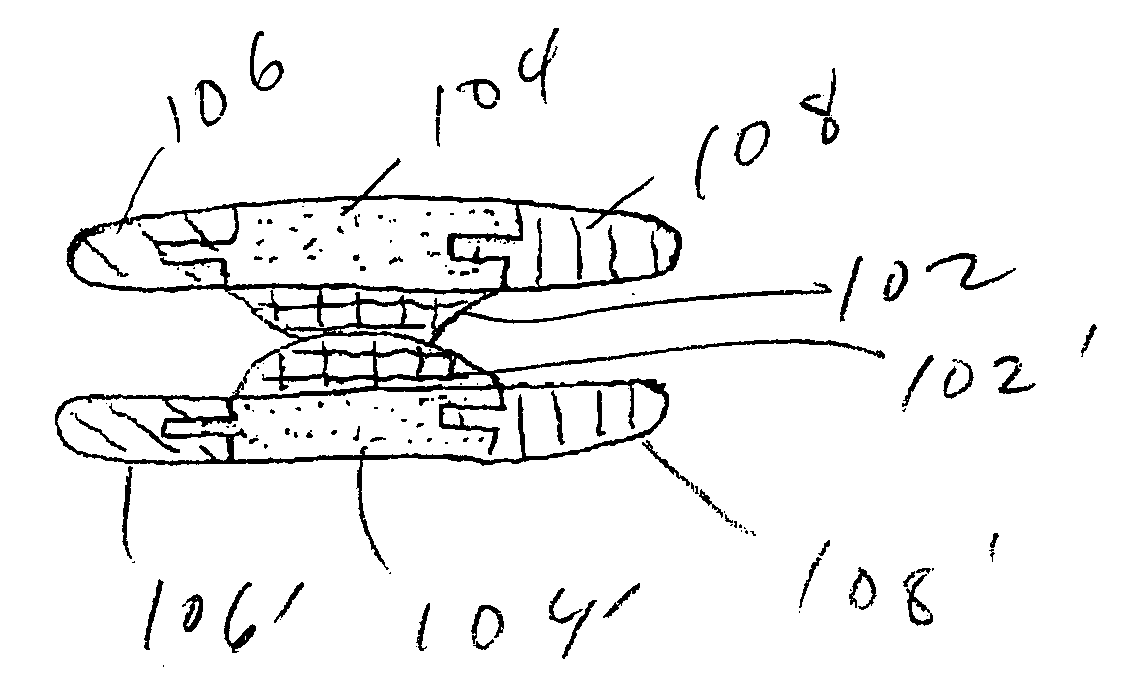
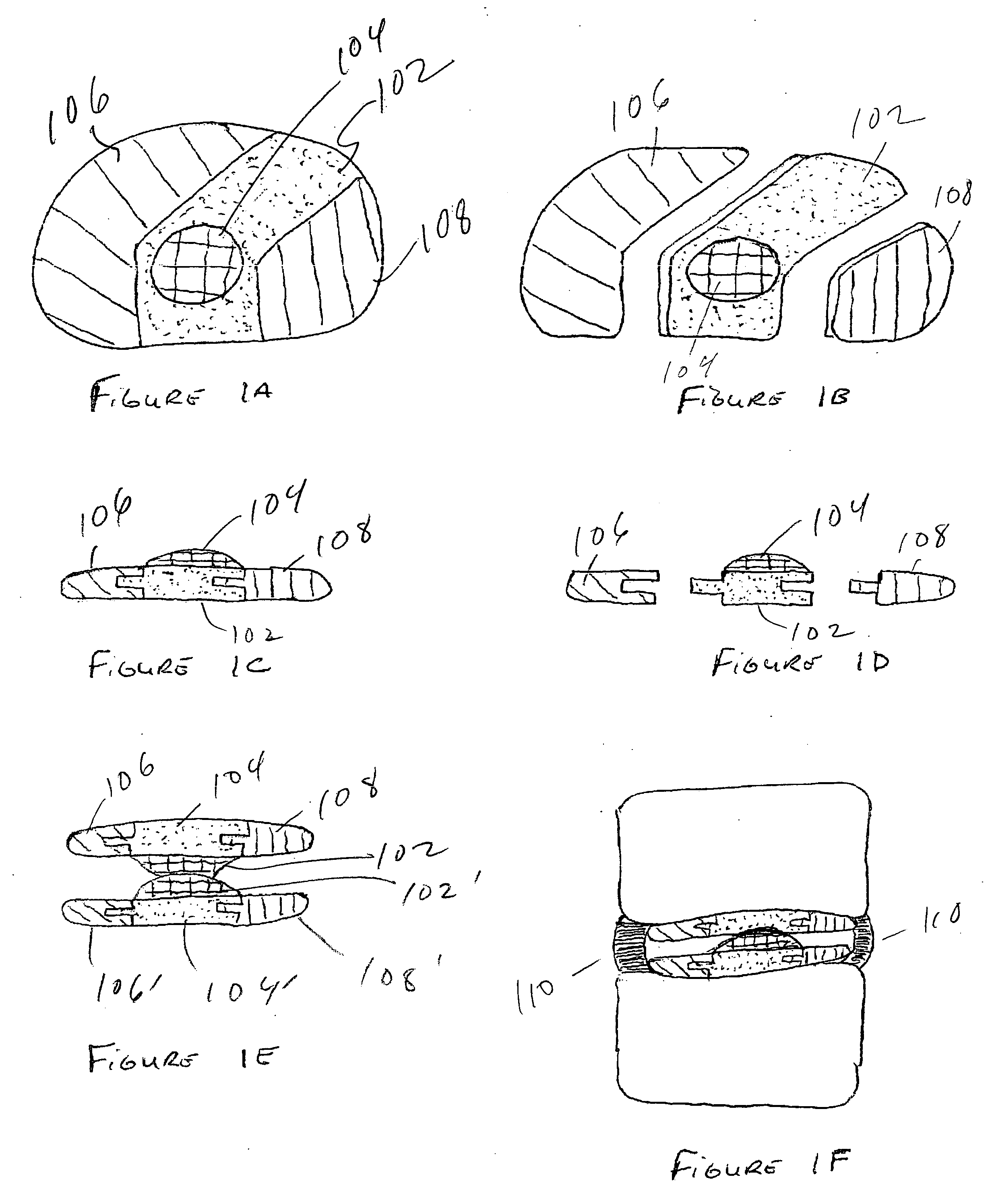
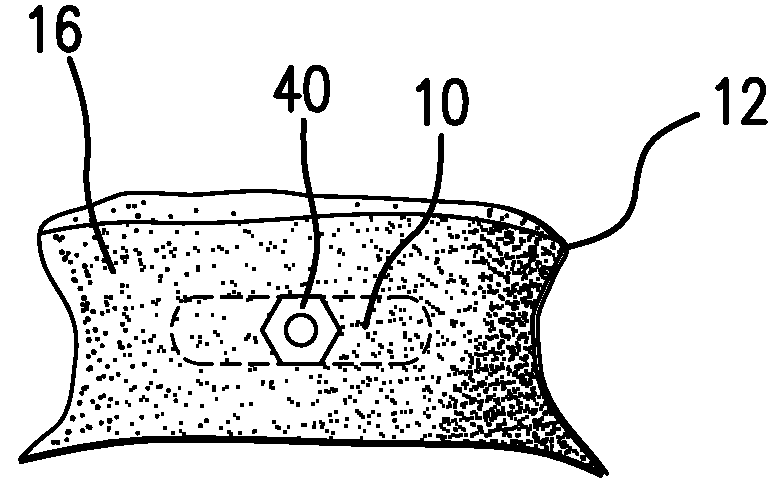
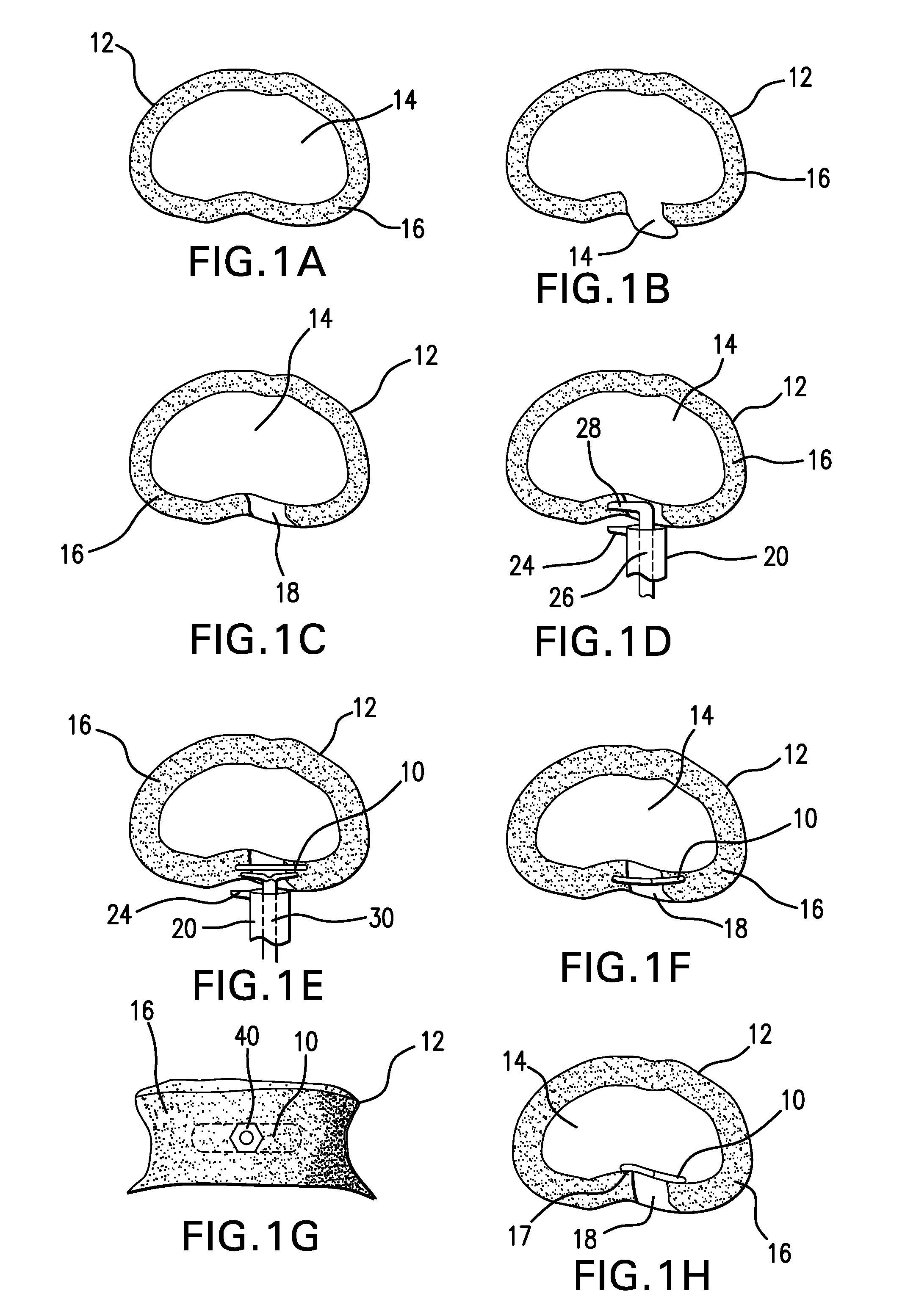
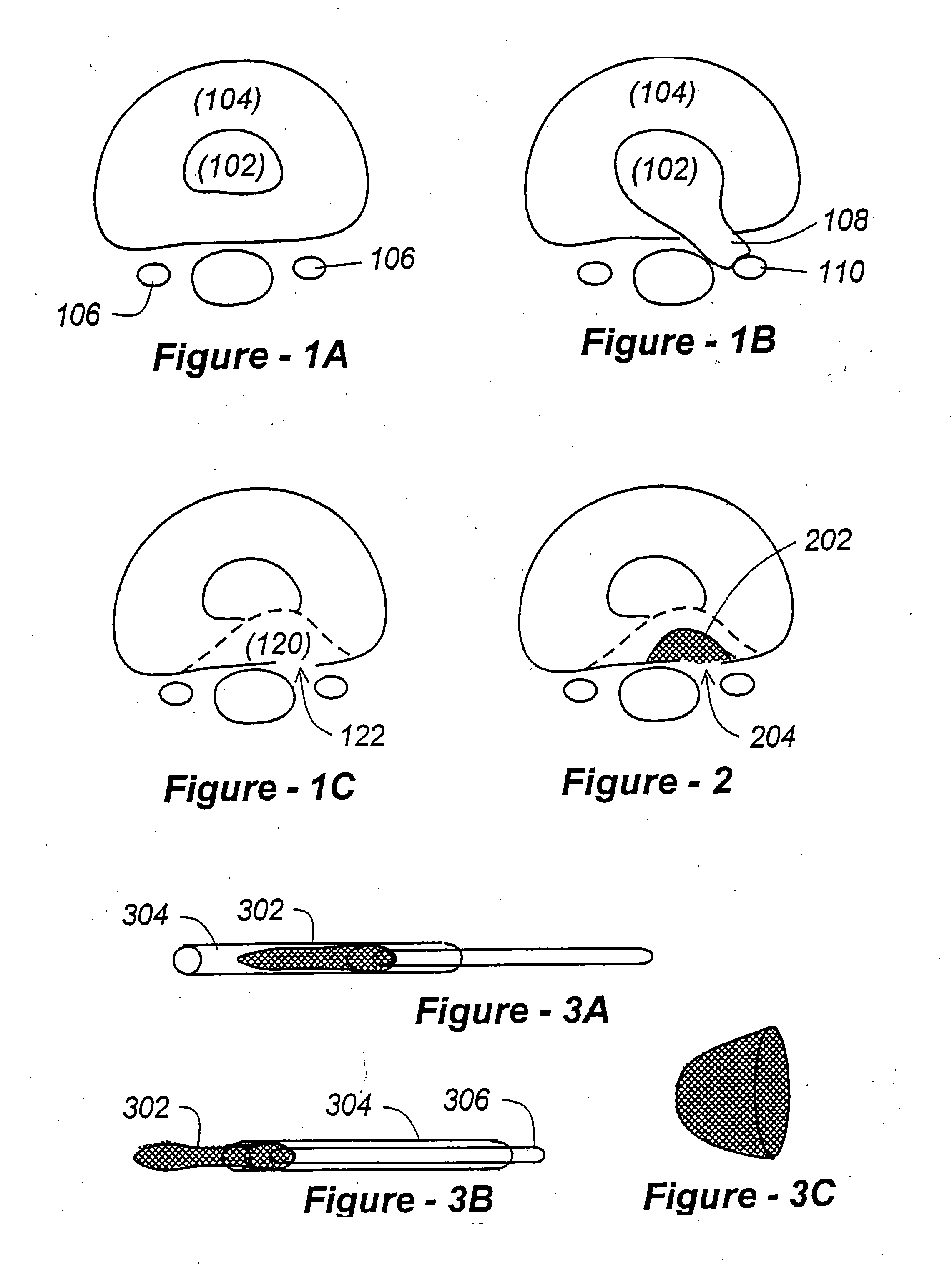
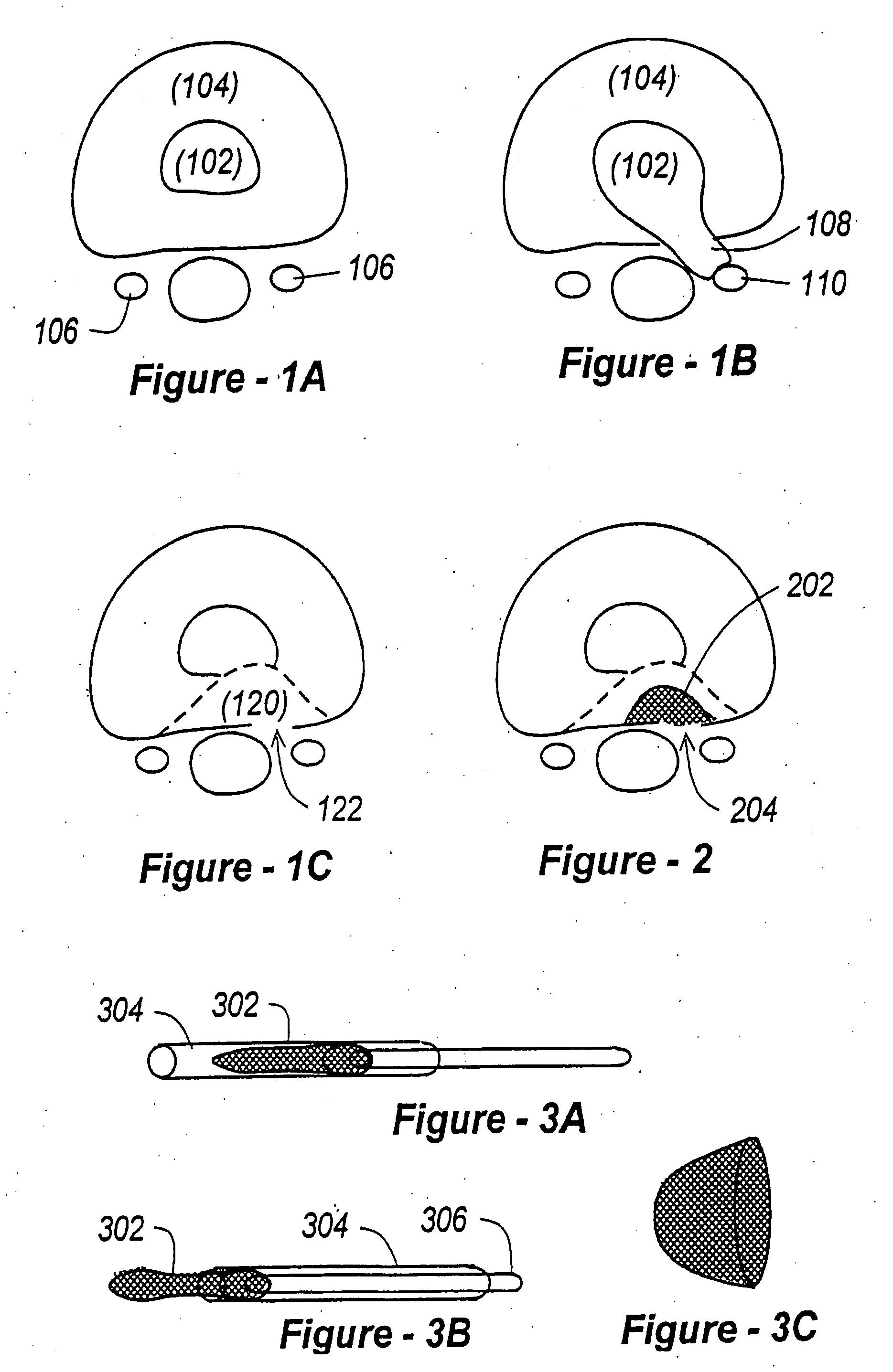
The Annulus Fibrosus Stent (AFS) is a platform of barriers used in the intervertebral disc. The barrier can be inserted into an intervertebral disc to act to reinforce and / or supplement the disc. The AFS can be inserted at any of the many different stages of disc pathology. The AFS can be inserted to prevent pressure and dissection of disc material which in and of itself can decrease and / or eliminate pain from damaged AF fibers. The AFS can be inserted before disc pathology progresses to a substantial event such as a disc herniation. It can be inserted at any time in the progression of the natural history of disc disease. It can even be inserted when substantially the entire intervertebral disc has been removed. It can be inserted in conjunction with another procedure such as a Nucleus Pulposus Replacement (NPR) or Total Disc Replacement (TDR), etc. The various shapes and material of the AFS in this patent are designed to address particular clinical situations, particular anatomy and particular stages of disc degeneration. The AFS can be inserted directly through the AF, or by a technique of detaching part of the AF with bone without actually cutting a substantial part of AF fibers or layers of fibers or by the Transosseous approach.
