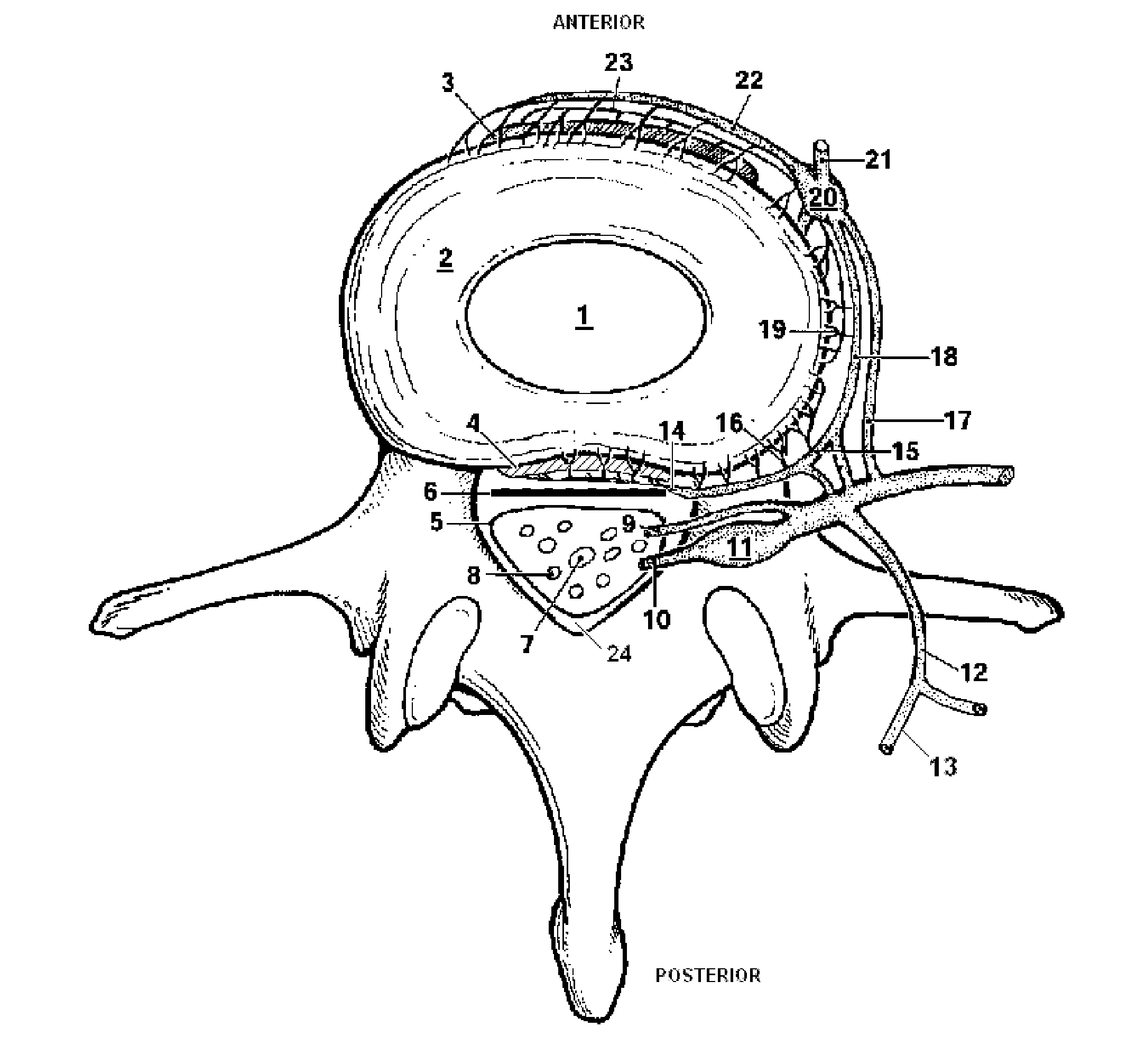
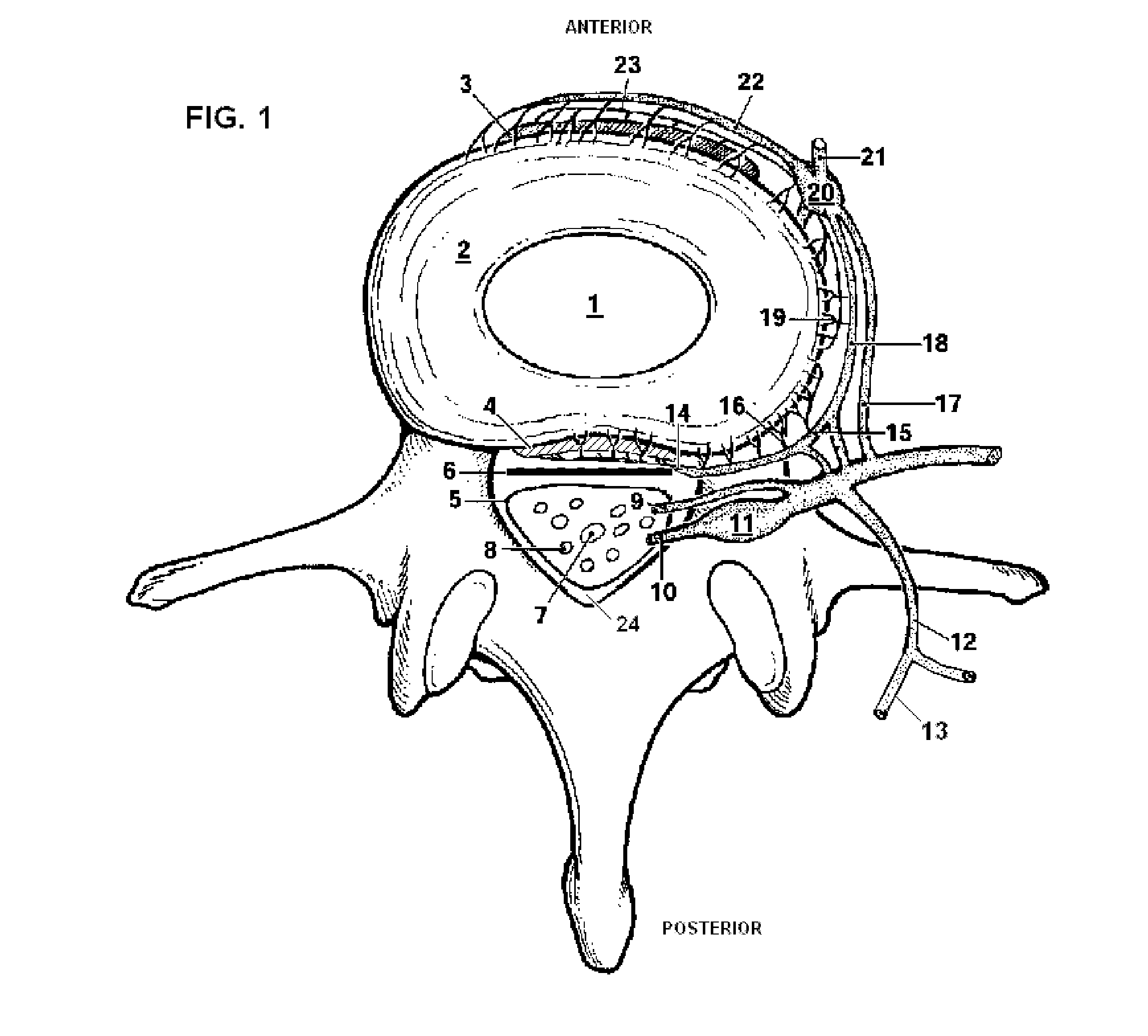
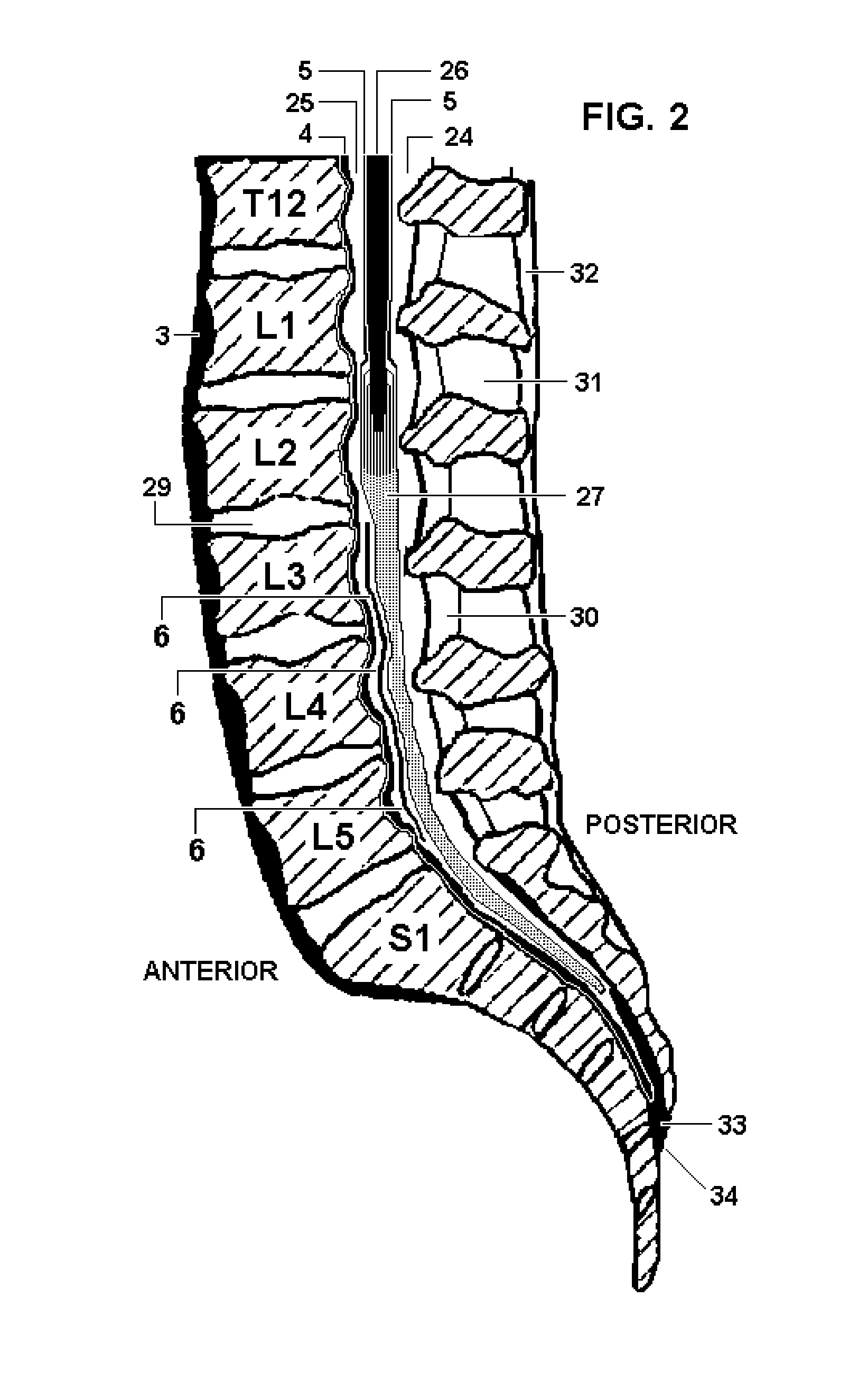
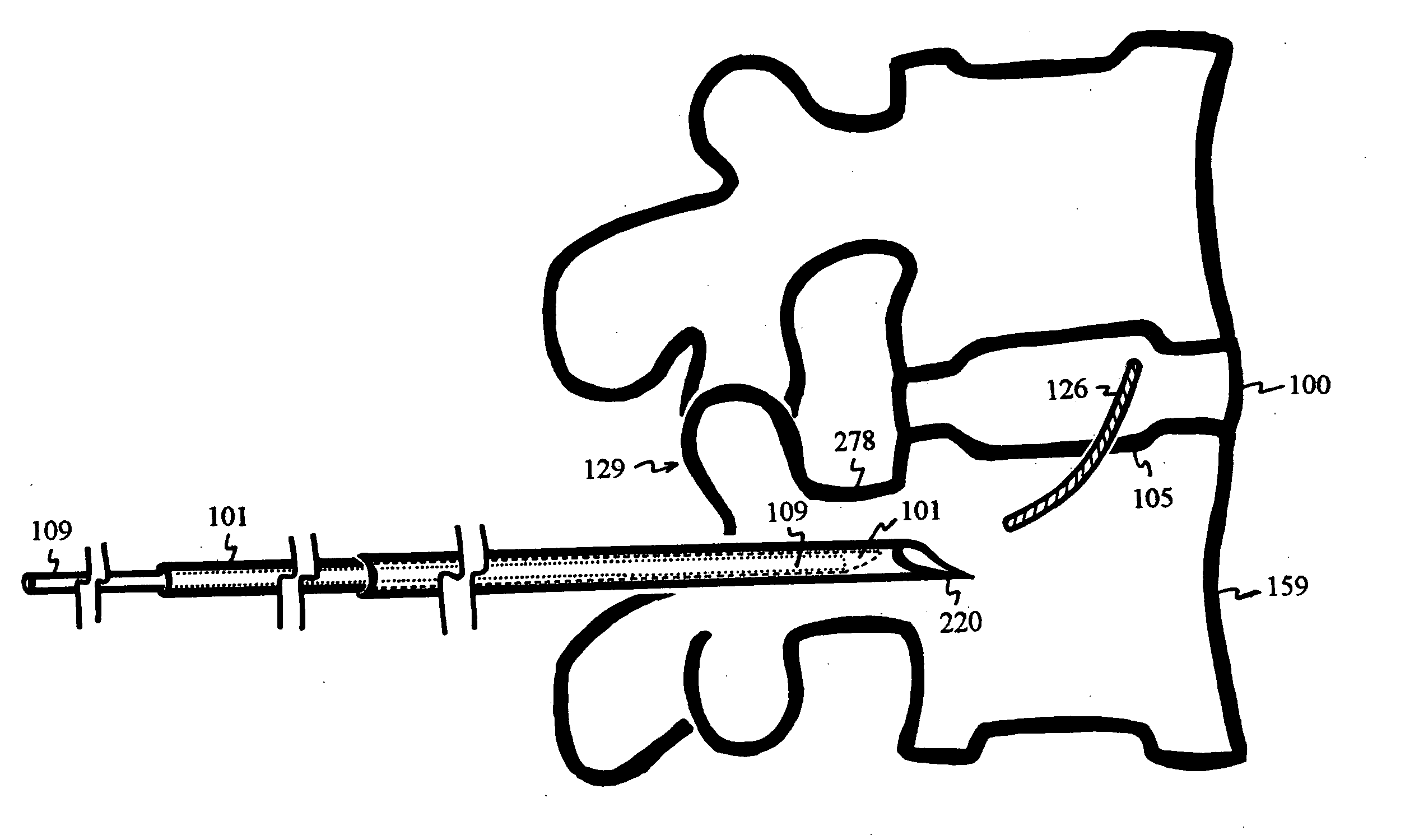
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
143results about How to "Relieve back pain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for repairing damaged intervertebral discs
InactiveUS7318823B2Reduce internal pressureReduce moistureBiocideOrganic chemistryIntervertebral discActive electrode
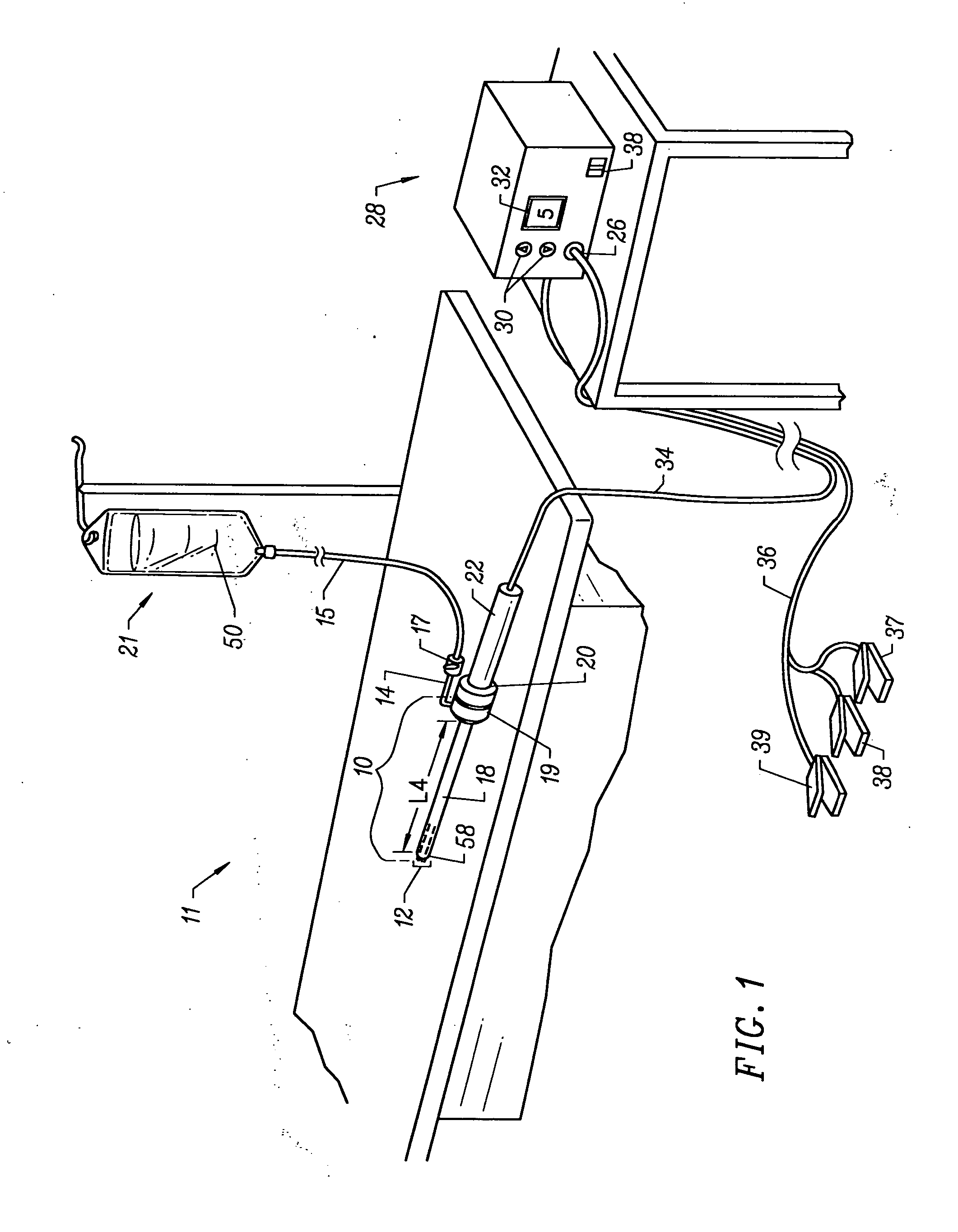
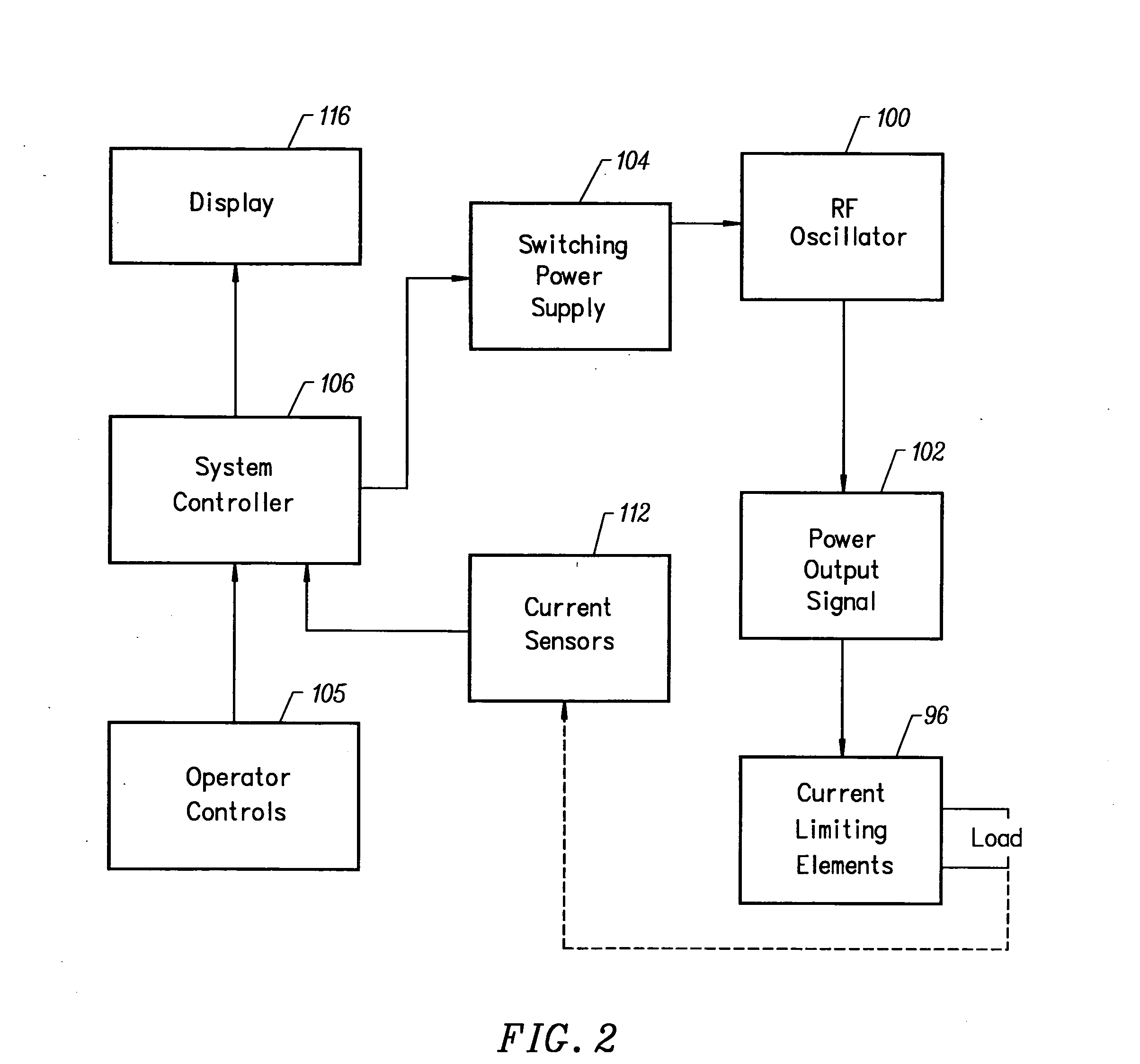