Owner:HYDE EDWARD ROBERT JR
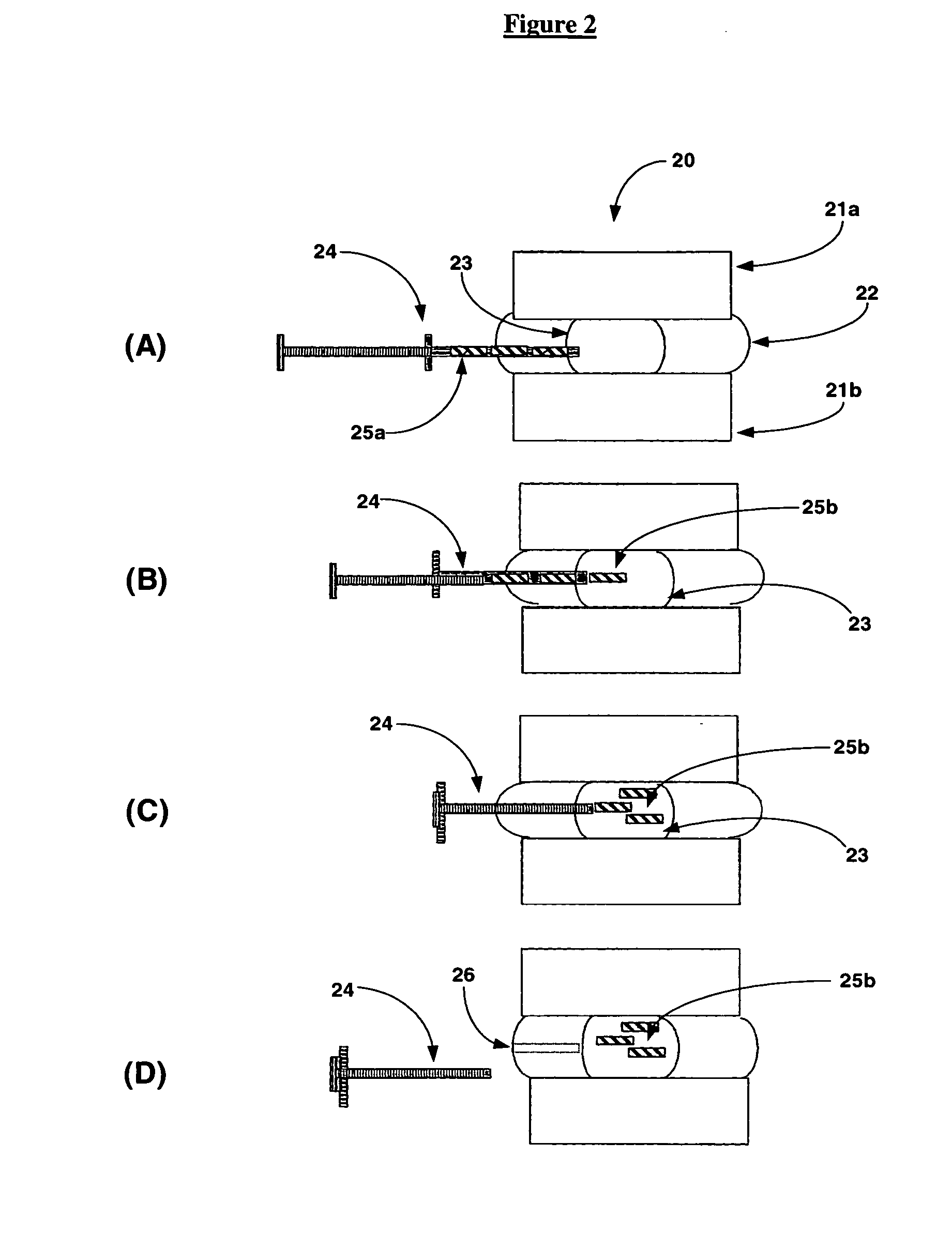
Apparatus and method for accessing and performing a function within an intervertebral disc
Apparatus and methods are disclosed for accessing the interior of an intervertebral disc to perform a function within the disc. The apparatus comprises a catheter having a lumen; and a guide wire having a distal portion and a proximal portion, and configured to be positioned within and moved relative to the lumen of the catheter; wherein the guide wire is capable of navigating itself within an intradiscal section of the intervertebral to a selected section of the disc and the catheter is capable of being advanced relative to the guide wire such that the catheter follows a path of the guide wire within the intradiscal section of the disc to the selected section. These apparatus and methods may be used for the treatment of intervertebral disc disorders such as sealing fissures of the annulus fibrosus, which may or may not be accompanied with contained or escaped extrusions. These apparatus and methods may also be used for the removal or addition of material to the intervertebral disc.
Owner:NEUROTHERM
Spinal implants, including devices that reduce pressure on the annulus fibrosis
InactiveUS20050256582A1Promote reconstructionPrevents herniationInternal osteosythesisBone implantFibrosisDiscectomy
The invention broadly facilitates reconstruction of the Annulus Fibrosus (AF) or the AF and the Nucleus Pulposus (NP). Such Reconstruction prevents recurrent herniation following Microlumbar Discectomy (MLD) other procedures. The invention may also be used in the treatment of herniated discs, annular tears of the disc, or disc degeneration, while enabling surgeons to preserve the contained NP. The methods and apparatus may be used to treat discs throughout the spine including the cervical, thoracic, and lumbar spines of humans and animals. In the preferred embodiment, a spinal repair system according to the invention comprises a first end portion adapted for placement within an intervertebral body, a second end portion adapted for placement within an adjacent intervertebral body, and a bridge portion connecting the first and second end portions, the bridge portion being adapted to span a portion of an intervertebral disc space and prevent excessive outward bulging.
Owner:ANOVA
Method for treatment of defects in the intervertebral disc
InactiveUS20050069571A1Easy to processPromote regenerationBiocideJoint implantsIntervertebral discDefect repair
A minimally invasive spinal disc defect repair method is disclosed. The method relates to insertion of disc defect repair materials that are substantially two-dimensionally shaped and which allow for insertion and preferably self retention within the disc defect with plugging of the annulus fibrosis.