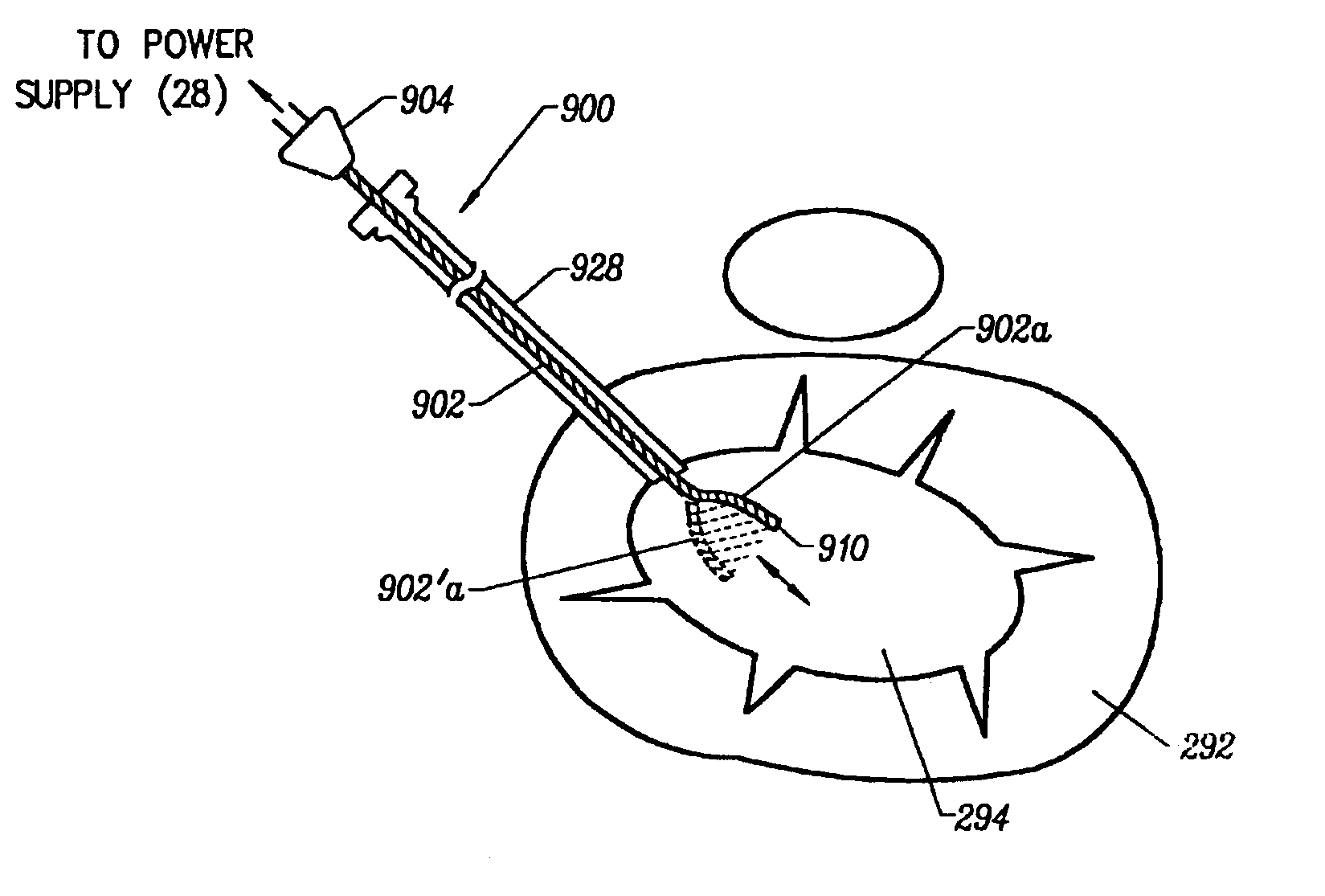
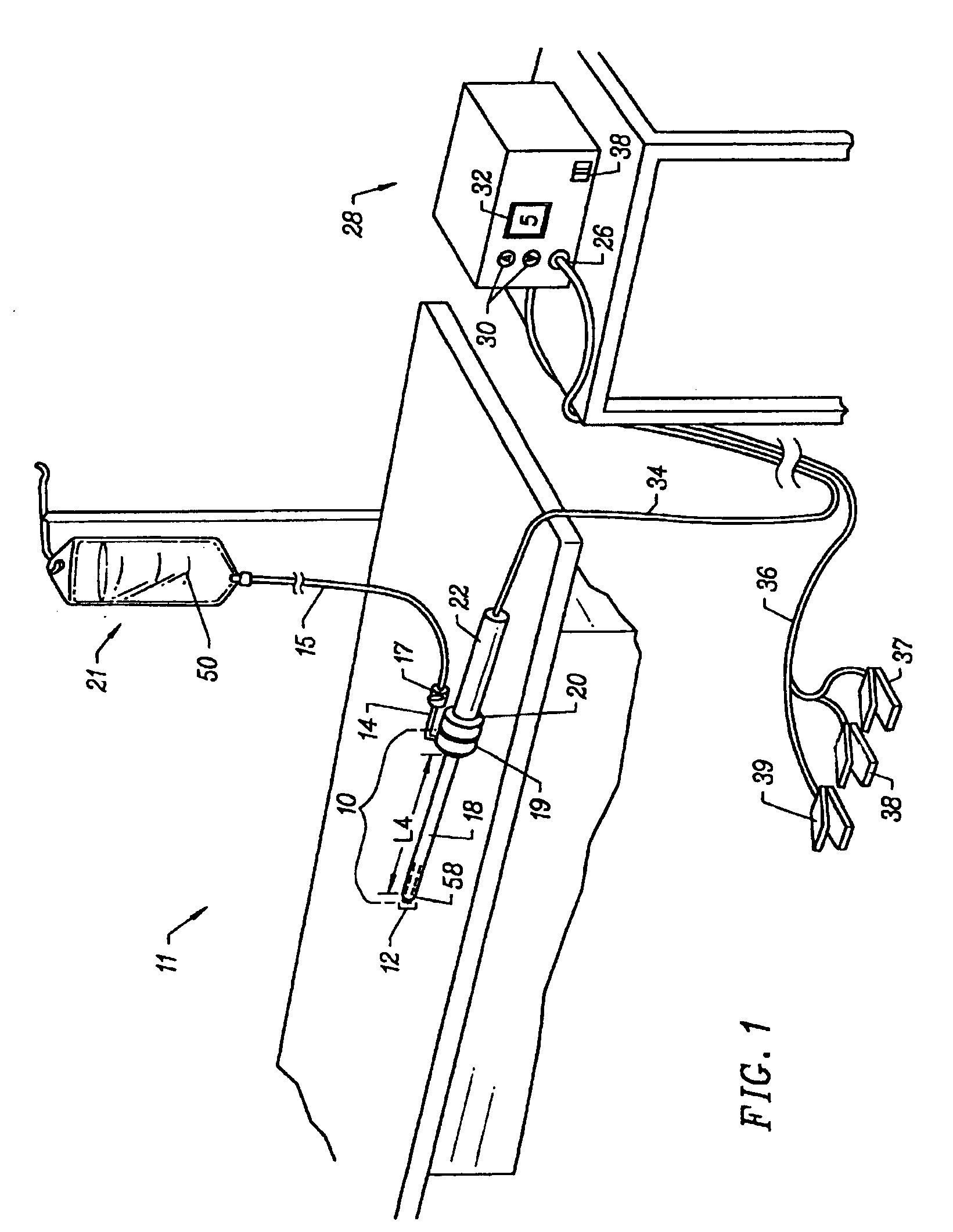
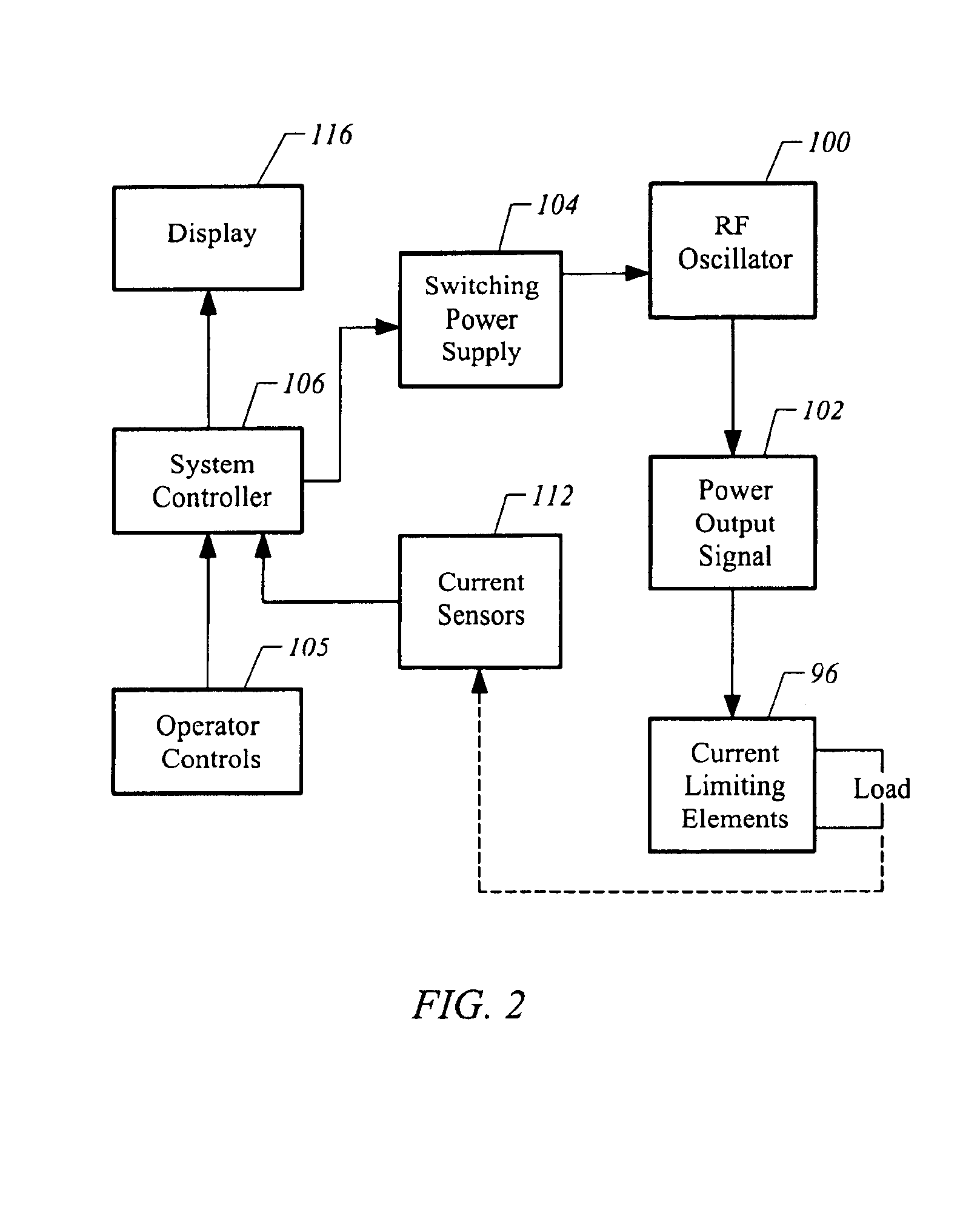
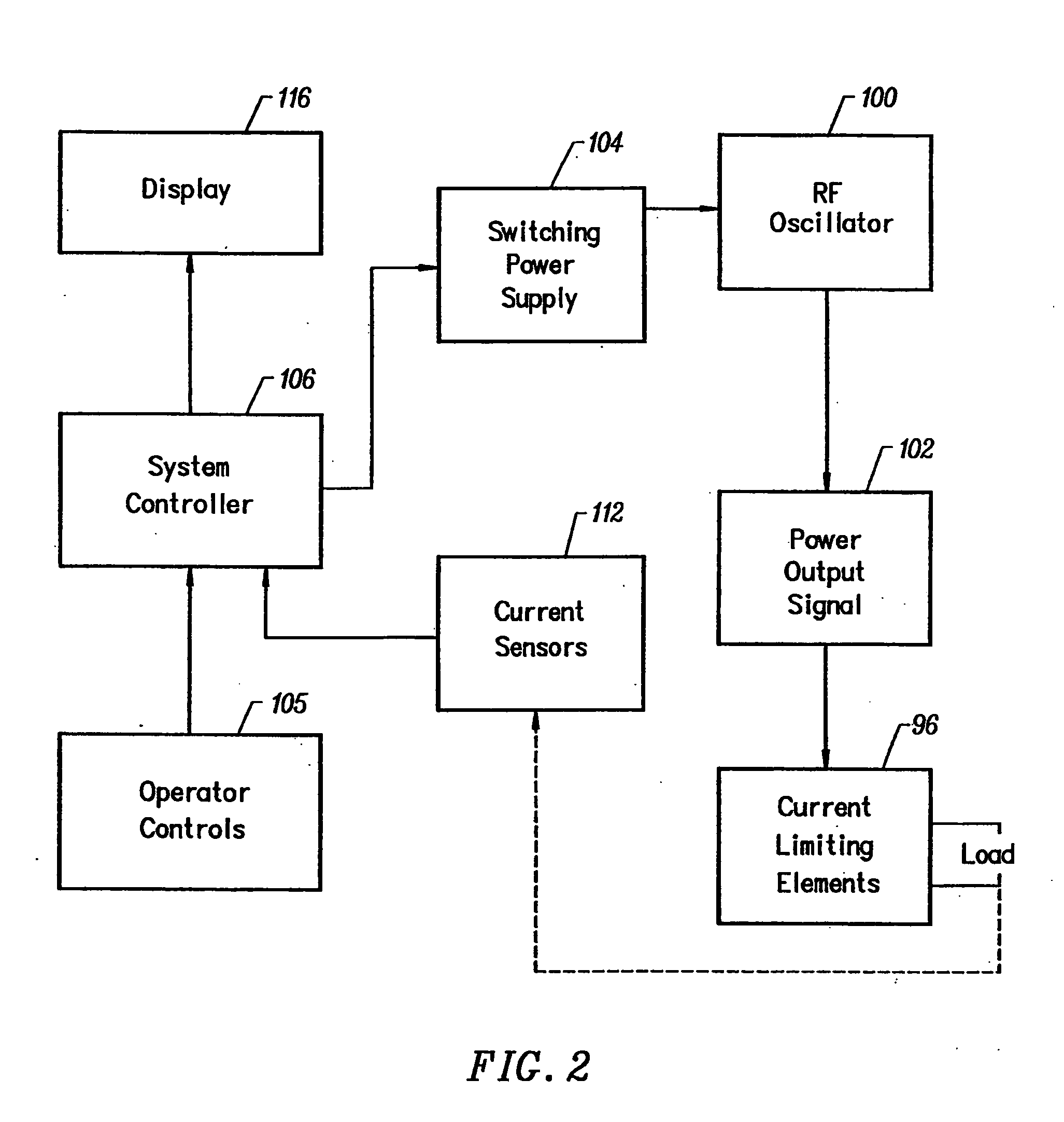
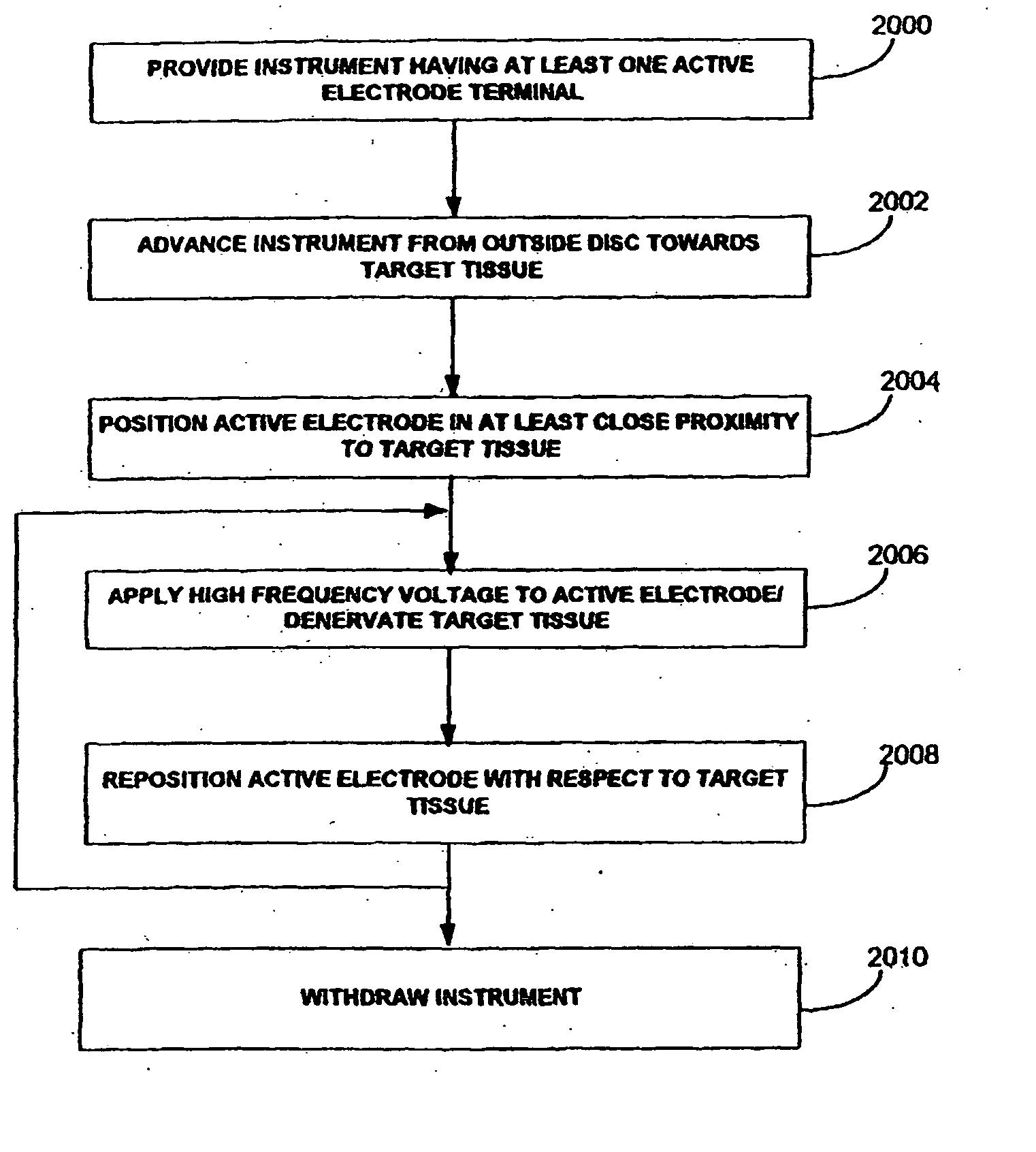
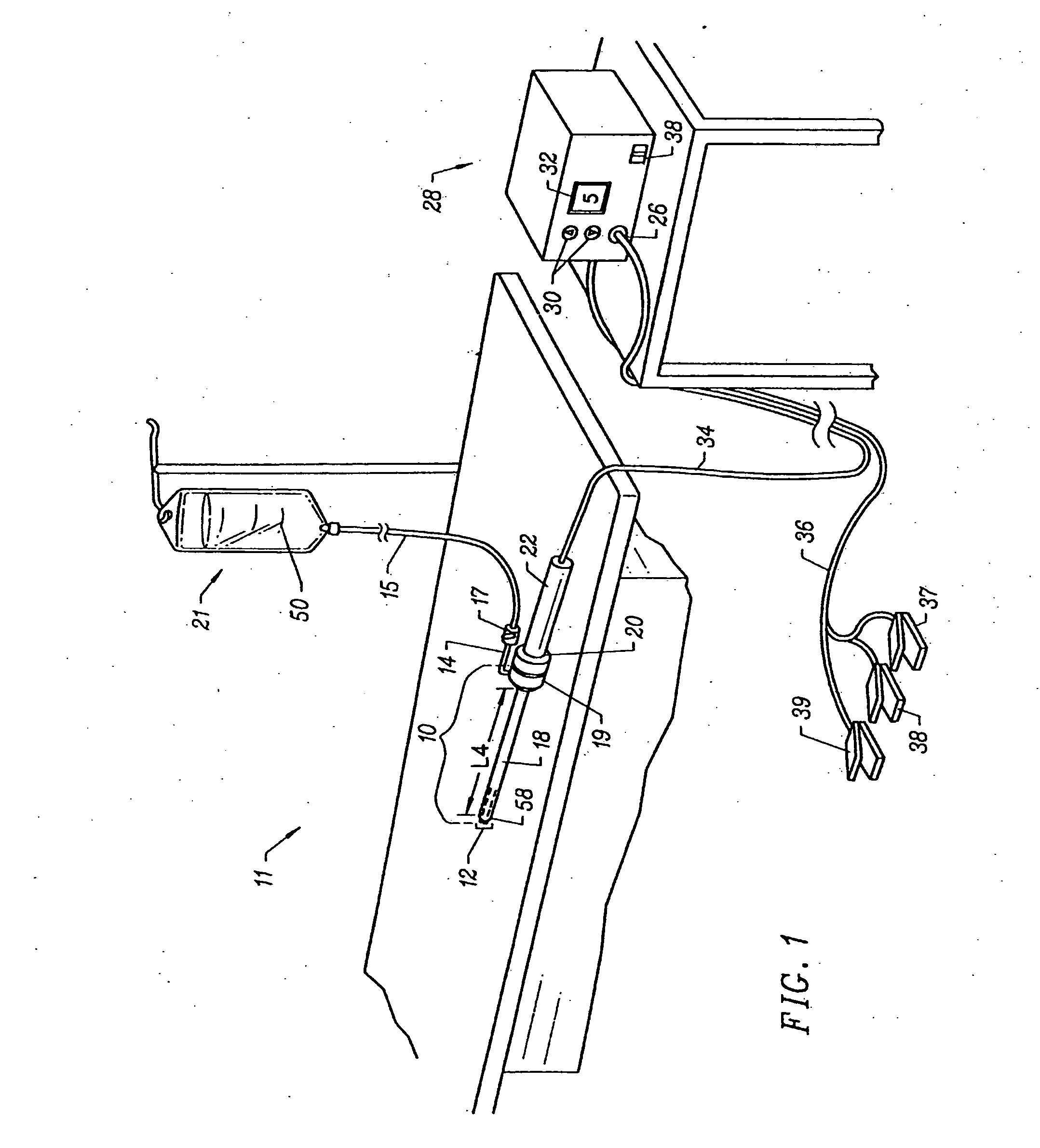
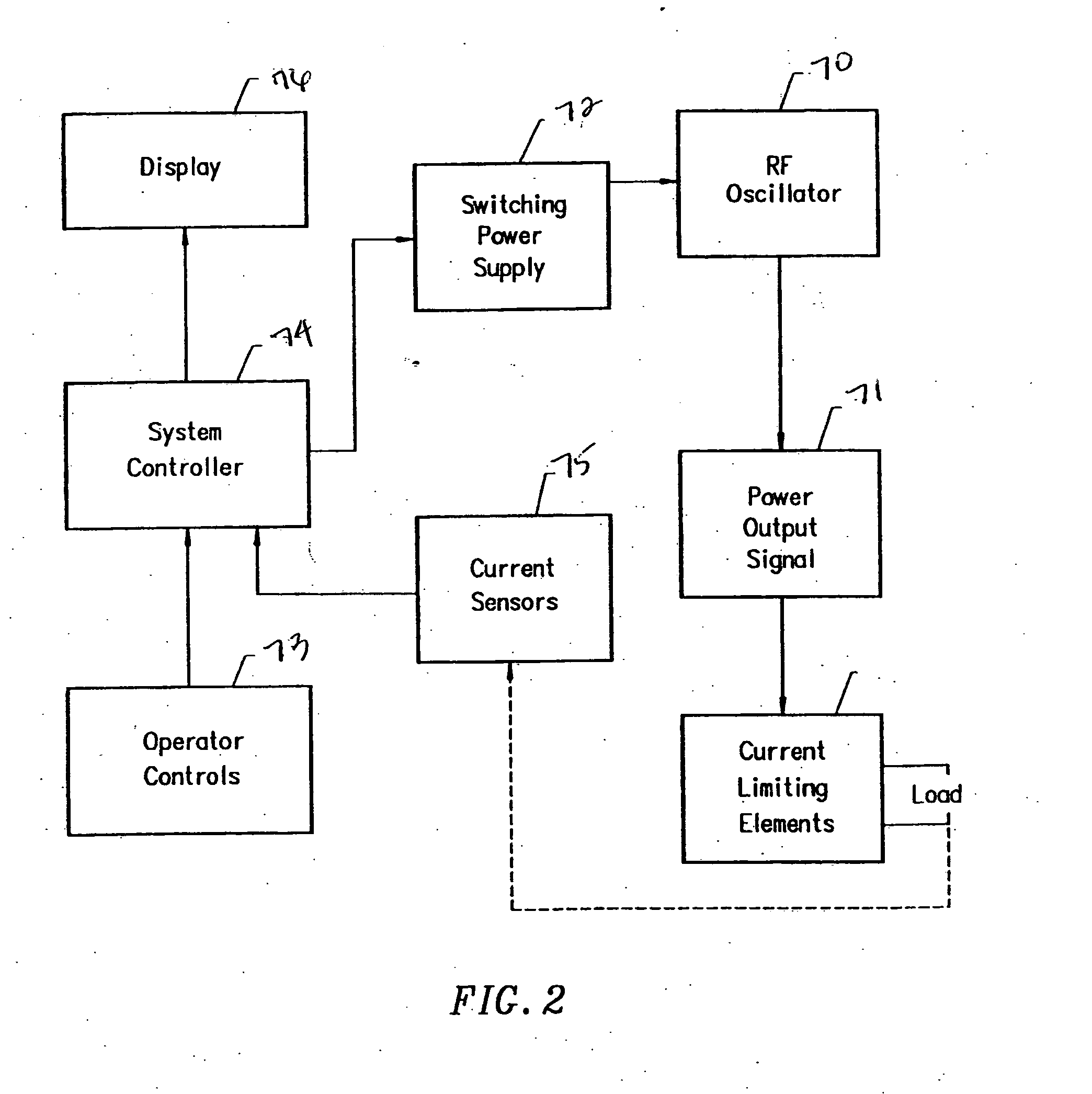
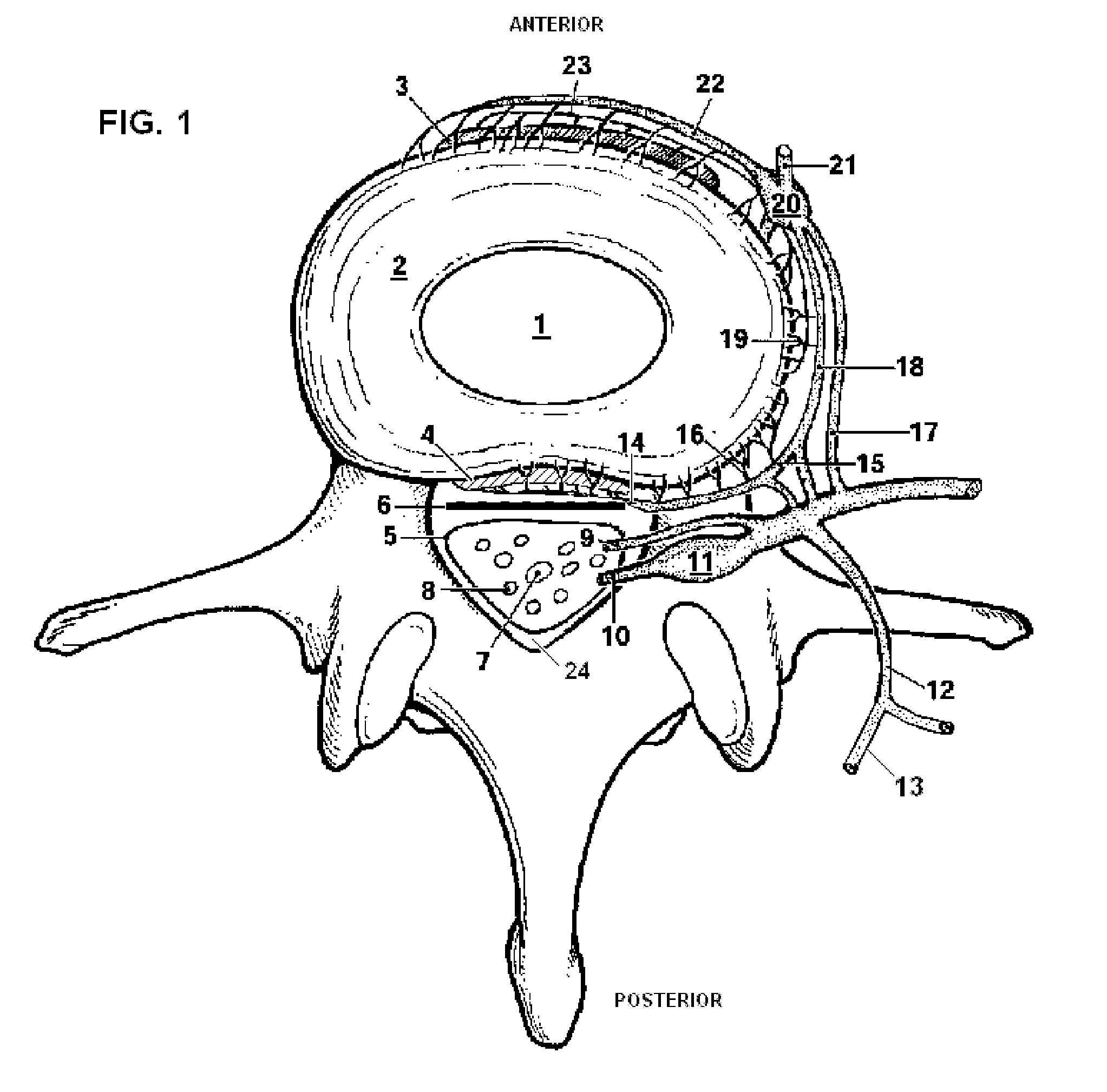
Apparatus and methods for treating an intervertebral disc by ablation of disc tissue. A method of the invention includes positioning at least one active electrode within the intervertebral disc, and applying at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s), wherein the volume of the nucleus pulposus is decreased, pressure exerted by the nucleus pulposus on the annulus fibrosus is reduced, and discogenic pain of a patient is alleviated. In other embodiments, a curved or steerable probe is guided to a specific target site within a disc to be treated, and the disc tissue at the target site is ablated by application of at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s). A method of making an electrosurgical probe is also disclosed.
Owner:ARTHROCARE
Methods and apparatus for treating intervertebral discs
InactiveUS20050010205A1Minimal and collateral damageLower the volumeDiagnosticsSurgical needlesMedicineIntervertebral disc
Apparatus and methods for treating a target tissue by delivering a fluid at a defined temperature to a patient's body. An apparatus of the invention includes a fluid delivery unit for delivering fluid in at least close proximity to the target tissue, an aspiration unit for withdrawing the fluid, and a fluid source unit for providing the fluid at the defined temperature. A method of the invention includes forming a void in at least close proximity to the target tissue, and circulating a preheated fluid through the void, wherein the target tissue undergoes adjustment from body temperature to a treatment temperature due to heat exchange between the fluid and the target tissue.
Owner:ARTHROCARE
Methods for repairing damaged intervertebral discs
InactiveUSRE40156E1Reduce internal pressureReduce moistureBiocideOrganic chemistryIntervertebral discActive electrode
Apparatus and methods for treating an intervertebral disc by ablation of disc tissue. A method of the invention includes positioning at least one active electrode within the intervertebral disc, and applying at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s), wherein the volume of the nucleus pulposus is decreased, pressure exerted by the nucleus pulposus on the annulus fibrosus is reduced, and discogenic pain of a patient is alleviated. In other embodiments, a curved or steerable probe is guided to a specific target site within a disc to be treated, and the disc tissue at the target site is ablated by application of at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s). A method of making an electrosurgical probe is also disclosed.
Owner:ARTHROCARE
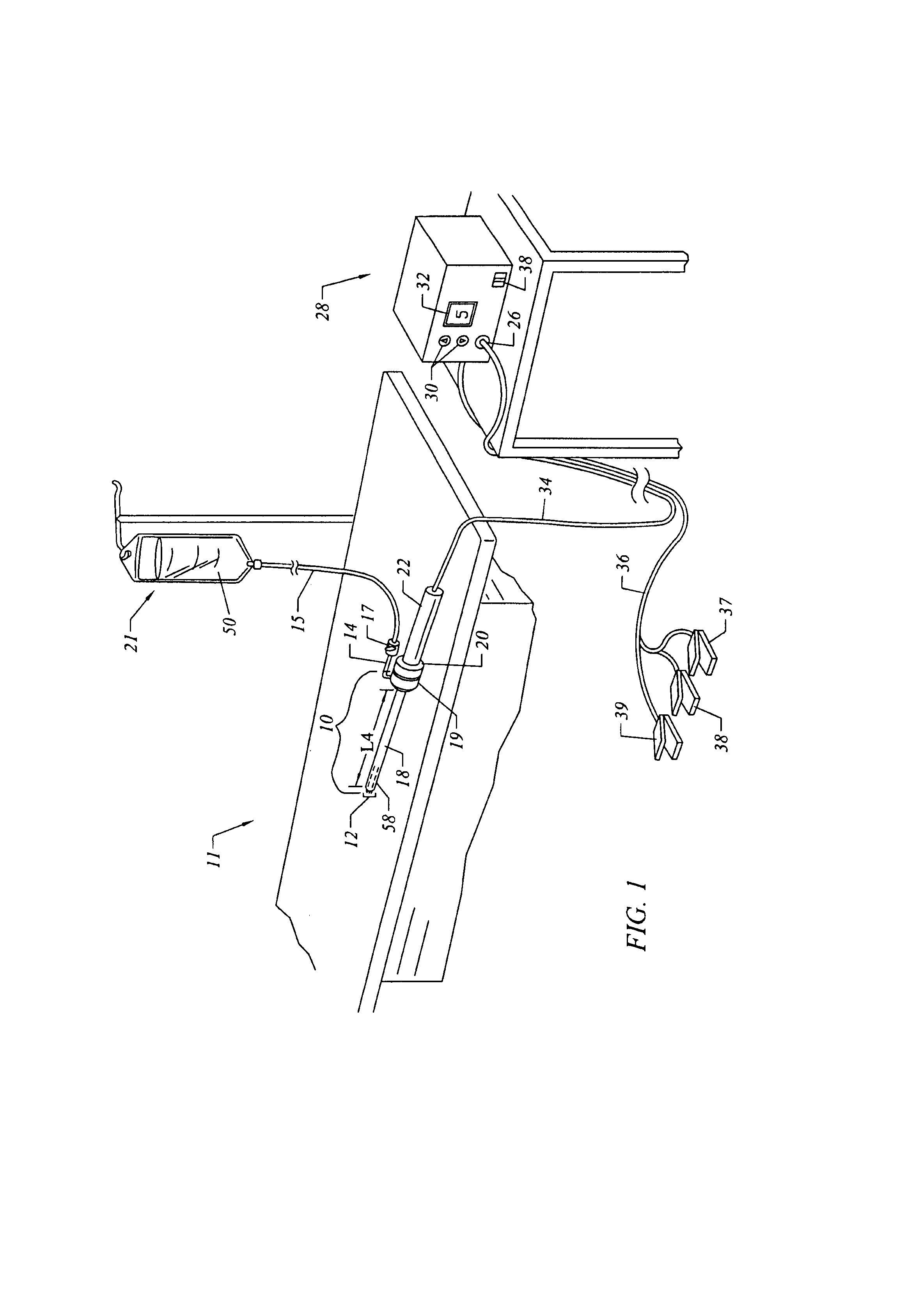
Methods and apparatus for treating back pain
Apparatus and methods for treating back pain of a patient by denervation of an intervertebral disc or a region of the posterior longitudinal ligament by the controlled application of heat to a target tissue. In one embodiment, the invention may include a procedure combining both decompression of a disc, and denervation of the annulus fibrosus. In one embodiment, a method of the invention includes positioning an active electrode of an electrosurgical instrument in at least close proximity to an intervertebral disc, and applying at least a first high frequency voltage between the active electrode and a return electrode, wherein nervous tissue within the annulus fibrosus is inactivated, and discogenic pain of the patient is alleviated. In one embodiment, the invention includes positioning a first electrode of a dual-shaft electrosurgical instrument at a first location in relation to a target disc, positioning a second electrode of the instrument at a second location, and applying a high frequency voltage between the first and second electrodes, wherein the first and second electrodes are disposed on separate shafts of the instrument.
Owner:ARTHROCARE
Methods and apparatus for treating intervertebral discs
InactiveUS7387625B2Reduce internal pressureReduce moistureDiagnosticsSurgical needlesIntervertebral discTreatment targets
Apparatus and methods for treating a target tissue by delivering a fluid at a defined temperature to a patient's body. An apparatus of the invention includes a fluid delivery unit for delivering fluid in at least close proximity to the target tissue, an aspiration unit for withdrawing the fluid, and a fluid source unit for providing the fluid at the defined temperature. A method of the invention includes forming a void in at least close proximity to the target tissue, and circulating a preheated fluid through the void, wherein the target tissue undergoes adjustment from body temperature to a treatment temperature due to heat exchange between the fluid and the target tissue.
Owner:ARTHROCARE
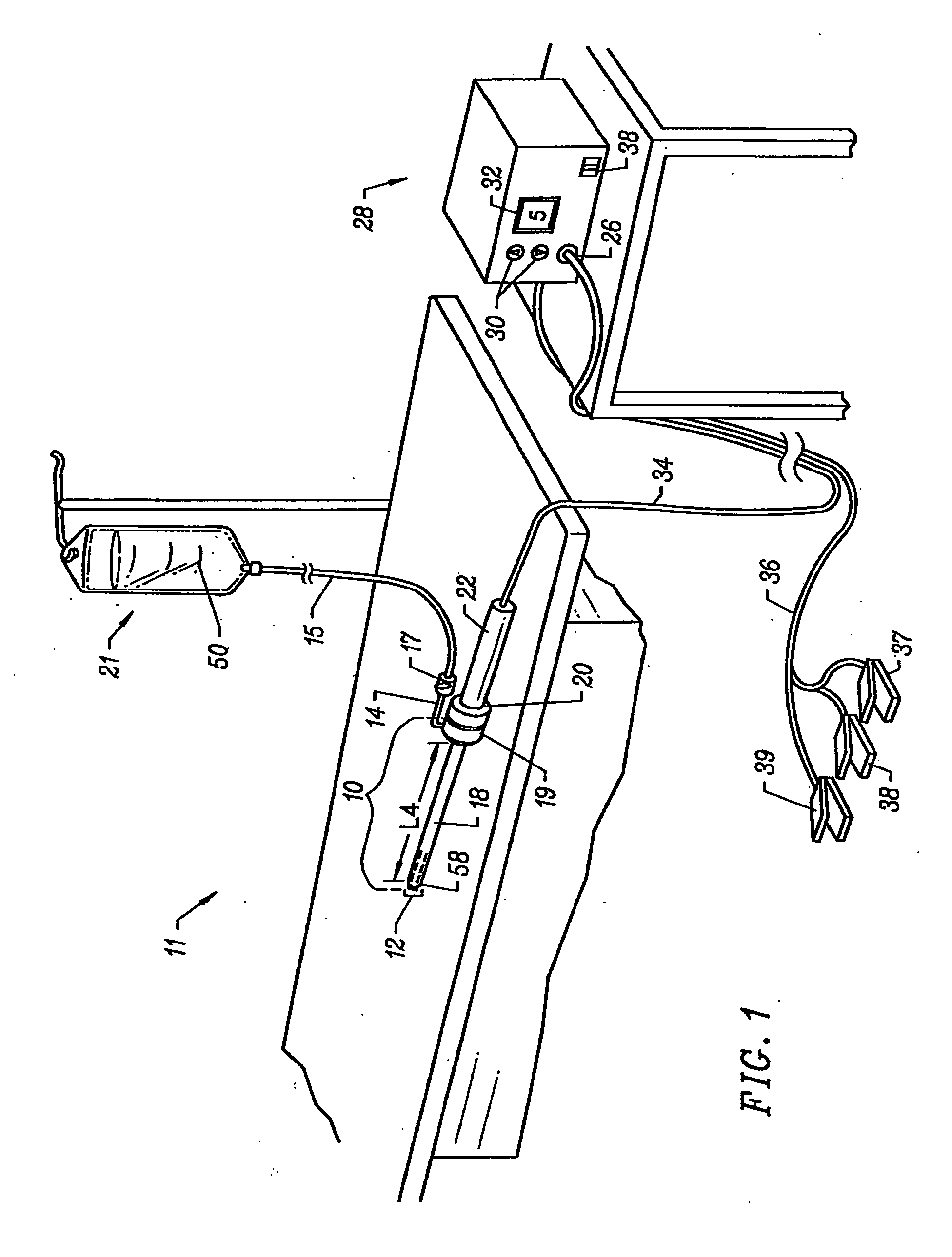
Methods and apparatus for treating back pain
InactiveUS20050261754A1Relieve back painSurgical instruments for heatingTherapeutic coolingIntervertebral discBACK DISCOMFORT
Apparatus and methods for treating back pain of a patient by denervation of an intervertebral disc or a region of the posterior longitudinal ligament by the controlled application of heat to a target tissue. In one embodiment, the invention may include a procedure combining both decompression of a disc, and denervation of the annulus fibrosus. In one embodiment, a method of the invention includes positioning an active electrode of an electrosurgical instrument in at least close proximity to an intervertebral disc, and applying at least a first high frequency voltage between the active electrode and a return electrode, wherein nervous tissue within the annulus fibrosus is inactivated, and discogenic pain of the patient is alleviated. In one embodiment, the invention includes positioning a first electrode of a dual-shaft electrosurgical instrument at a first location in relation to a target disc, positioning a second electrode of the instrument at a second location, and applying a high frequency voltage between the first and second electrodes, wherein the first and second electrodes are disposed on separate shafts of the instrument.
Owner:ARTHROCARE
Methods and apparatus for treating back pain
Apparatus and methods for treating back pain of a patient by denervation of an intervertebral disc or a region of the posterior longitudinal ligament by the controlled application of heat to a target tissue. In one embodiment, the invention may include a procedure combining both decompression of a disc, and denervation of the annulus fibrosus. In one embodiment, a method of the invention includes positioning an active electrode of an electrosurgical instrument in at least close proximity to an intervertebral disc, and applying at least a first high frequency voltage between the active electrode and a return electrode, wherein nervous tissue within the annulus fibrosus is inactivated, and discogenic pain of the patient is alleviated. In one embodiment, the invention includes positioning a first electrode of a dual-shaft electrosurgical instrument at a first location in relation to a target disc, positioning a second electrode of the instrument at a second location, and applying a high frequency voltage between the first and second electrodes, wherein the first and second electrodes are disposed on separate shafts of the instrument.