Owner:DEPUY ACROMED INC
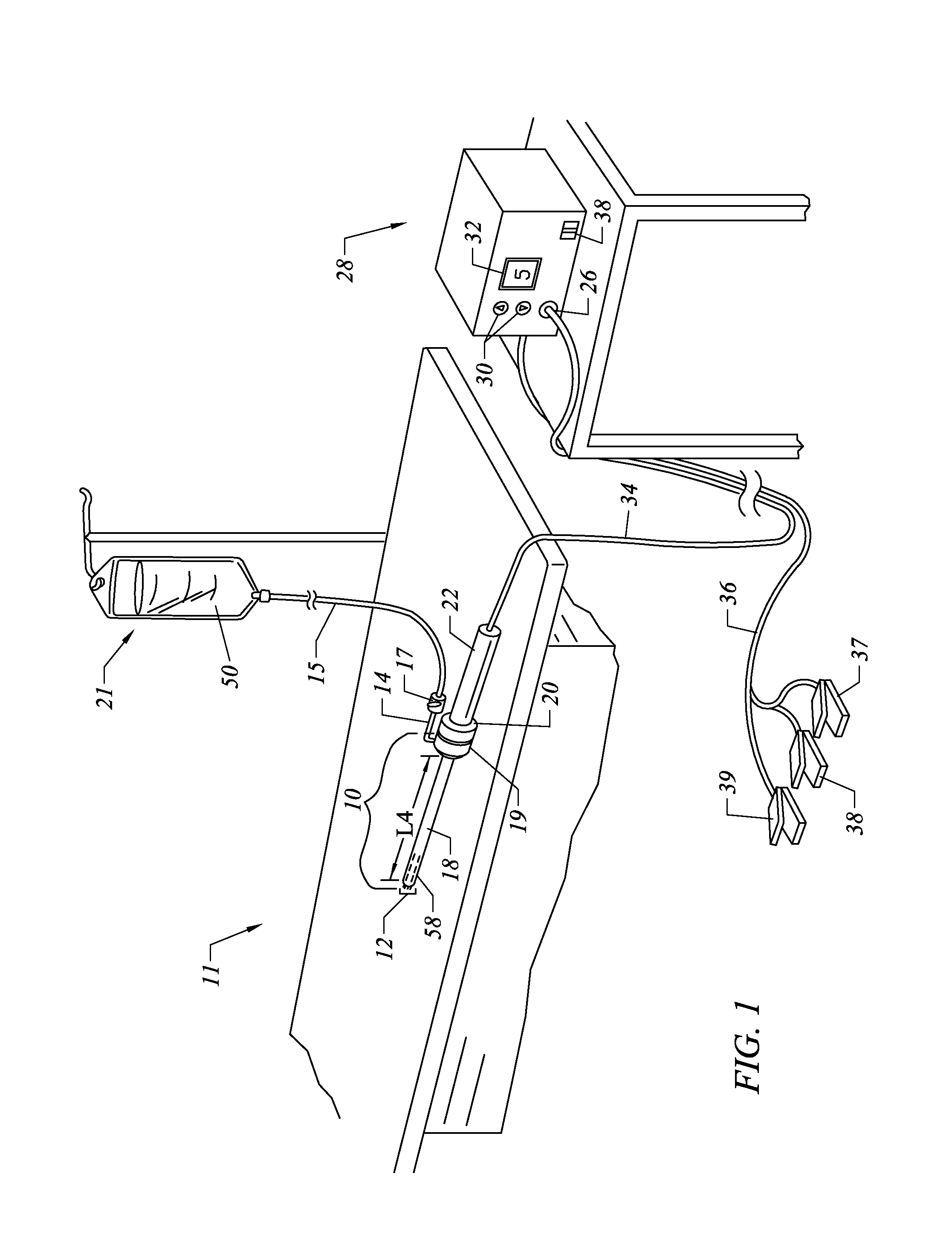
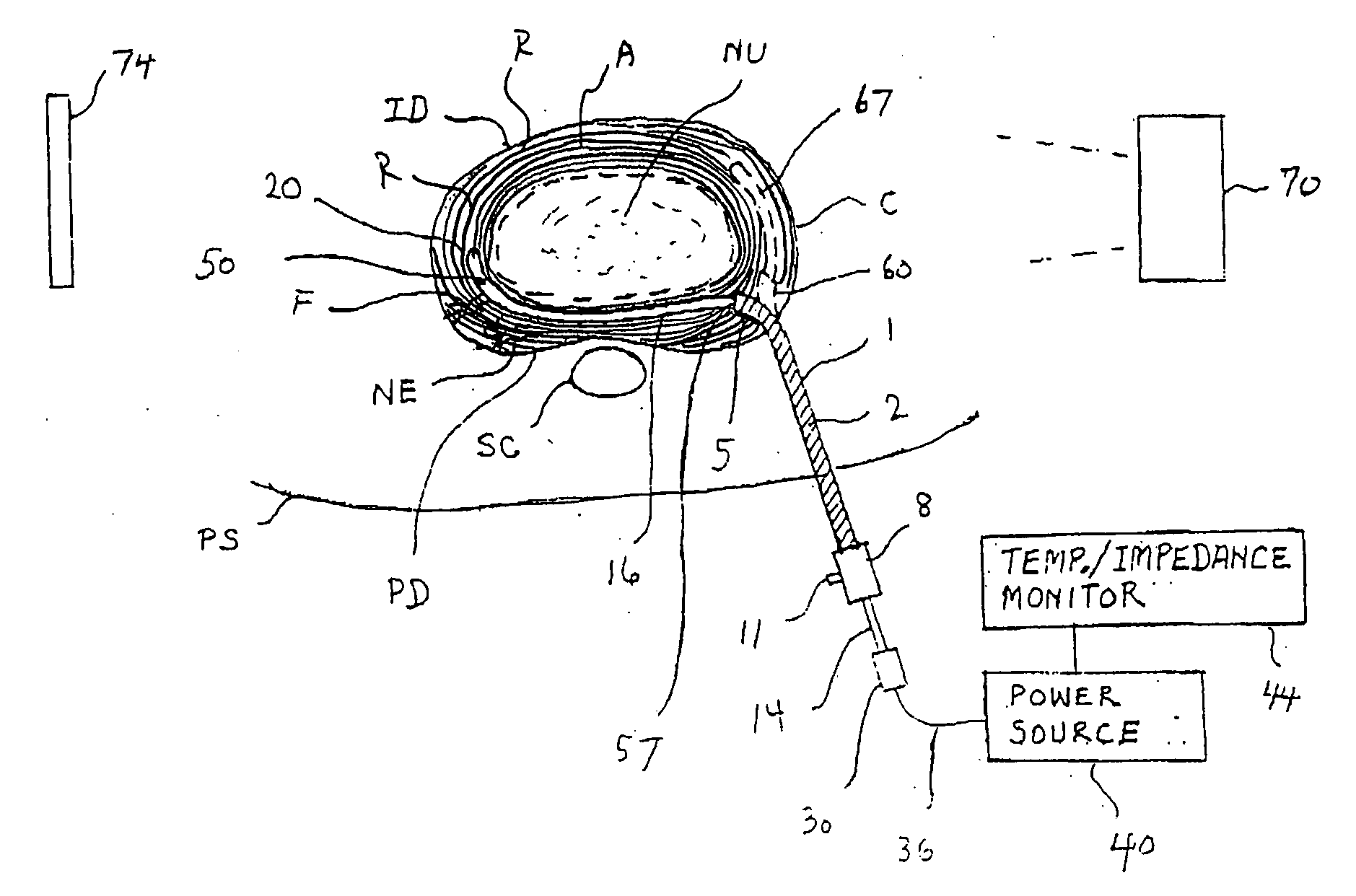
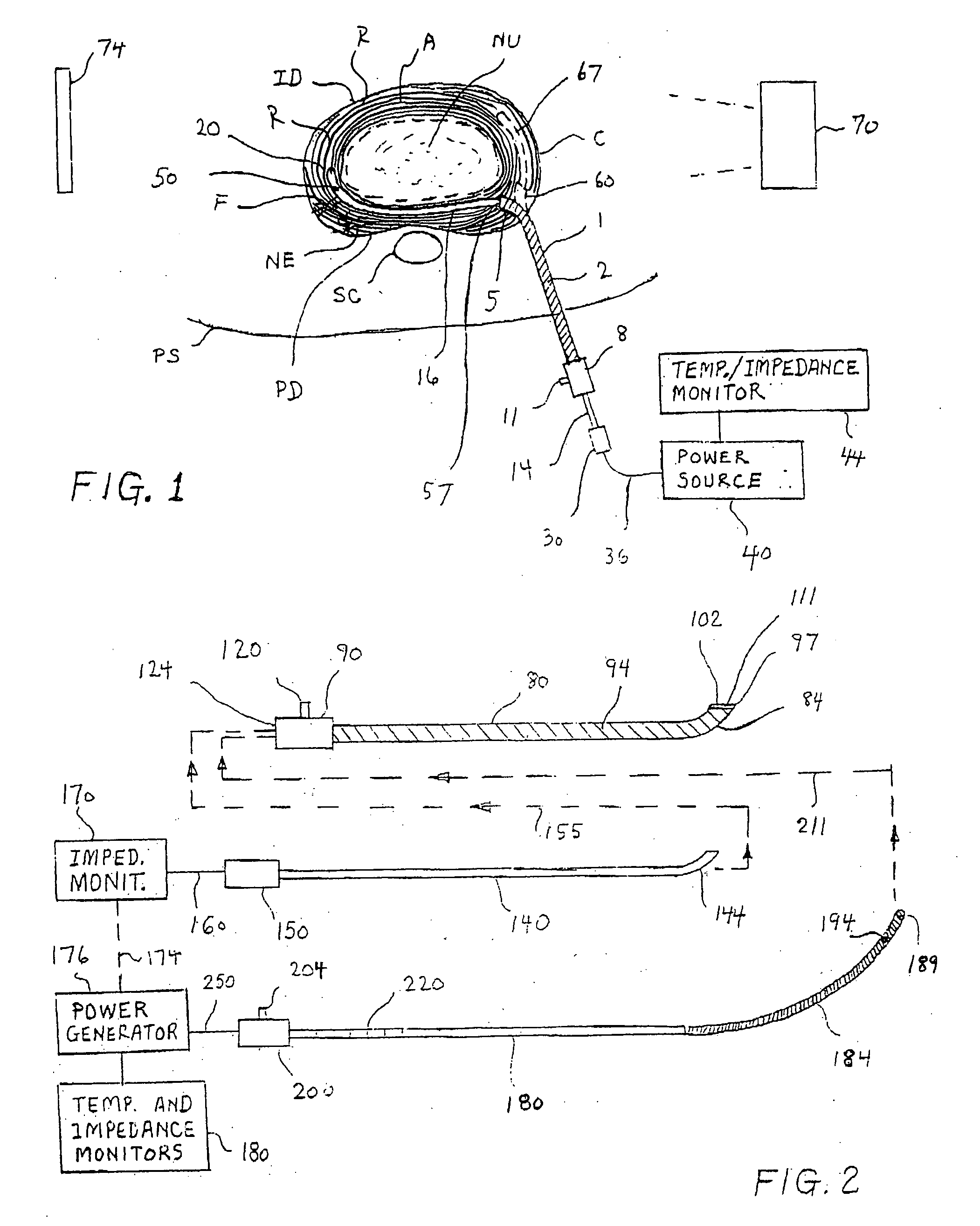
Methods and apparatus for treating back pain
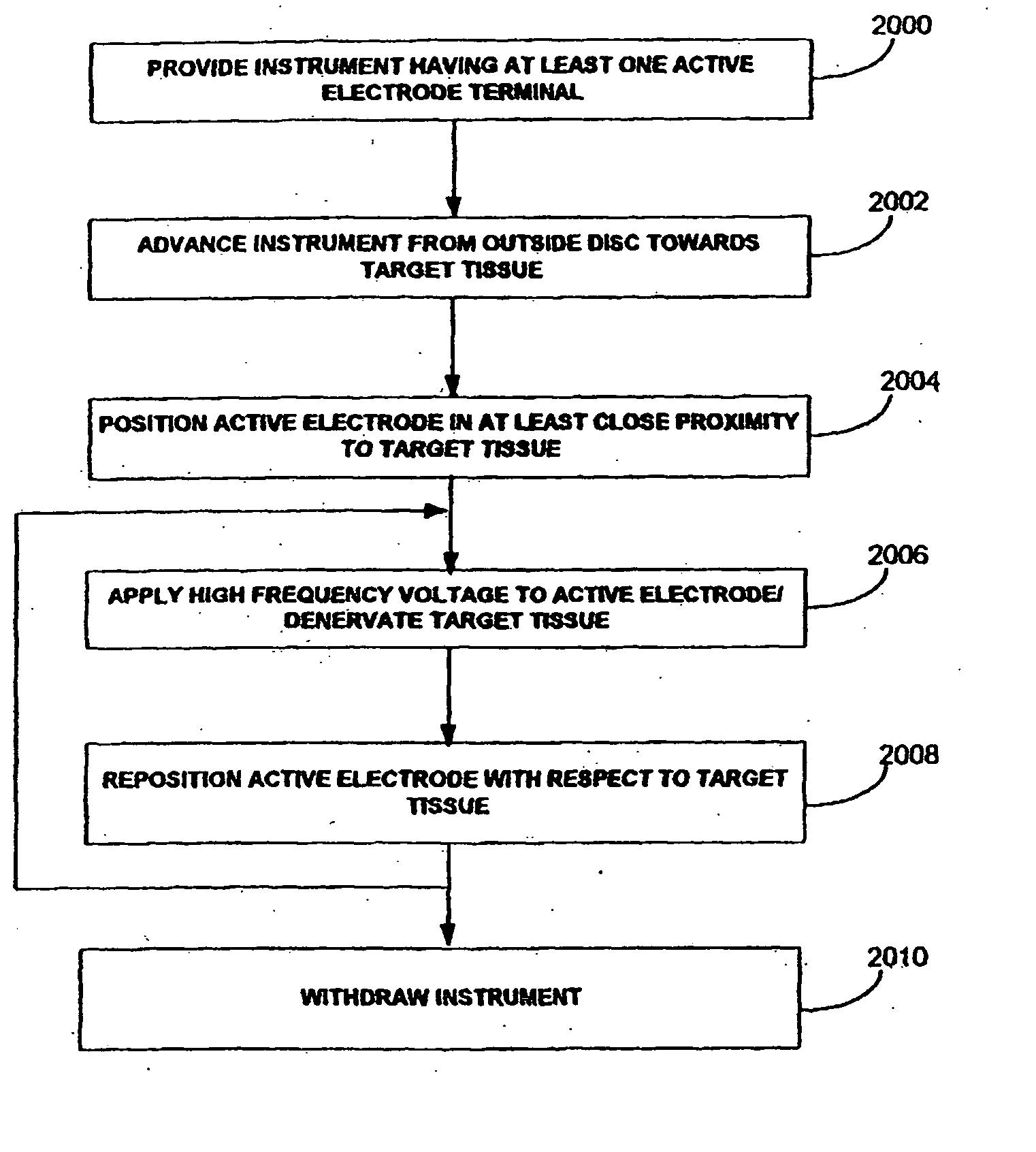
Apparatus and methods for treating back pain of a patient by denervation of an intervertebral disc or a region of the posterior longitudinal ligament by the controlled application of heat to a target tissue. In one embodiment, the invention may include a procedure combining both decompression of a disc, and denervation of the annulus fibrosus. In one embodiment, a method of the invention includes positioning an active electrode of an electrosurgical instrument in at least close proximity to an intervertebral disc, and applying at least a first high frequency voltage between the active electrode and a return electrode, wherein nervous tissue within the annulus fibrosus is inactivated, and discogenic pain of the patient is alleviated. In one embodiment, the invention includes positioning a first electrode of a dual-shaft electrosurgical instrument at a first location in relation to a target disc, positioning a second electrode of the instrument at a second location, and applying a high frequency voltage between the first and second electrodes, wherein the first and second electrodes are disposed on separate shafts of the instrument.
Owner:ARTHROCARE
Percutaneous methods for injecting a curable biomaterial into an intervertebral space
A method for treating a spinal disc having an outer relatively intact annulus defining a disc space and an inner defective nucleus pulposus within the disc space, comprises the steps of: determining the integrity of the annulus by subjecting the annulus to a first pressure applied internally of the annulus; providing access to the nucleus pulposus through the annulus without removing any tissue from the annulus or from the nucleus pulposus; and sealably injecting curable biomaterial through the annulus access directly into the nucleus pulposus at a second pressure correlated with the first pressure. The integrity of the annulus may be determined by a pre-operative discogram using a contrast medium that has a viscosity substantially similar to the viscosity of the biomaterial to be injected. The needle is placed initially within the center of the nucleus pulposus and then withdrawn during the injection to approximately the inner border of the annulus. The second pressure is then maintained until the biomaterial is substantially cured. In steps, the curable biomaterial has strong adhesive properties and is capable of injection under pressure to fill fissures in the nucleus pulposus. The curable biomaterial may be injected under a pressure sufficient to distract opposing vertebral bodies communicating with the disc space.
Owner:SPINEWAVE
Method and apparatus for the treatment of the intervertebral disc annulus
InactiveUS20090259260A1Shorten the lengthSuture equipmentsBone implantPresent methodIntervertebral disk
This disclosure presents methods and devices for treating a tear, rent, incision, defect, aperture or delamination of the annulus fibrosus of an intervertebral disc. The methods and devices can employ fixation delivery apparatuses, fixation apparatuses, patch delivery tools and patches positioned, at least in part, in or on aspects of an intervertebral disc for treatment of the intervertebral disc or its components. In some aspects, these techniques include the use of this includes a fixation apparatus that includes at least one bone anchor connected to at least one disc anchor by a shortenable elongate member.