Owner:ARTHROCARE
System and Method for Electrical Stimulation of the Lumbar Vertebral Column
ActiveUS20120215218A1Inhibit migrationPrevent rotationSpinal electrodesSurgical instruments for heatingElectrical impulseElectrical stimulations
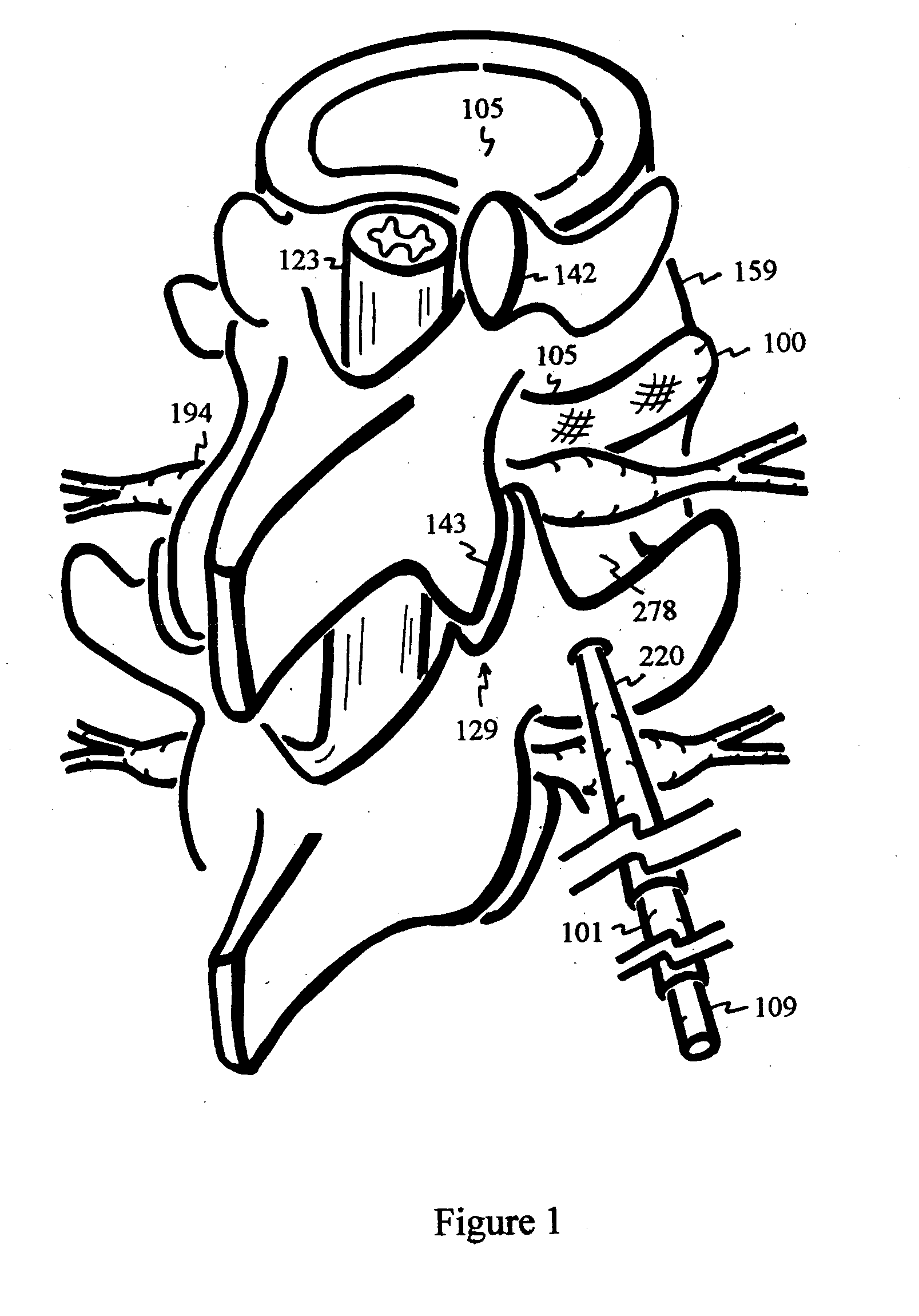
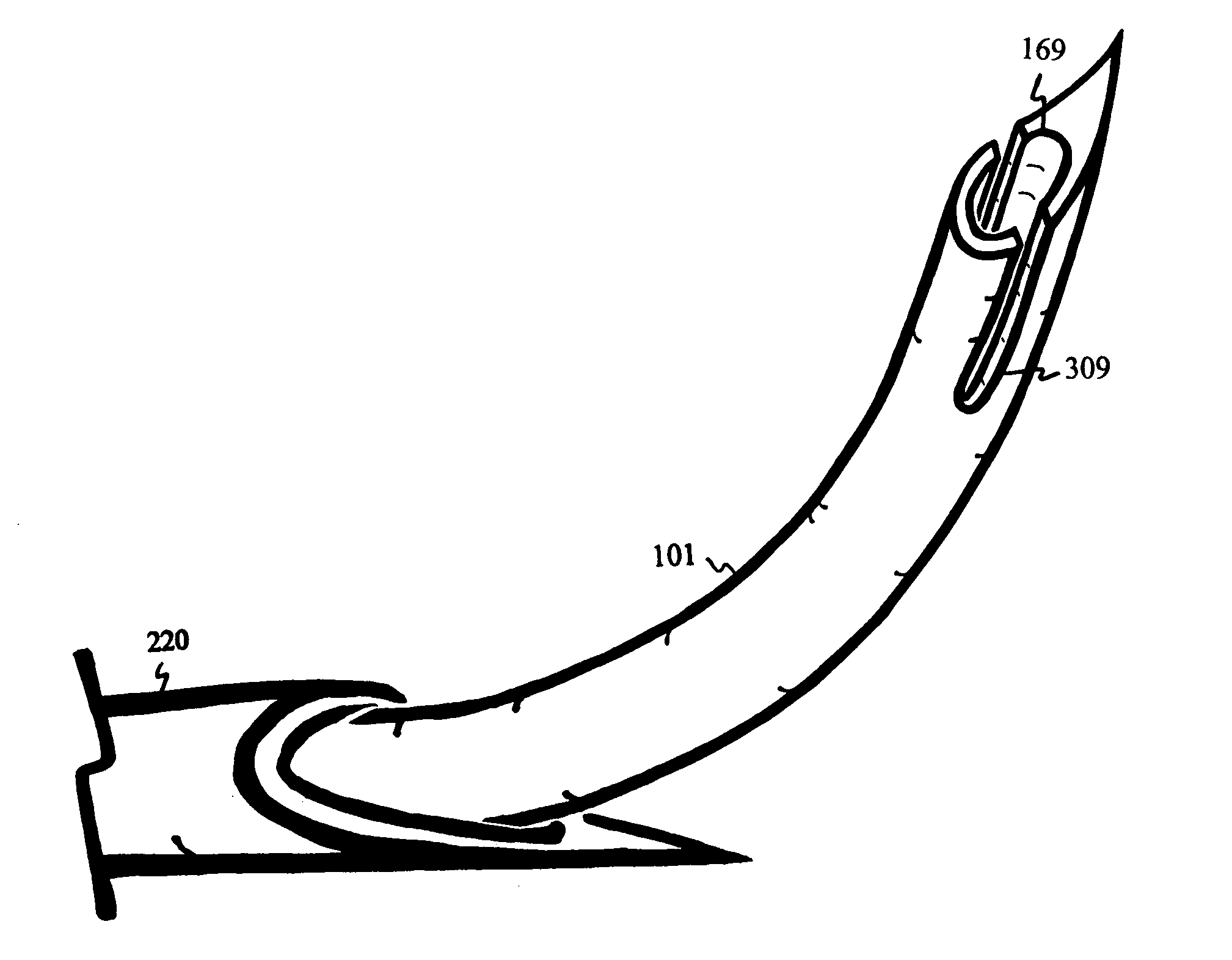
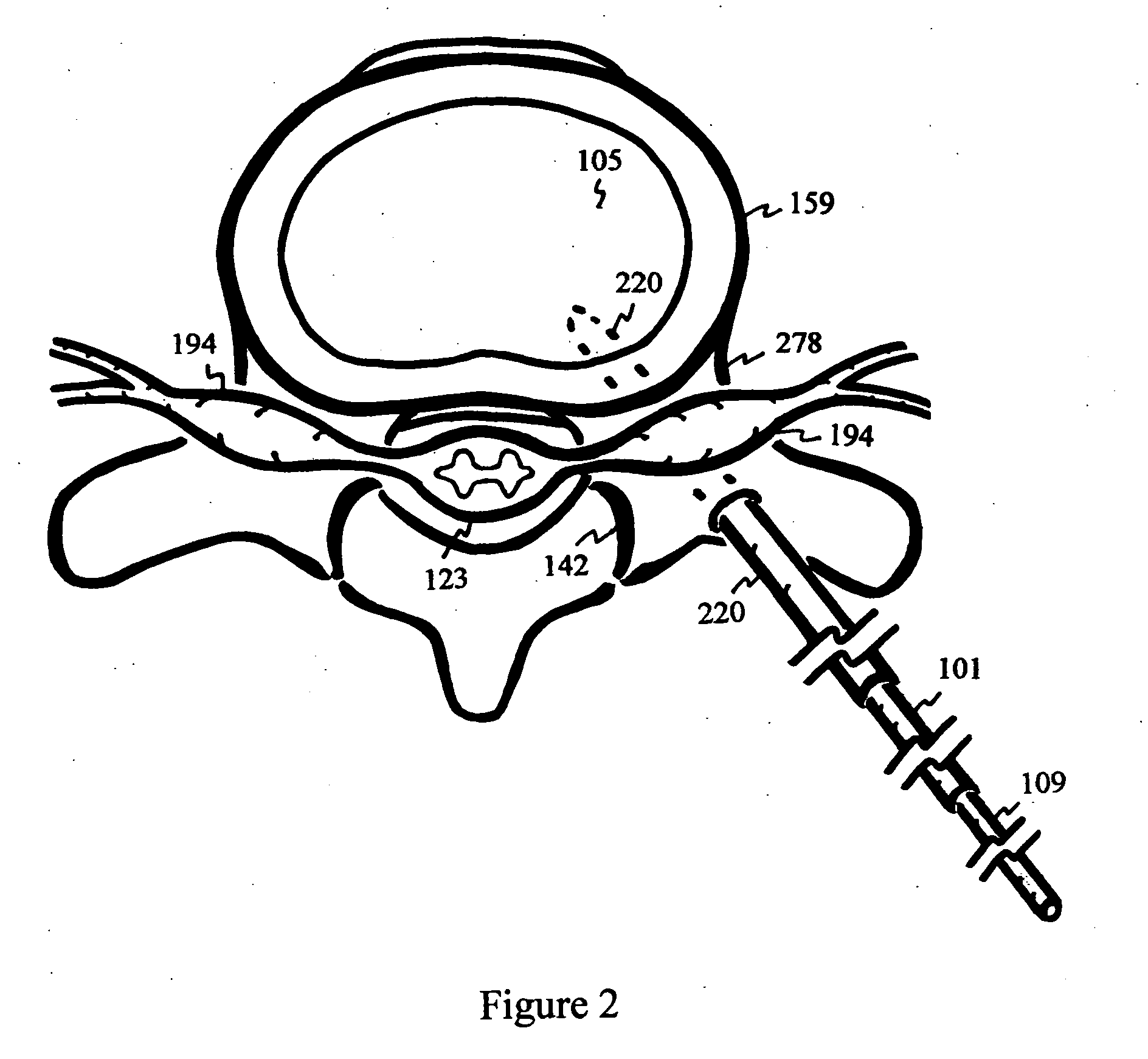
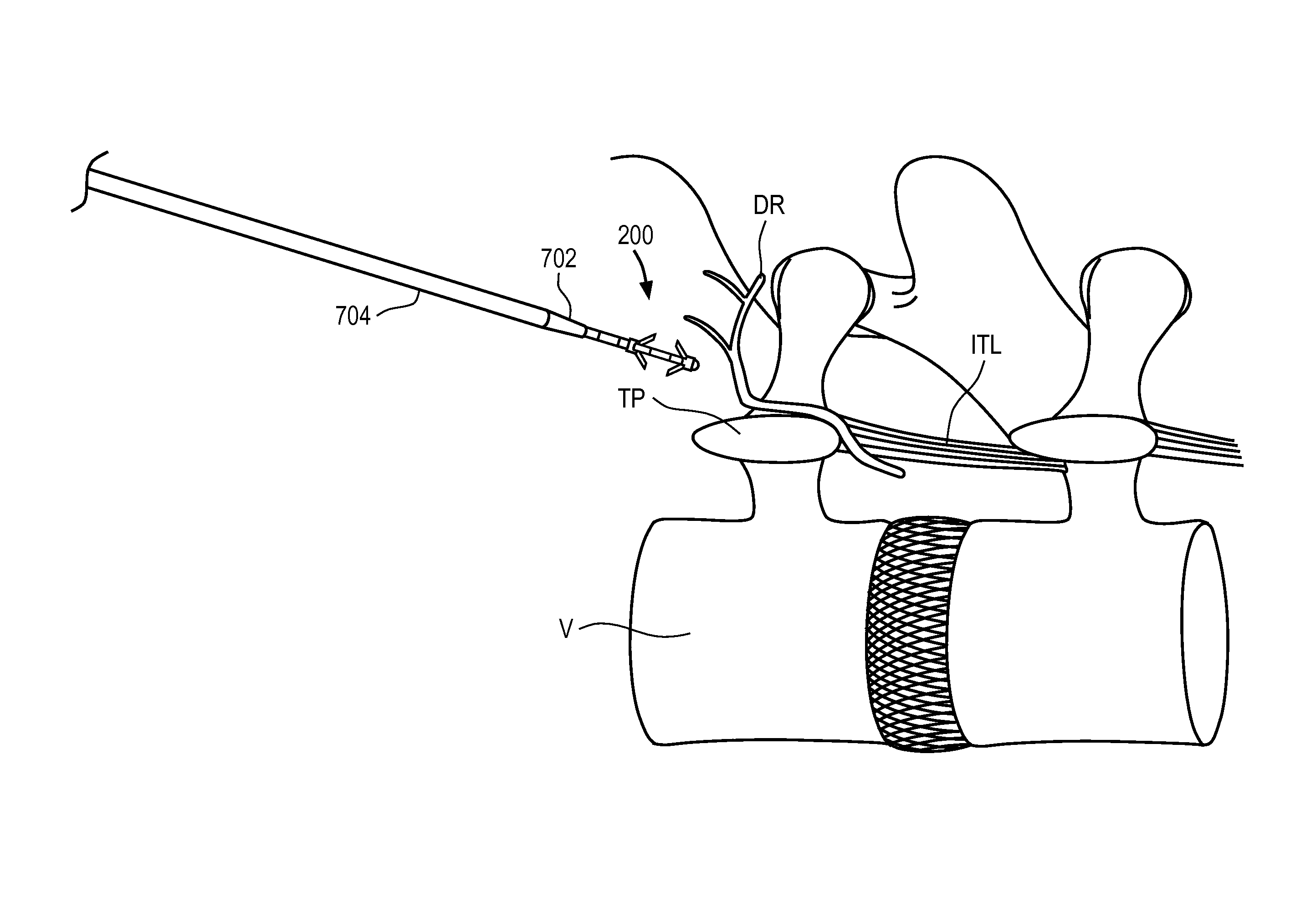
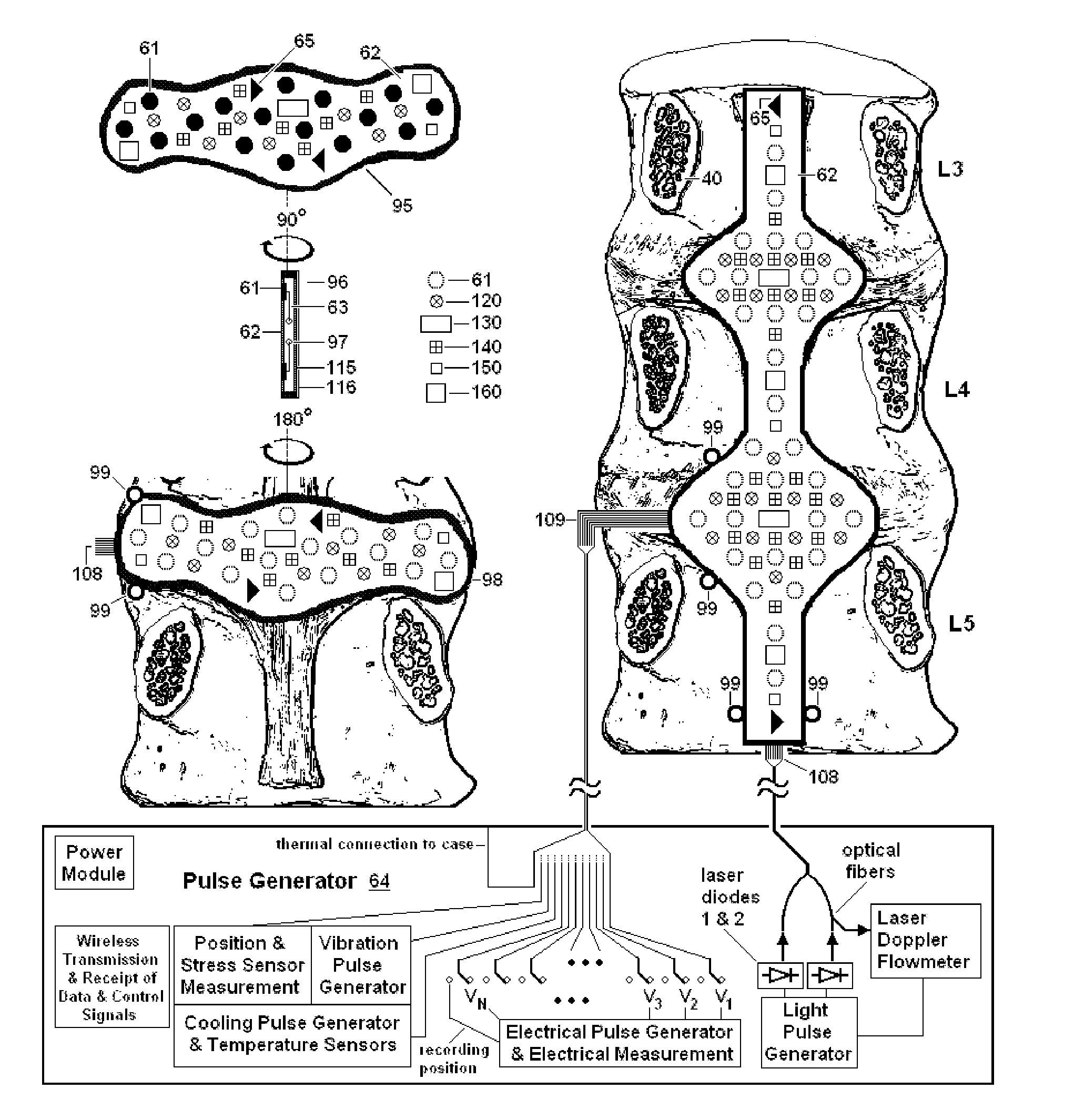
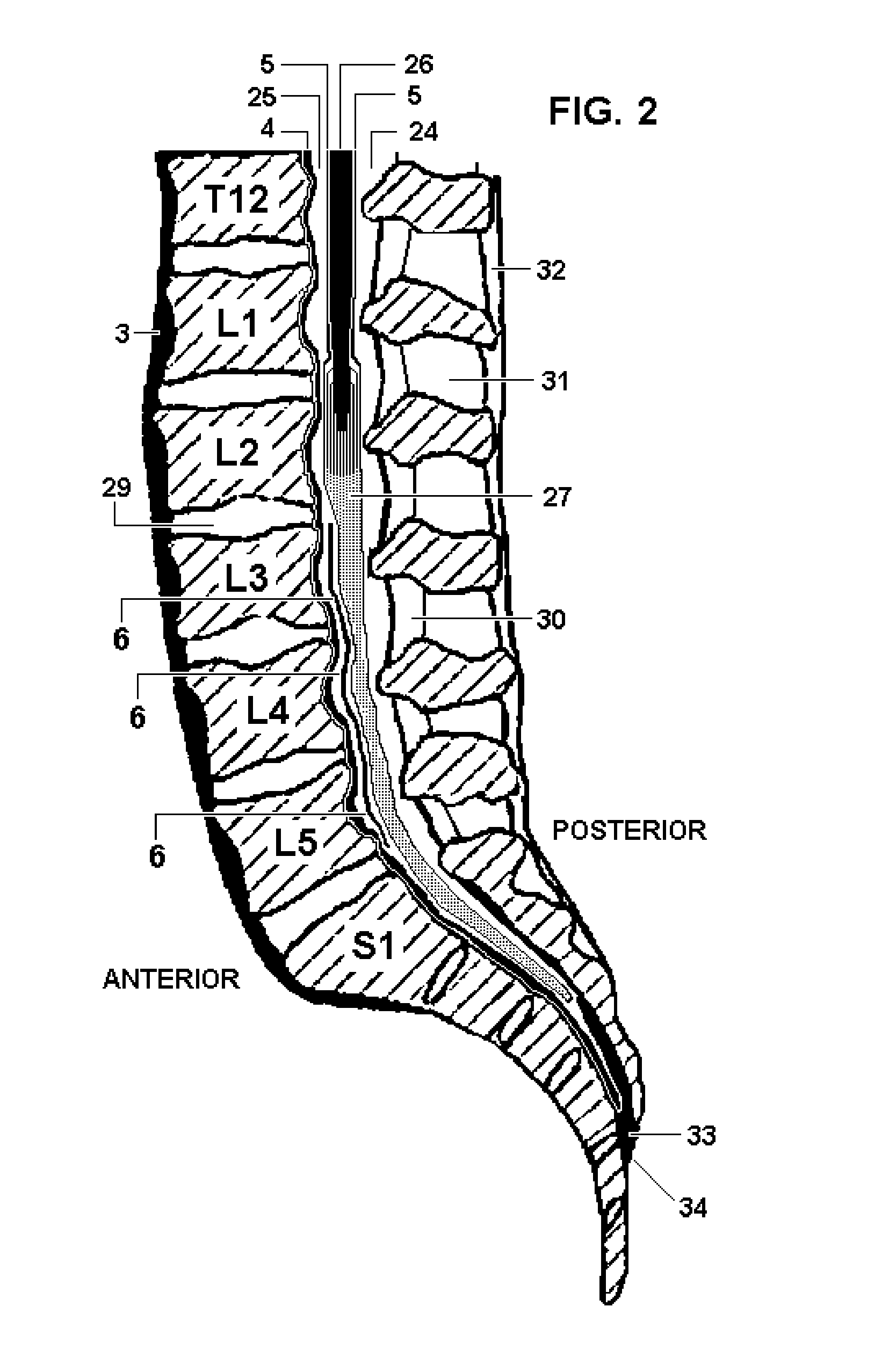
Disclosed methods and devices treat chronic lower back pain from degenerated or injured intervertebral discs. Electrodes connected to a pulse generator deliver electrical impulses to nerves located within the posterior longitudinal ligament and annulus fibrosus of lumbar intervertebral discs. The stimulation reduces back pain reversibly, adjustably, and with almost complete coverage of the pain-generating region. A temporary percutaneous lead and a permanent paddle lead are used. The percutaneous lead, designed to prevent inappropriate stimulation of the thecal sac, is inserted using a specially-designed introducer cannula and lead blank. The paddle lead is configured individually for implantation in the anterior epidural space of each patient. Electrical stimulation parameters may also be selected so as to ablate the nerves, using non-thermal irreversible electroporation, or using joule heating wherein a thermal insulator covers substantially all of the thecal sac, thereby shielding the thecal sac from potential heat damage.
Owner:LIPANI JOHN D
Disc shunt for treating back pain
InactiveUS20050246023A1Reduce stressRelieve stressInternal osteosythesisDiagnosticsMedicineBack pain
The intervertebral disc is avascular. With aging, nutrients and oxygen transporting through the endplates diminish. The disc degenerates, and pain ensues. Conduits are delivered through a pedicle or vertebral body into the intervertebral disc to re-establish the exchange of nutrients and waste between the disc and bodily circulation to slow, stop or reverse disc degeneration and relieve pain. Endplate plugs may be deployed to seal gaps between the conduits and the endplates to prevent immune responses to the nucleus pulposus and to preserve the hydrostatic pressure within the disc.
Owner:YEUNG JEFFREY E
Disc shunt delivery devices
InactiveUS20050240201A1Reduce stressRelieve stressInternal osteosythesisEar treatmentNutrientOxygen transport
The intervertebral disc is avascular. With aging, nutrients and oxygen transporting through the endplates diminish. The disc degenerates, and pain ensues. Delivery devices are used to deliver a conduit through a pedicle or vertebral body into the intervertebral disc to re-establish the exchange of nutrients and waste between the disc and bodily circulation to slow, stop or reverse disc degeneration and relieve pain.
Owner:YEUNG JEFFREY E
System and Methods for Diagnosis and Treatment of Discogenic Lower Back Pain
ActiveUS20160074661A1Relieve back painAvoid stimulationSpinal electrodesChiropractic devicesClosed loopElectrical impulse
Methods and devices to treat discogenic lumbar back pain are disclosed. Electrodes are implanted within the anterior epidural space of the patient. A pulse generator that is connected to the electrodes delivers electrical impulses to sympathetic nerves located within the posterior longitudinal ligament (PLL) of the lumbar spine and outer posterior annulus fibrosus of the intervertebral disc. In alternate embodiments, energy directed to nerves in the PLL may be from light or mechanical vibrations, or the nerves may be cooled. The electrodes may also be used diagnostically to correlate spontaneous nerve activity with spinal movement, fluctuations in autonomic tone and the patient's experience of pain. The electrodes may also be used to generate diagnostic evoked potentials. The diagnostic data are used to devise parameters for the therapeutic nerve stimulation. Automatic analysis of the data may be incorporated into a closed-loop system that performs the nerve stimulation automatically.
Owner:LIPANI JOHN D
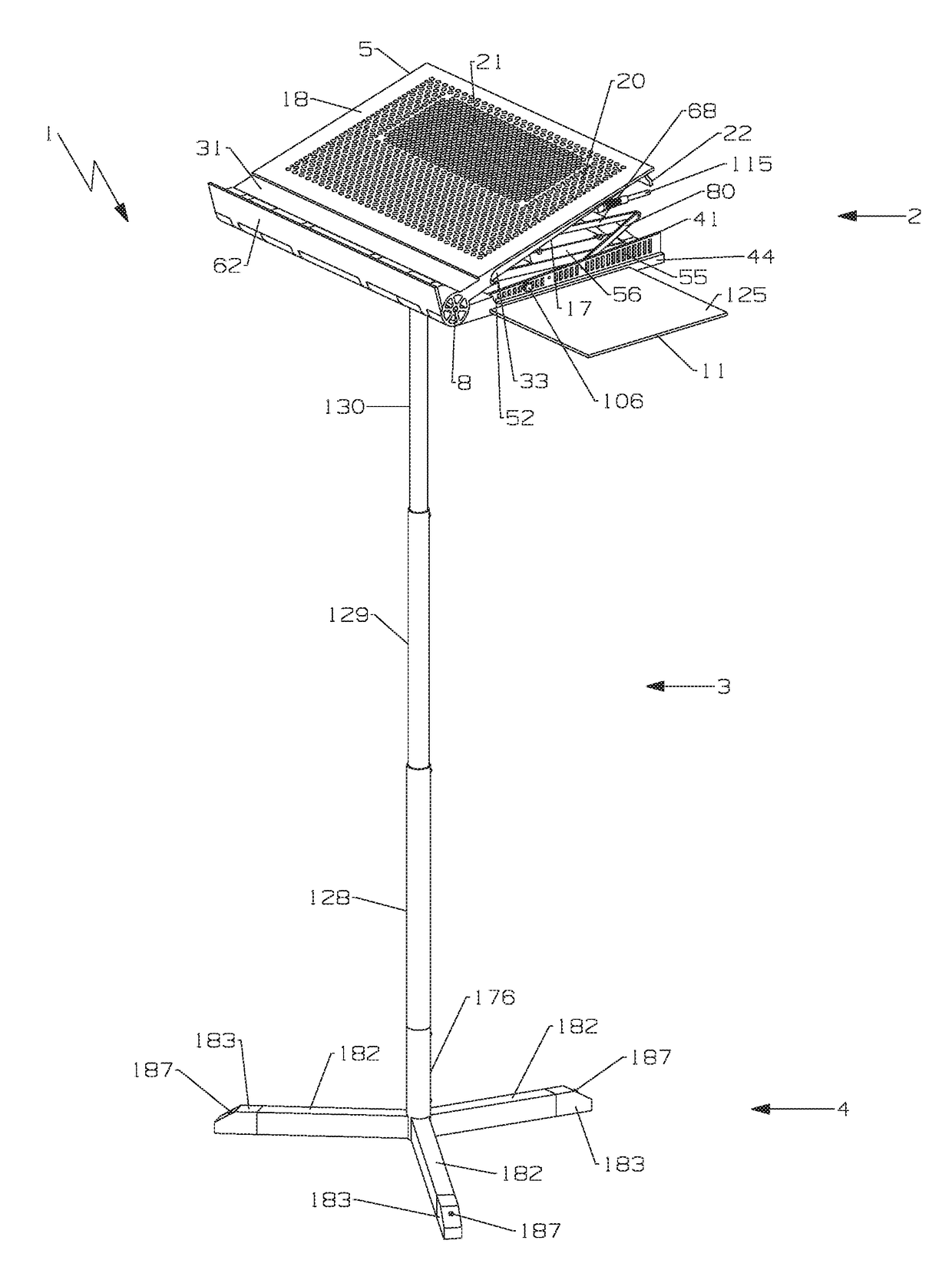
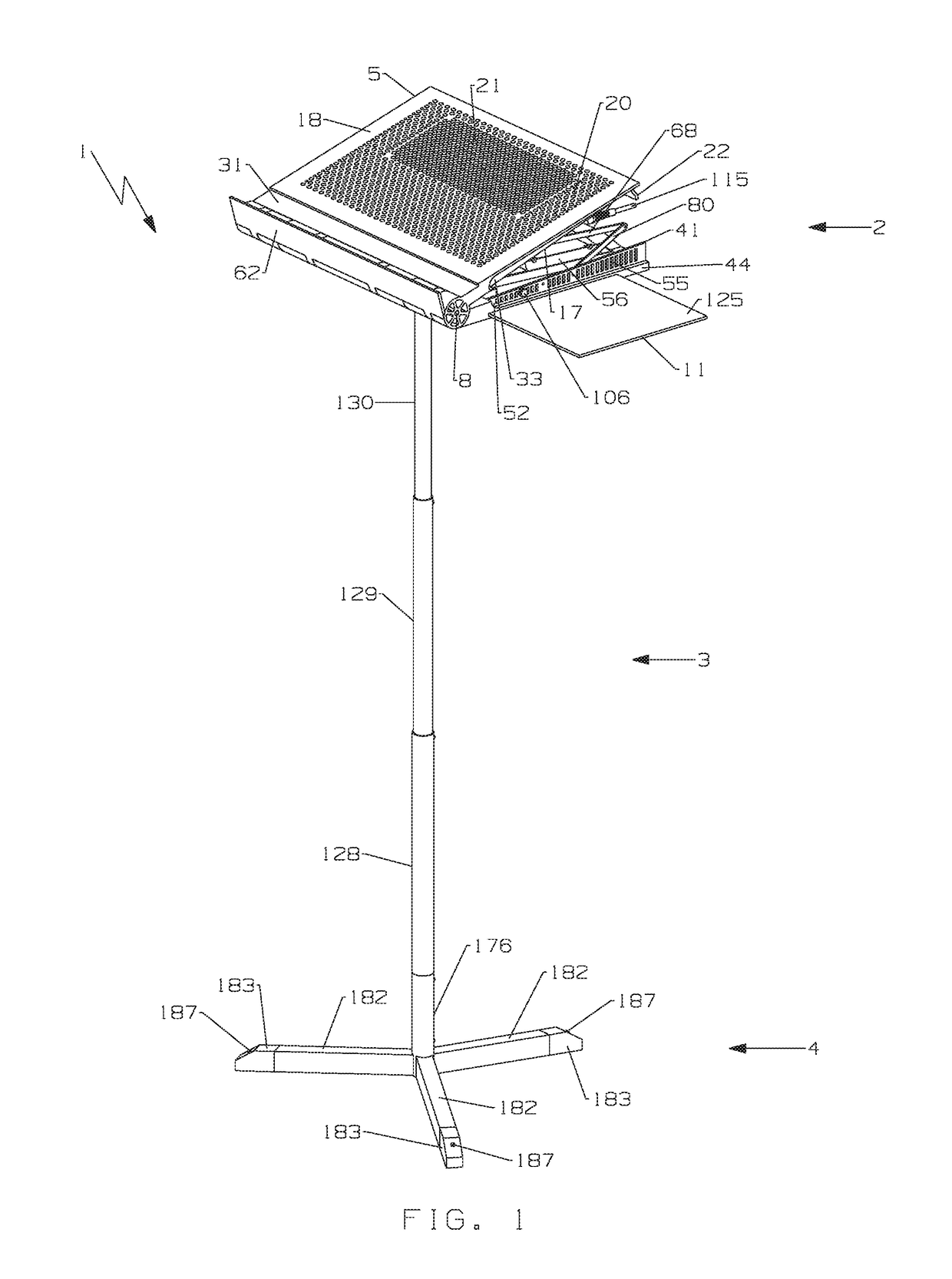
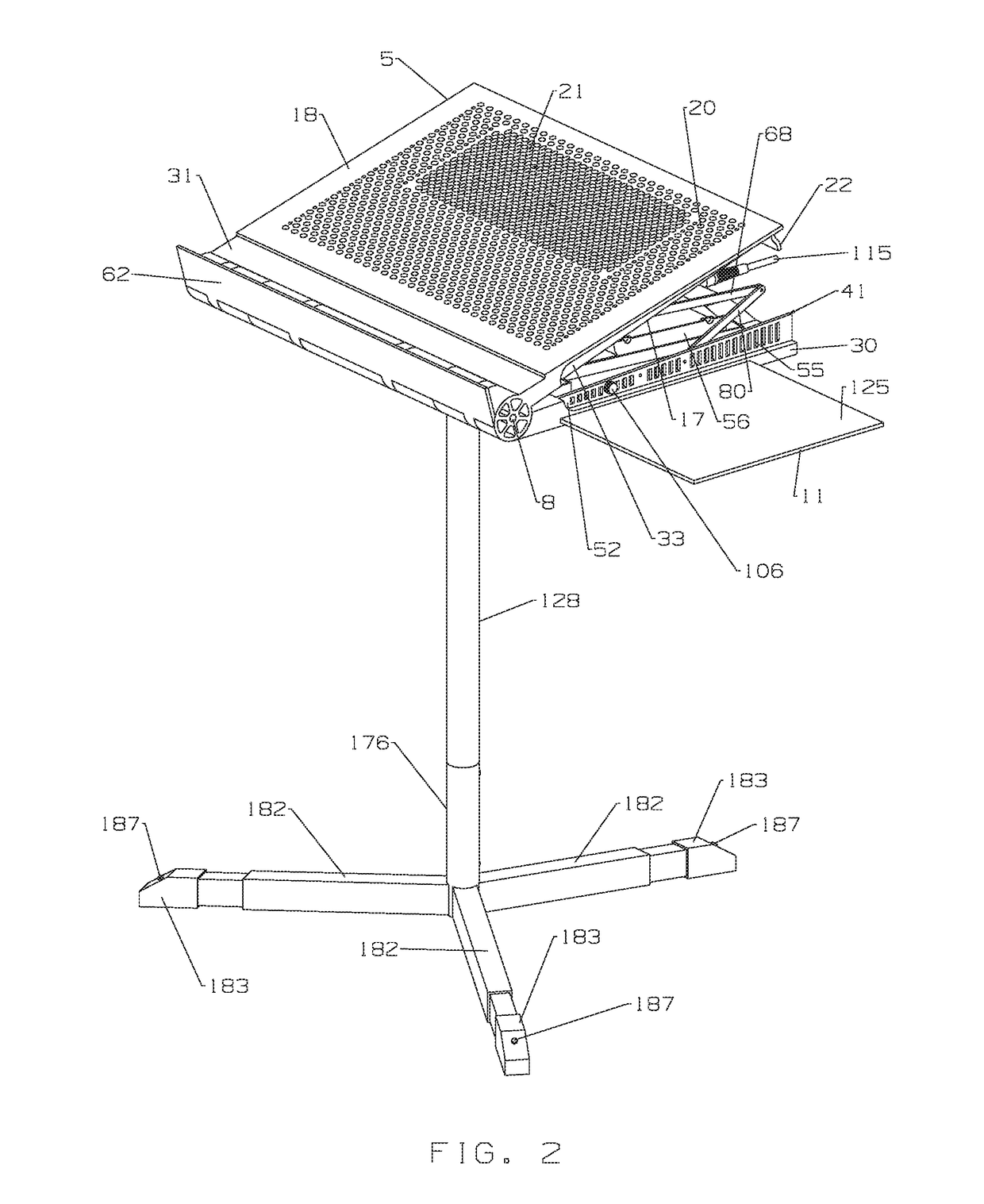
Multi-function travel-friendly workstation with cooling and ventilation
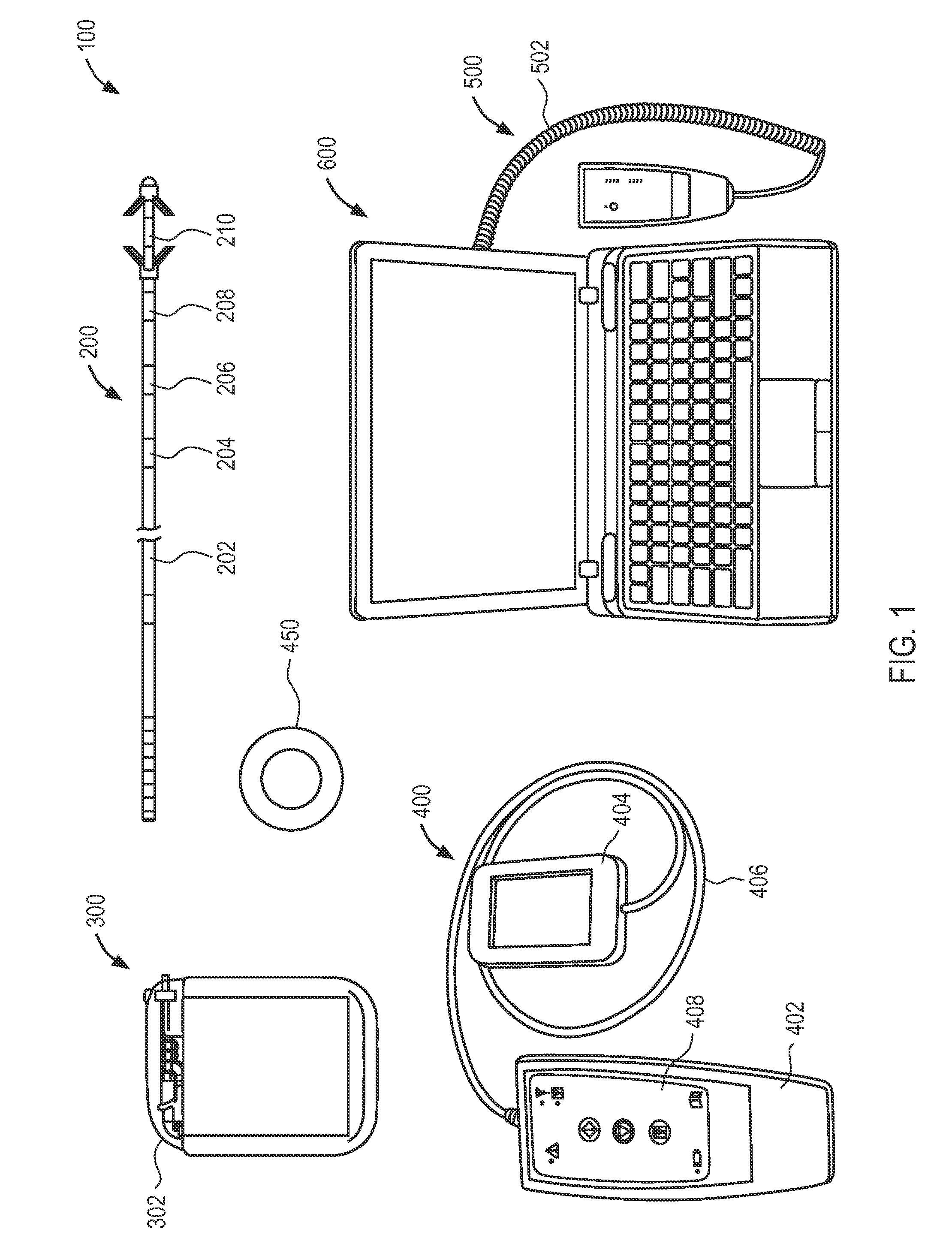
An ergonomically designed space saving, collapsible multi-function travel-friendly modular workstation for supporting a broad range of electronic systems, reading materials and the like for users while standing, sitting or on-the-go, and fits in anywhere, anytime is presented. The workstation comprises a support unit, telescopic rod and tripod and is designed to provide needed cooling and ventilation for electronic systems, support healthy postures, and complete comfort and versatility of a multi-function workstation with all the important things needed when working at a desk, thereby improving a user's comfort when using the workstation. Further, the workstation is designed for easy transportation, storage and set up, as well as provide a versatile workspace for a user in many different environments, for everyday use such as note taking, writing, reading, presentations, performing arts and rehearsing while playing a musical instrument, music or conductor stand, etc.