Owner:KRT INVESTORS
Methods for injecting a curable biomaterial into an intervertebral space
A method for treating a diseased or damaged spinal disc comprises the steps of: (a) providing access to the nucleus pulposus through the annulus; (b) removing at least a portion of the nucleus pulposus to create an intradiscal space; determining the size of the intradiscal space; and (c) sealably introducing under pressure a curable biomaterial through the annulus directly into the intradiscal space. The method may include the additional steps of applying a force to distract the opposing vertebral bodies about the intradiscal space and then removing the distraction force after the biomaterial has cured. The step of determining the size of the intradiscal space may be accomplished by expanding a compliant balloon within the intradiscal space using a contrast medium capable of visualization under fluoroscopy. The curable material is sealably introduced through a vented needle inserted through the opening. The curable biomaterial is introduced until a quantity of the material flows into the vent.
Owner:SPINEWAVE
Spinal nucleus replacement implants
InactiveUS20100185287A1Spinal implantsInstruments for stereotaxic surgeryReplacement implantSpinal cord
A multi-piece intervertebral disc augmentation implant that may be assembled within the annulus fibrosus is disclosed. The implant may be guided into a precise location through a relatively small opening in the annulus fibrosus with the aid of an elongated guide.
Owner:WARSAW ORTHOPEDIC INC
Apparatus and method for accessing and performing a function within an intervertebral disc
Apparatus and methods are disclosed for accessing the interior of an intervertebral disc to perform a function within the disc. The apparatus comprises a catheter having a lumen; and a guide wire having a distal portion and a proximal portion, and configured to be positioned within and moved relative to the lumen of the catheter; wherein the guide wire is capable of navigating itself within an intradiscal section of the intervertebral to a selected section of the disc and the catheter is capable of being advanced relative to the guide wire such that the catheter follows a path of the guide wire within the intradiscal section of the disc to the selected section. These apparatus and methods may be used for the treatment of intervertebral disc disorders such as sealing fissures of the annulus fibrosus, which may or may not be accompanied with contained or escaped extrusions. These apparatus and methods may also be used for the removal or addition of material to the intervertebral disc.
Owner:NEUROTHERM
Apparatus for thermal treatment of an intervertebral disc
InactiveUS20040015218A1Surgical instruments for heatingTherapeutic coolingThermal energyThermal probe
An apparatus and method for treating an intervertebral disc having an inner nucleus pulpous and an outer annulus fibrous includes a thermal probe defining proximal and distal ends and having a guidable region adjacent the distal end thereof. The guidable region is characterized by having sufficient rigidity to advance within the annulus fibrous of the intervertebral disc in response to an axial force exerted on the proximal end of the thermal probe while having sufficient flexibility to substantially follow and conform to an azimuthal course defined by the natural striata of the annulus fibrous. The thermal probe is adapted for connection to a thermal energy source to provide thermal energy to the annulus fibrous to alleviate pain associated with the intervertebral disc.
Owner:JTC TRUSTEES +1
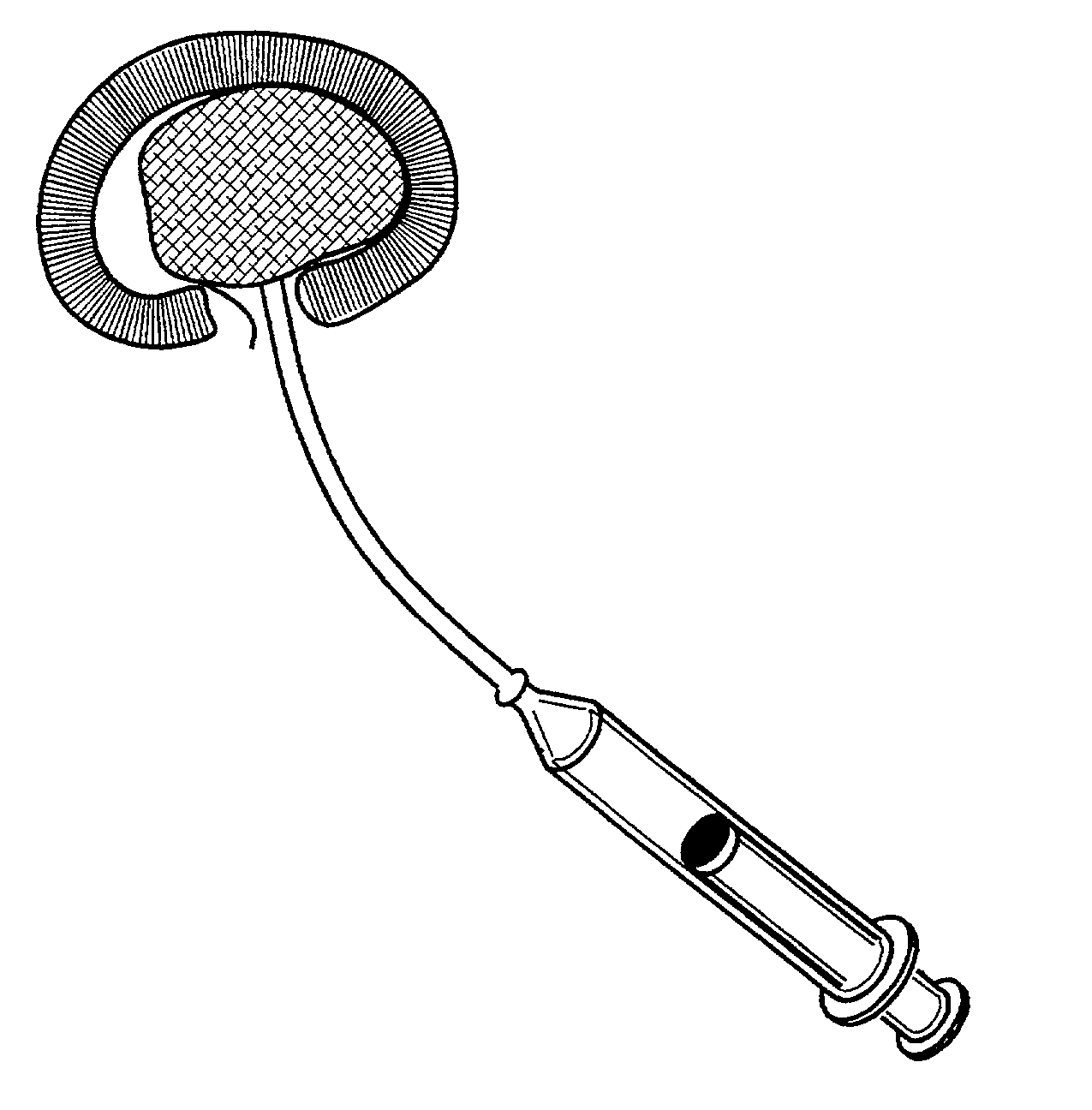
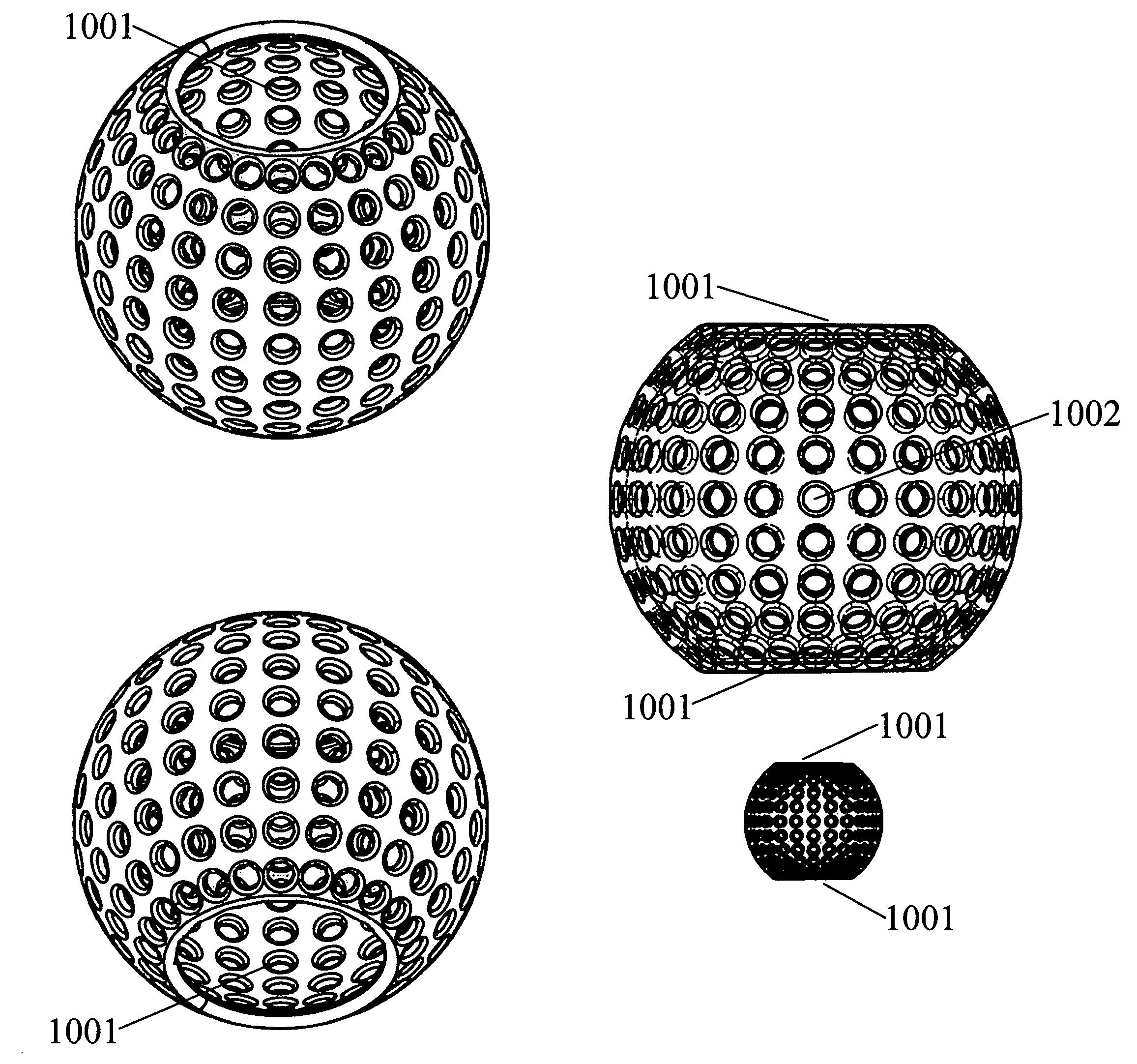
Method and apparatus for implanting a hydrogel prosthesis for a nucleus pulposus
An instrument for inserting an elongated hydrogel prosthesis into an intervertebral disc includes an insertion cannula that is inserted through the annulus fibrosus of an intervertebral disc to provide access to the nucleus region of the disc, an elongated hydrogel prosthesis packaged within a tubular container adapted to be coupled to a proximal end of the insertion cannula, and a source of fluid pressure adapted to be coupled to a proximal end of the tubular container. Auxiliary instruments for use in convenient insertion of the insertion cannula through the nucleus pulposus and providing for a complete and controlled passage of the hydrogel prosthesis through the insertion cannula are provided in a kit with the insertion cannula. A sizing apparatus for determining the volume of prosthesis to be inserted includes a catheter or cannula capable of being inserted through the insertion cannula into the nucleus region of the intervertebral disc and having at its distal end a balloon capable of being inflated within the intervertebral disc with a measurable volume of a fluid in order to determine the amount of hydrogel prosthesis to be injected.
Owner:REGELTEC INC
Supplementation or replacement of a nucleus pulposus of an intervertebral disc
ActiveUS20080312744A1Avoid damageFunction increaseDiagnosticsBone implantMedicineIntervertebral disc
A degenerated nucleus pulposus located in a central core region of an intervertebral disc within the annulus fibrosus is supplemented or replaced by a method wherein an amount of a biocompatible material is introduced into the central core region by a process including the steps of 1) forming a channel through a vertebral body adjacent to said intervertebral disc, extending from an exterior surface of the vertebral body to the central core region of the annulus fibrosus; 2) introducing an amount of a biocompatible material through the channel into the central core region of the annulus fibrosus; 3) pressurizing the biocompatible material through the channel to a postsurgical pressure sufficient to alleviate symptoms caused by the degenerated nucleus pulposus; and 4) sealing the channel while maintaining the sufficient postsurgical pressure. After sealing the channel, a vertebroplasty may optionally be performed in the vertebra.