Owner:ASANTE JAMES NATHANIEL
U-shaped disc shunt and delivery device
InactiveUS20070142791A1Reduce frictionRelieve back painInternal osteosythesisSurgical needlesSwelling pressureIrritation
The intervertebral disc contains no blood vessels. Nutrients and waste are diffused mainly through adjacent vertebral bodies. As we age, calcified layers form between the disc and vertebral bodies, blocking diffusion. The disc begins to starve and flatten. The weight shifts abnormally from disc to the facet joints causing strain and back pain. Under anaerobic conditions, lactic acid is produced causing acidic irritation and unspecific pain. A U-shaped disc shunt is delivered into and sealed within the degenerated disc simply by needle puncturing and withdrawal, to draw nutrients from bodily circulation into the avascular disc. A continual supply of nutrients increases biosynthesis of the water-retaining sulfated glycosaminoglycans, hence swelling pressure within the disc. The weight is re-shifted from the facet joints to the regenerated disc, alleviating back pain. With oxygen transported through the shunt, anaerobic production of lactic acid is minimized. In addition, the residual lactic acid is expelled through the U-shaped shunt during disc compression into bodily circulation to alleviate unspecific pain.
Owner:ALEEVE MEDICAL INC
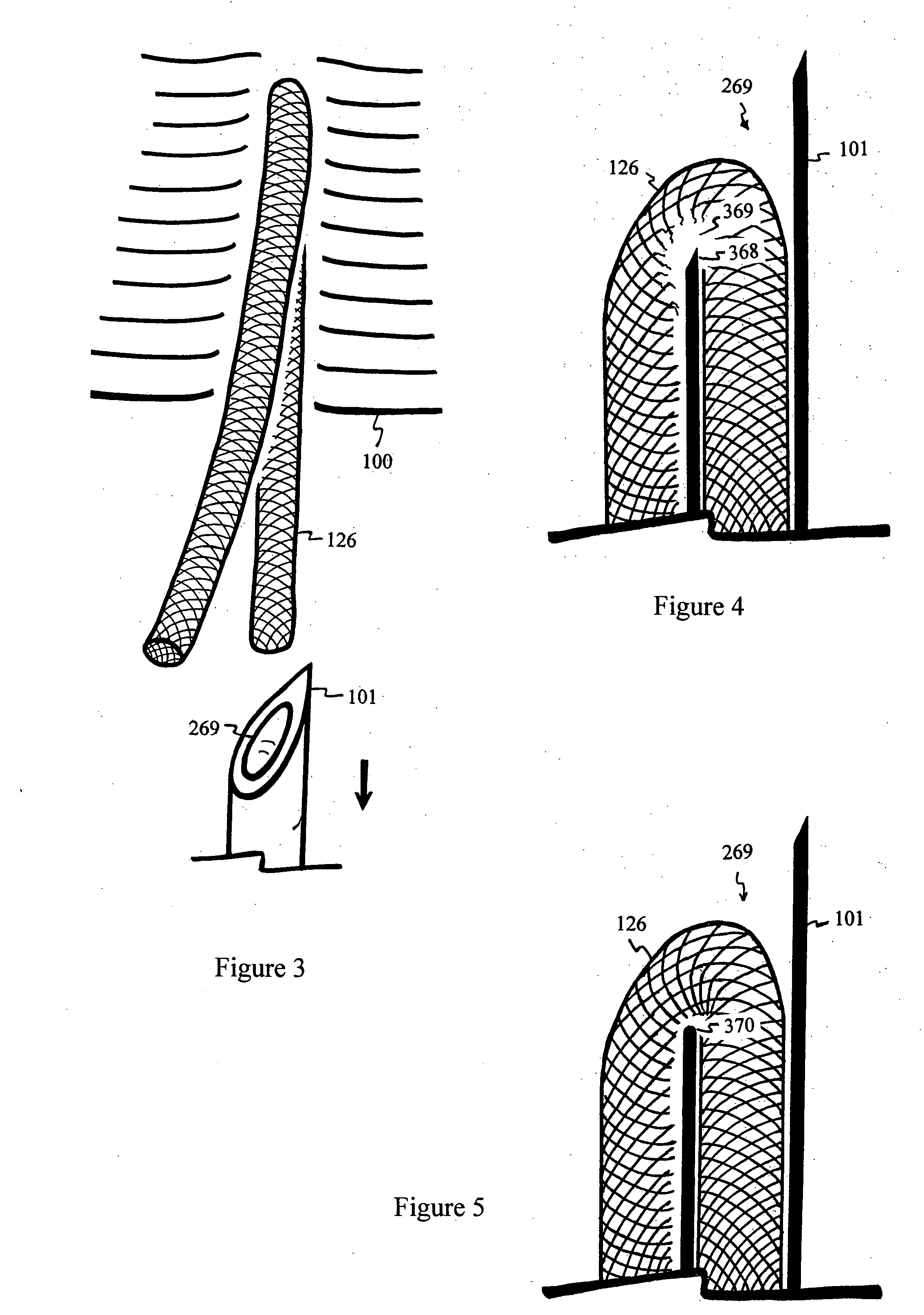
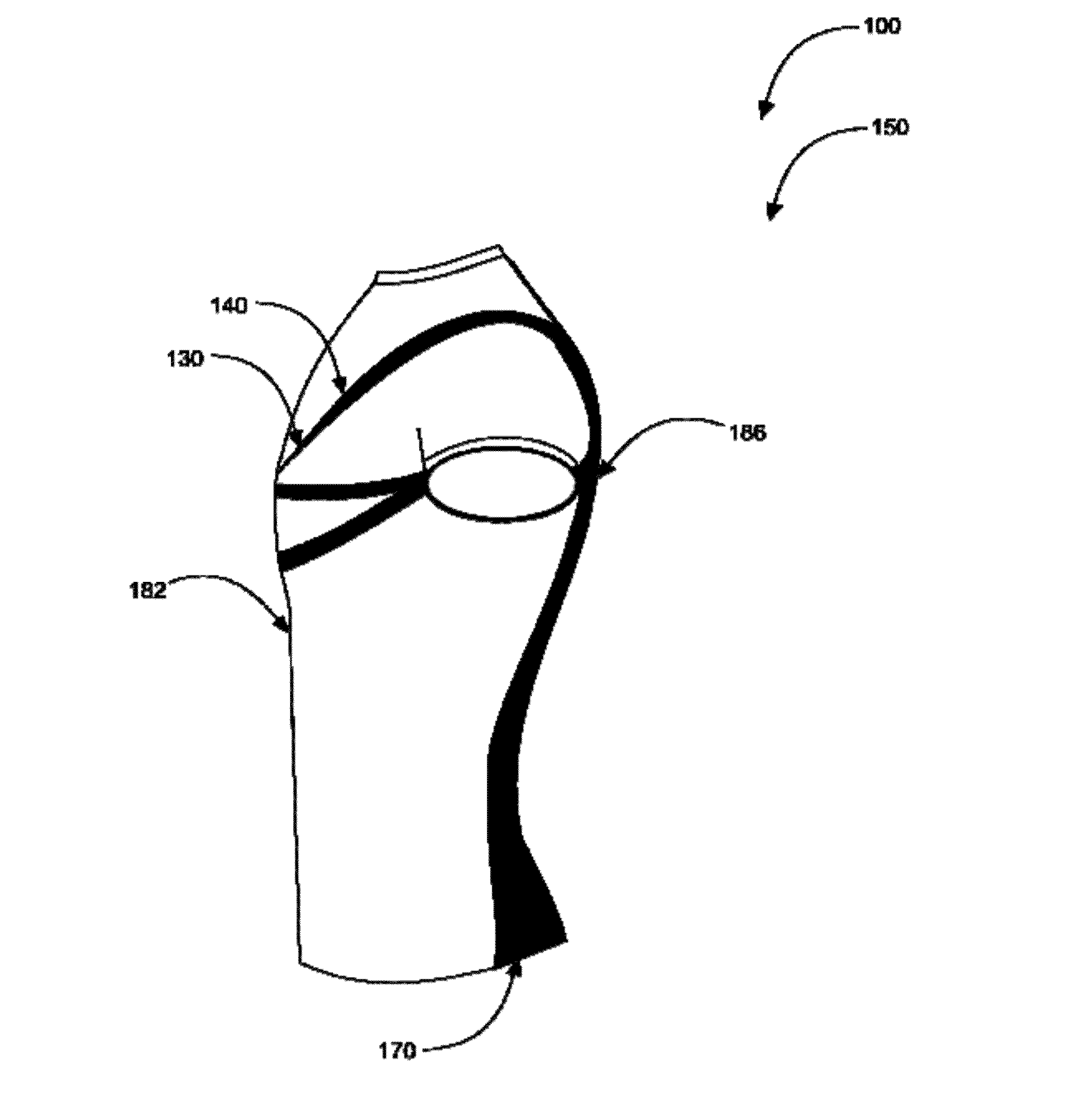
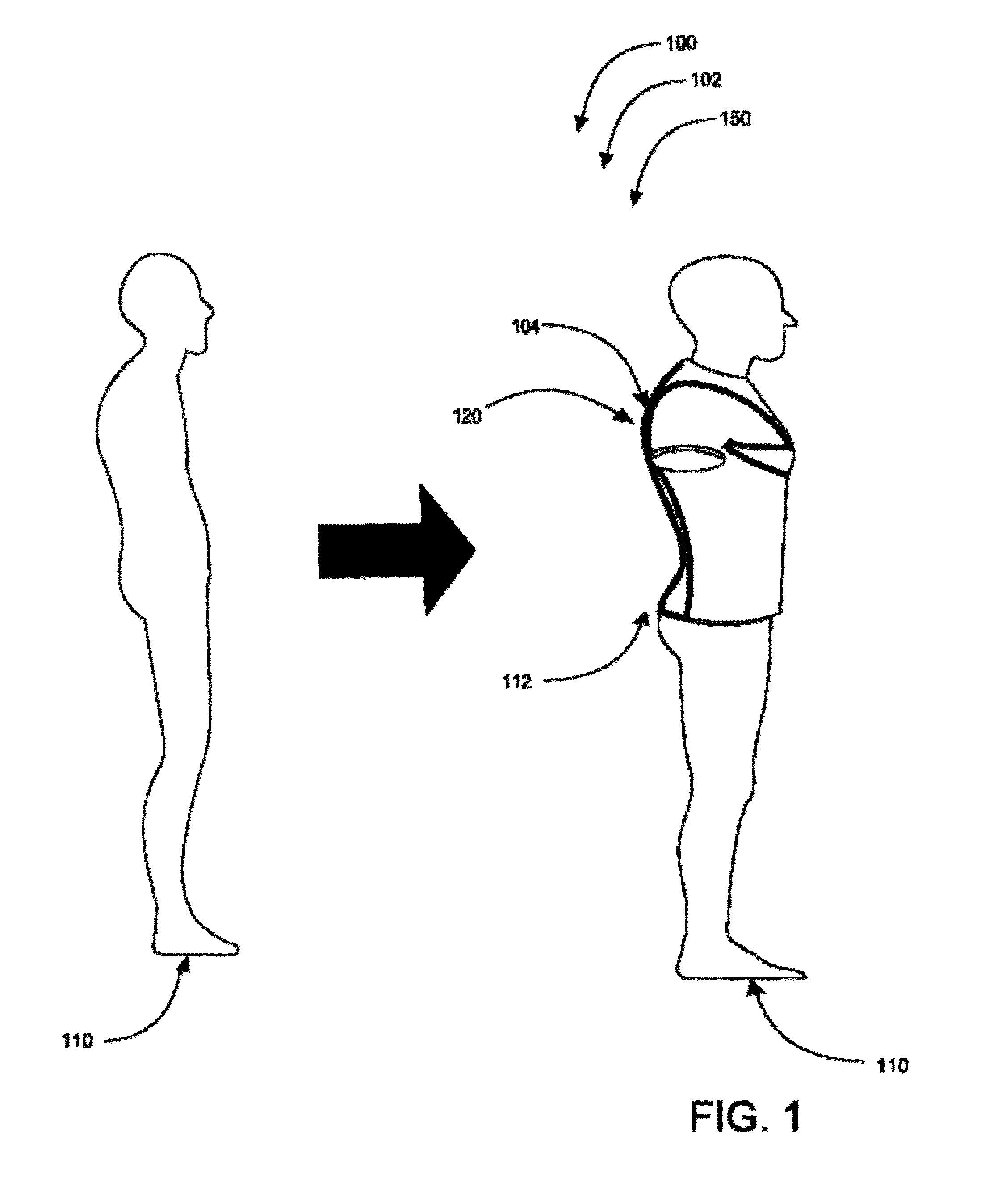
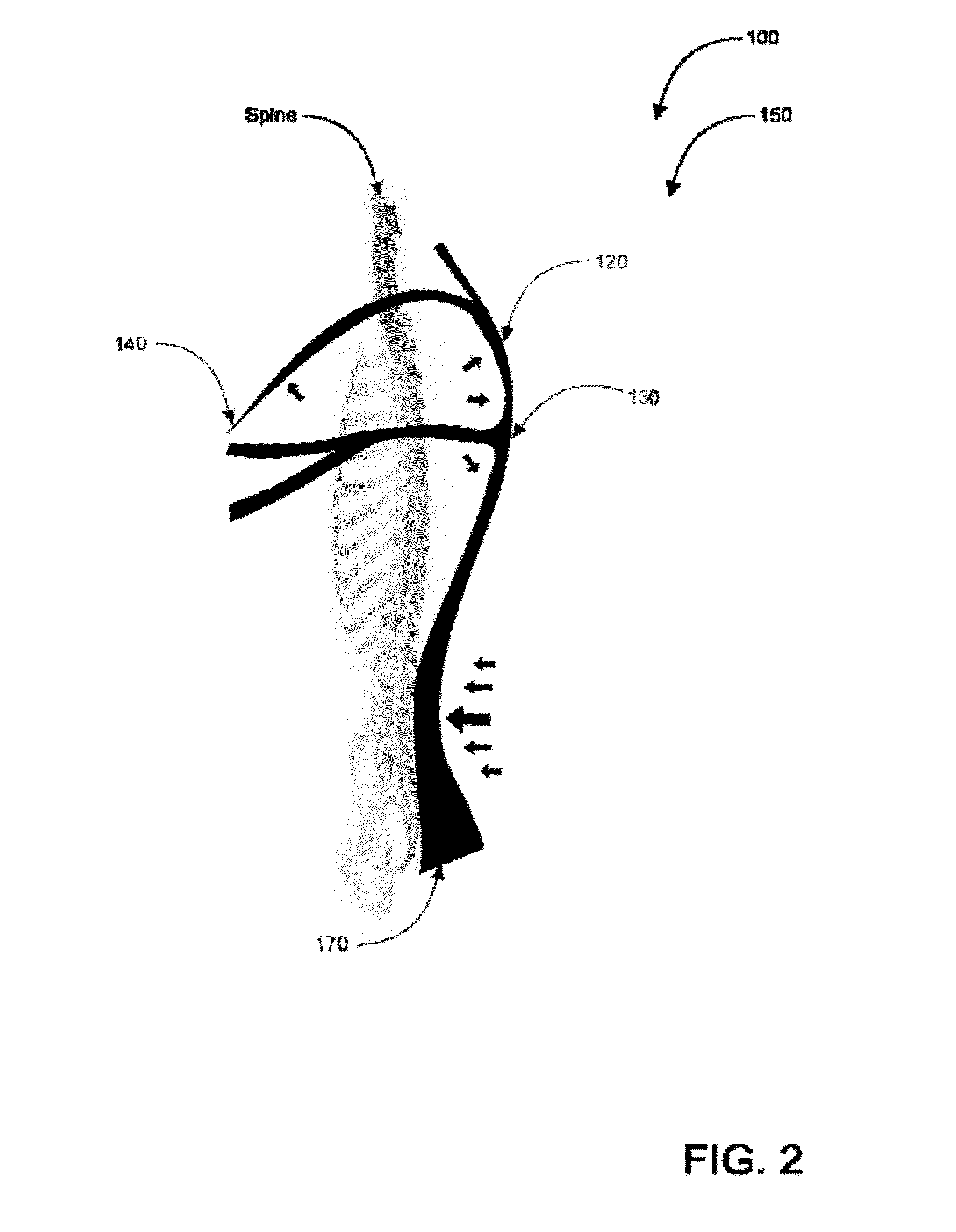
Spine-align garment systems
A posture-supporting, spine-aligning garment to support and correct body alignment and general positioning in its wearer-users; the garment being aesthetically pleasing. Posture-support, spine-align shirt with “Backboard” Technology is designed to alleviate back pain by promoting proper lumbar and thoracic alignment, core stabilization, corrective shoulder positioning, and upper body support. Posture-support spine-align garment system comprises an upper body apparel assembly having an elastic retention system; flexible stretch bands; anchors; a backboard, spine-specific support; and at least one fabric cover. The device may be manufactured with all or without one or more of the mentioned items. Posture-supporting, spine-aligning garment may comprise a posture shirt in preferred embodiments.
Owner:KAYSER ANDREW
Sportsman's lightweight adjustable one legged stool and walking aid
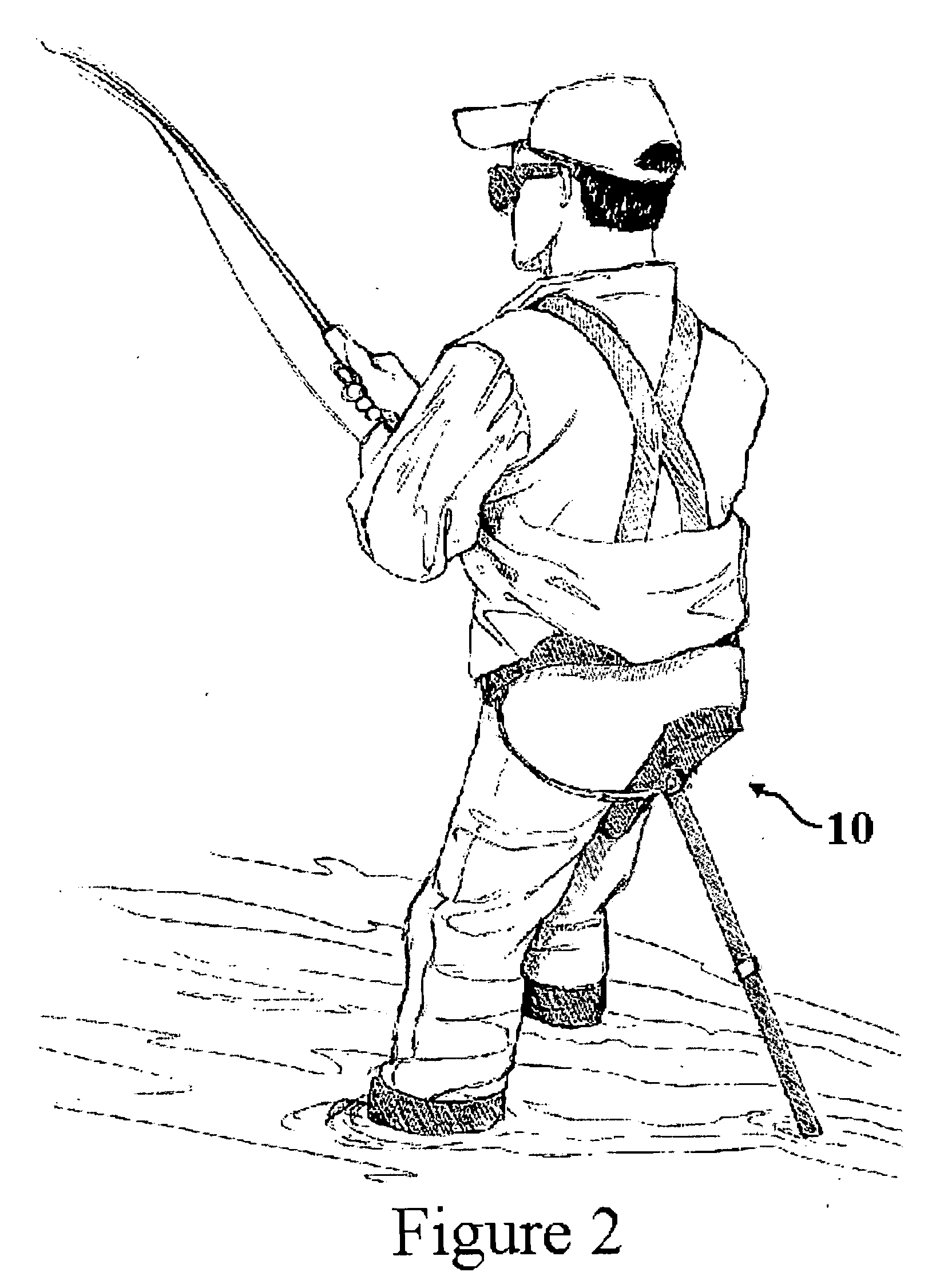
InactiveUS20050242630A1Great opportunityRelieve back painWalking sticksOffice stoolsEngineeringHeavy duty