Owner:SYNTHES USA
Methods and apparatus for treating disc herniation and preventing the extrusion of interbody bone graft
InactiveUS20070027471A1Minimized in sizeAvoiding delicateBone implantJoint implantsFibrosisAppendage
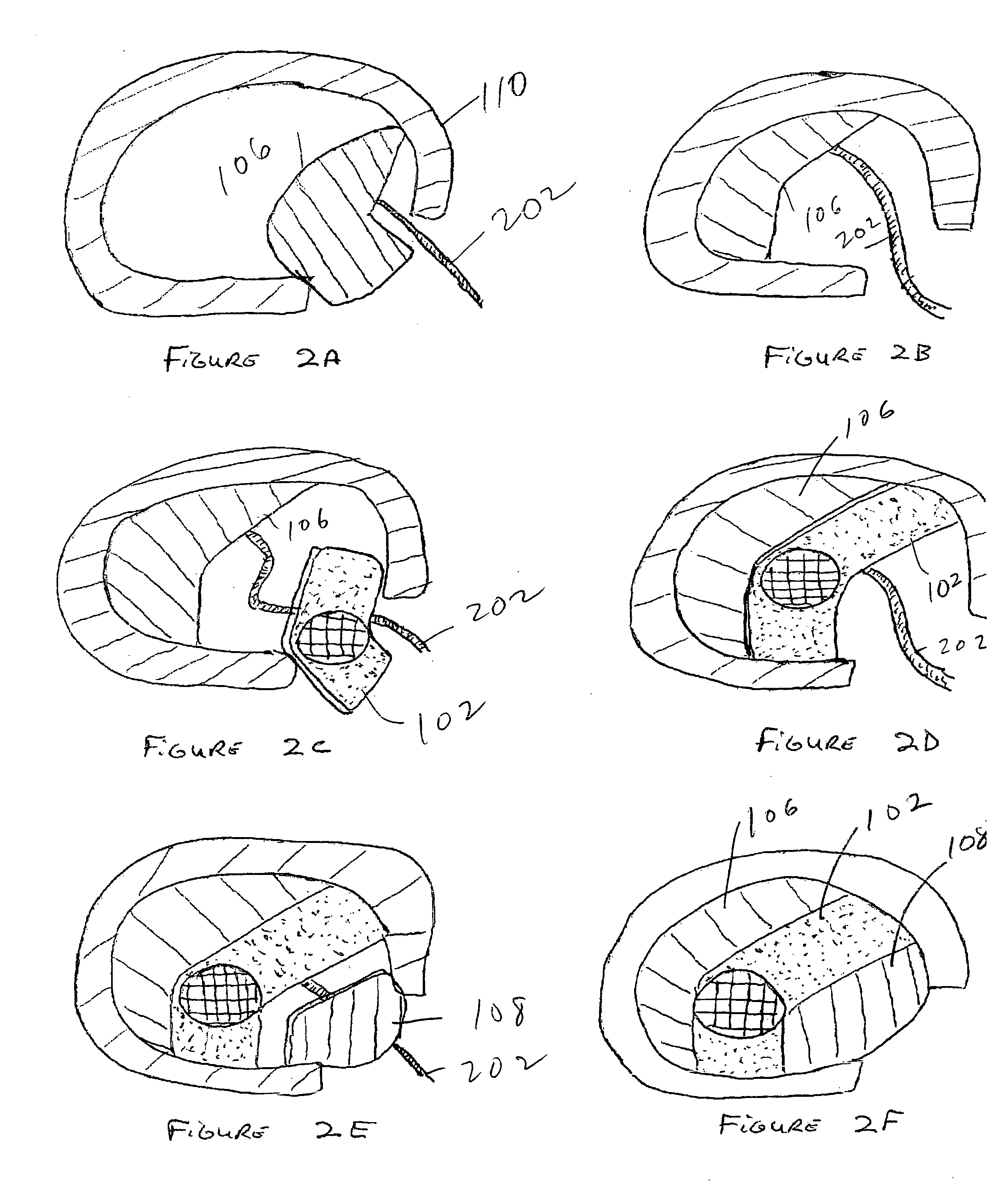
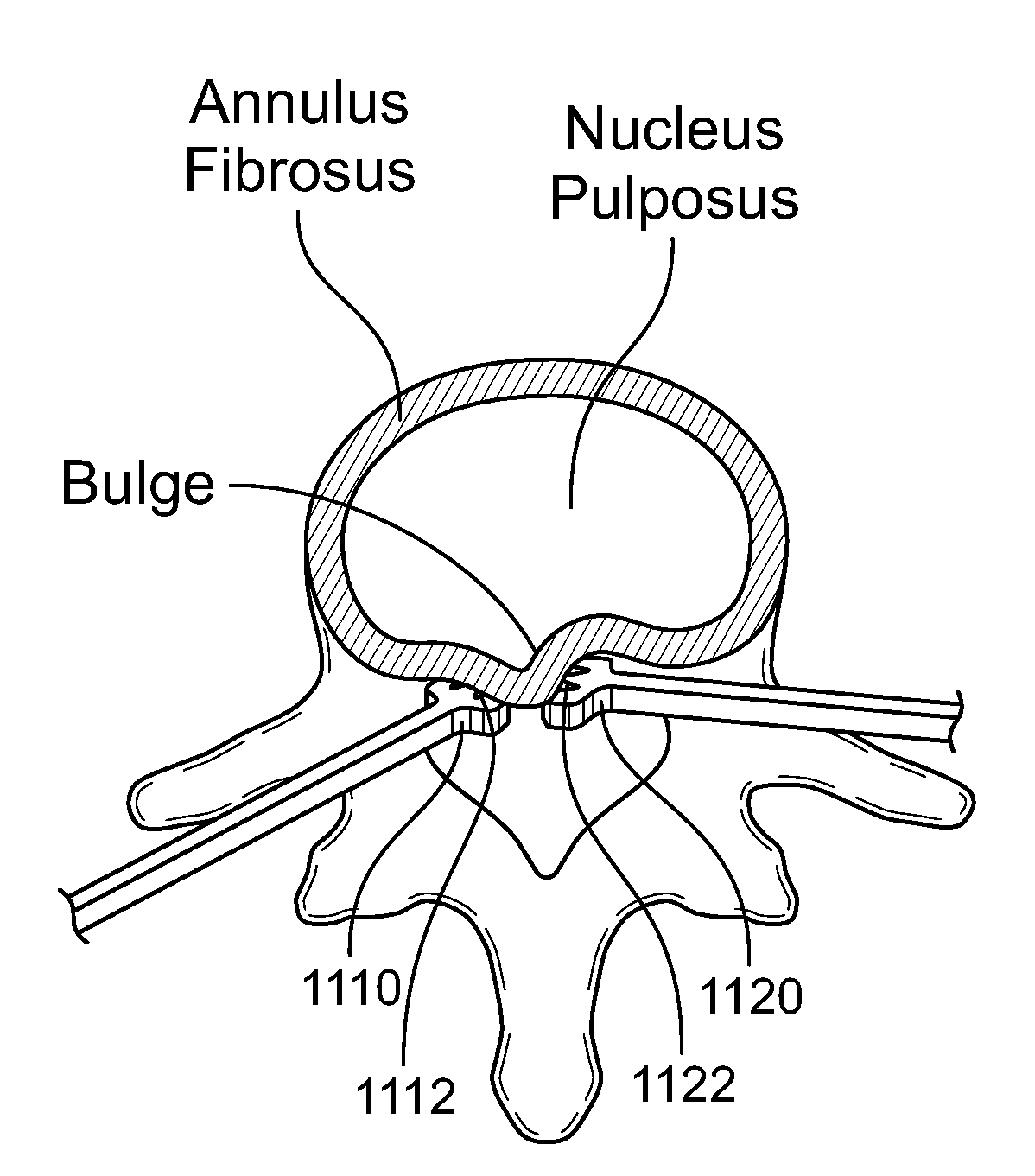
Methods for treating an annulus fibrosis having a defect include inserting a flexible device into the defect. The flexible device is advanced distally beyond an outer layer of the annulus fibrosus. The flexible device is then expanded such that a width of the flexible device is larger than the defect, where the flexible device prevents escape of nucleus pulposus through the defect. The flexible device may have at least two appendages made from a shape-memory metal. Alternatively, the flexible device may have a U-shaped structure that includes a central portion and two legs. The flexible device may also be anchored to the annulus fibrosis and / or the vertebrae.
Owner:SUTURE CONCEPTS
Methods and apparatus for reconstructing the annulus fibrosis
InactiveUS20070055375A1Promote reconstructionPrevents recurrent herniationBone implantSpinal implantsFibrosisEngineering
Methods and devices for fixing a defect in the annulus fibrosis of a patient. The devices include first and second vertical components extending from the middle region of the horizontal component, each of the first and second vertical components having a width and an end. The middle region of the horizontal component of the device to block the defect in the annulus fibrosis. The first vertical component is attached to the upper vertebra and the second vertical component is attached to the lower vertebra. The horizontal component can be positioned beyond at least an outer layer of the annulus fibrosis, alternatively positioned beyond the innermost layer of annulus fibrosis, alternatively positioned between adjacent layers of annulus fibrosis, or alternatively positioned on the exterior of the annulus fibrosis.
Owner:ANOVA
Spacerless artificial disc replacements
InactiveUS20080027548A9More wear-resistantMore flexiblyJoint implantsSpinal implantsAlloyAxial rotation
Spacerless artificial disc replacements (ADRs) are disclosed. One preferred embodiment includes two saddle-shaped components to facilitate more normal spinal flexion, extension, and lateral bending while limit axial rotation, thereby protecting the facet joints and the annulus fibrosus (AF). Either or both the superior and inferior components are made of a hard material such as chrome cobalt, titanium, or a ceramic including alumina, zirconia, or calcium phosphate. The articulating surfaces of the ADR are also preferably highly polished to reduce friction between the components. Metals, alloys or other materials with shape-memory characteristics may also prove beneficial.
Owner:HOWMEDICA OSTEONICS CORP
Assembled disc spacers
InactiveUS20060004454A1Reduce pressureImproves upon disc spacers (DS)Joint implantsSpinal implantsPosterior approachLamina terminalis
Disc spacers (DS) are assembled within a disc space, allowing them to be inserted through smaller openings in the Annulus Fibrosus (AF). The large size of the assembled DS decreases the pressure on the vertebral endplates. Embodiments of the invention may be used in the cervical, thoracic, or lumbar spine. The DS may be inserted through an anterior, lateral, or posterior approach to the spine using annulus preserving techniques. The devices are preferably made of biocompatible materials such as titanium, chrome cobalt, and ceramic. Alternative materials, including materials with shape memory properties, such as Nitinol, may be used to form one or more components of the device.
Owner:FERREE BRET A +1
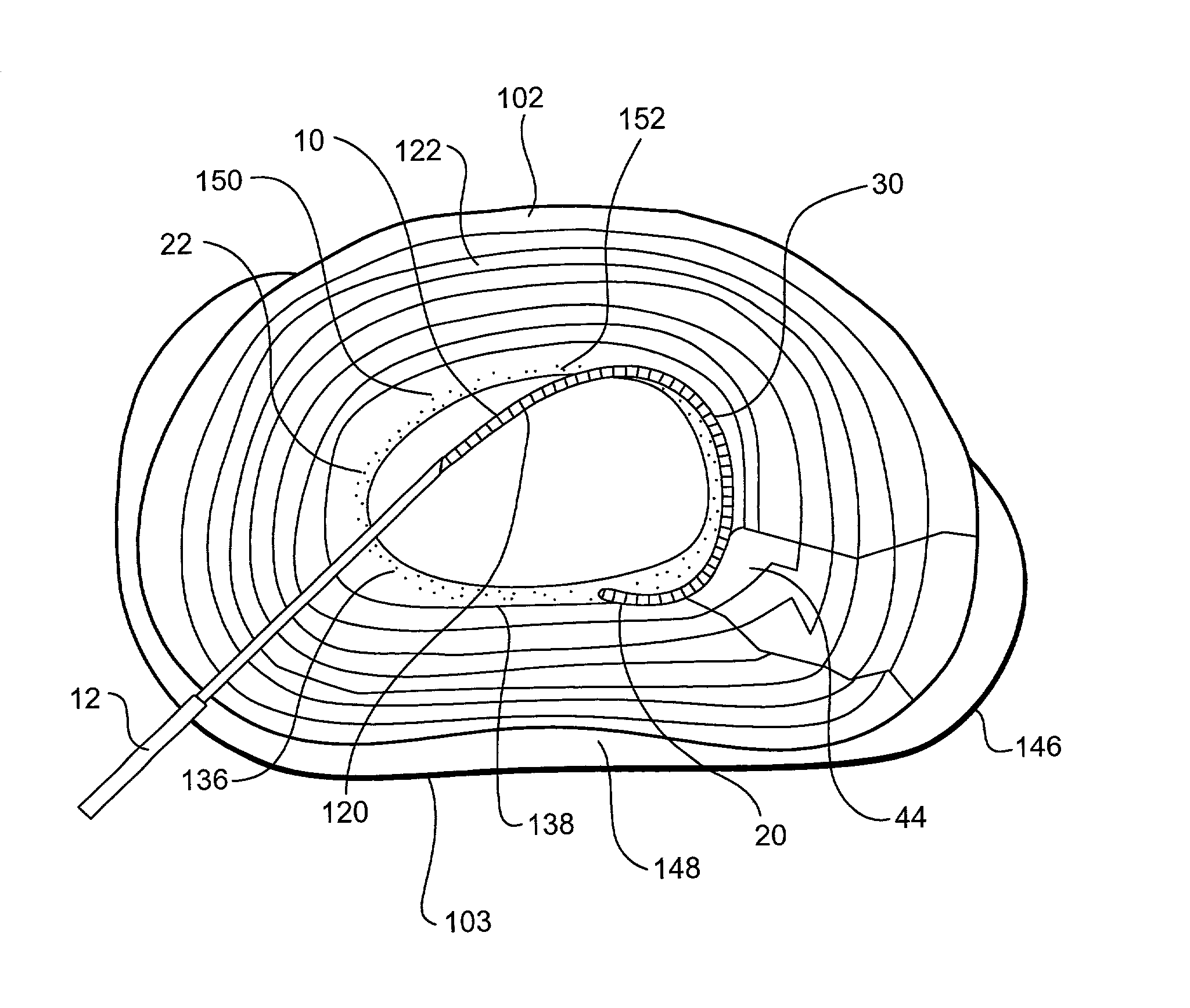
Annular repair device and method
ActiveUS20100185285A1Occlude any subsequent leakage of nucleus pulposusImprove sealingSpinal implantsOsteosynthesis devicesDevice implantIntervertebral disc
Provided is a device and method for repairing a damaged intervertebral disc in the spine of a patient. The device provided having at two slidably engaged components that permit ease of insertion into the void created in the annulus fibrosis of the damaged disc. Also provided is a method of implanting the device into the annulus fibrosis of a damaged disc.
Owner:PERKINS RICHARD
Methods for treating a defect in the annulus fibrosis
InactiveUS20070156152A1Minimized in sizeAvoiding delicateInternal osteosythesisBone implantFibrosisDiscectomy
Methods and apparatus for treating disc herniation provide a conformable device which assumes a first shape associated with insertion and a second shape or expanded shape to occlude the defect which typically follows partial discectomy. The device may take different forms according to the invention, including patches size to cover the defect or plugs adapted to fill the defect. In a preferred embodiment, however, the device is a gel or other liquid or semi-liquid which solidifies to occlude the defect from within the body of the disc itself. In another preferred embodiment, a mesh screen is collapsed into an elongated form for the purposes of insertion, thereby minimizing the size of the requisite incision while avoiding delicate surrounding nerves. Such a configuration also permits the use of instrumentation to install the device, including, for example, a hollow tube or sheath adapted to hold the collapsed screen, and a push rod to expel the collapsed device out of the sheath for use in occluding the disc defect. A device according to the invention may further include one or more anchors to assist in permanently affixing the device with respect to the defect.
Owner:ANOVA
Annular repair devices and methods
Methods and devices are described for occluding openings in an annulus fibrosis to prevent conditions such as disc herniation and recurrent disc herniation. An occluding device having an intradiscal component, an extradiscal component, and a barrier element disposed between the intradiscal and extradiscal components is provided. The intradiscal component has first and second arms having a collapsed state with a horizontal dimension less than the width of the opening and an expanded state having a horizontal dimension greater than the width of the opening. The barrier element and the intradiscal component in the collapsed state are inserted through the annulus fibrosis opening, the extradiscal component being positioned adjacent an outer surface of the annulus fibrosis. The intradiscal component assumes the expanded state in the intradiscal space, the first and second arms urging the barrier element against the inner wall of the annulus fibrosis adjacent the opening, thereby occluding the opening.
Owner:ANOVA
Treatment of spinal tissue
ActiveUS20180317997A1Small sizeElectrotherapySurgical instruments for heatingEnergy basedIntervertebral disk
A method of treating an intervertebral disc without piercing the disc involves navigating a treatment element to the intervertebral disc and contacting the treatment element with an exterior of the intervertebral disc. The method further involves applying an energy-based treatment to the exterior of the intervertebral disc to modify a property of a target tissue of the intervertebral disc. The target tissue may be, for example, annulus fibrosus and / or nucleus pulposus tissue. The treatment element is then removed, and the property remains modified after the treatment element is removed and the target tissue heals, thereby treating the intervertebral disc.
Owner:AERIN MEDICAL
Methods and apparatus for treating disc herniation and preventing the extrusion of interbody bone graft
InactiveUS20070142839A1Minimized in sizeAvoiding delicateSuture equipmentsBone implantMedicineFibrosis
Methods and apparatus for treating defects in the annulus fibrosis are described. The methods include providing first and second elongate fastening members, each having a first end and an anchor on the first end that is substantially transverse when deployed. The first end of the first and second elongate fastening members are positioned distal of the outer layer and at least one inner layer of the annulus fibrosis. The defect is then closed by interlocking the first and second elongate fastening members. Alternatively, second ends of the elongate fastening members may be linked, resulting in reducing the size of the opening of the defect. Devices having first and second elongate fastening members, each having a first end, a second end, and an anchor on the first end that is substantially transverse when deployed; and a connector that links the first and second elongate fastening members are also described.
Owner:ANOVA
Degenerative disc regeneration techniques
InactiveUS20070093905A1Reducing TNF-a productionLowering IL-1 productionSpinal implantsTissue regenerationIntervertebral diskDegenerative disc disease
In the repair / regenerate of the intervertebral disc, all or a portion of the nucleus pulposus or annulus fibrosus is excised and treated for reinsertion into the disc or adjacent or alternate level disc. Alternatively certain bioactive agents may be injected into the degenerative disc without excising disc material.
Owner:DEPUY SPINE INC (US) +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com