A sportsman's lightweight, adjustable, heavy-duty, one legged stool and walking aid includes a pair of telescoping rods, a locking member to secure the rods together, an adjustable seat at an upper end of the pair of telescoping rods, wherein the seat has a handle portion therein, and a tether to secure the stool to the user. The seat may be in the form of a bicycle type seat whereby the handle is formed by a narrow forward end of the seat. The stool, or leaning post, has the seat in an operative position at all times and it need not be unfolded to be utilized.
Owner:MILLER JASON
Rehabilitation exercise device and method for persons with injuries causing limited ranges of motion to one or more limbs
InactiveUS20130143723A1Reduce riskRelieve back painResilient force resistorsRange of motionEngineering
A method and apparatus permitting a user to perform rehabilitation exercises despite having limited range of motion. Provided is a rehabilitation exercise device having a belt portion, which is to be fixed as a conventional belt to the waist of the user. The belt portion has laterally adjustable connectors for the attachment of elastic members / straps which are connected to the legs of the user, for various rehabilitary exercises based on the application of force required for the stretching of the elastic straps.
Owner:BENDER JONATHAN R
Passive back extensor device to treat trigger point - back pain
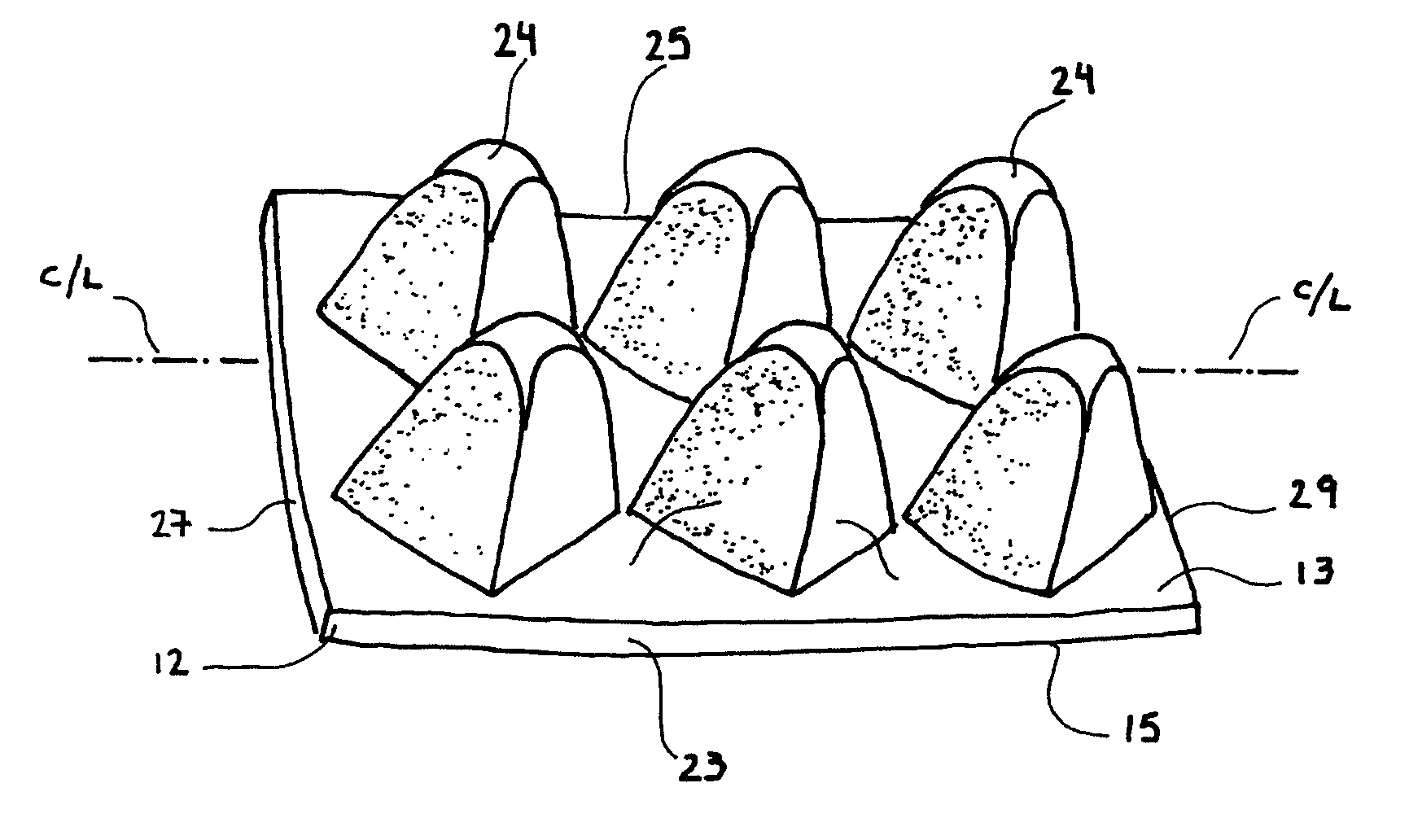
InactiveUS20050165450A1Relieve painLess discomfortChiropractic devicesGenitals massageBack painEngineering
A passive back extensor device for treatment of trigger-point back pain has a base substrate having upper and lower generally planar surfaces. A plurality of protuberances are integral with the base substrate and project from the upper generally planar surface and are arranged in substantially evenly spaced relation and define a pair of spaced rows so as to collectively define an elongate space between the rows. Each of the protuberances is of generally pyramid configuration having a rounded end oriented for passive manual pressure engagement with the paraspinal muscles along an individual's back on each side of the spine and with the elongate space receiving the bony protuberances of the spine. Each of the protuberances is arranged with one of its corners located adjacent a side edge or an end edge of the base substrate so that the planar surfaces and base is oriented in angular relation with the side and end edges of the base substrate.
Owner:PEREZ TORRENS YNGRID
Systems and methods for restoring muscle function to the lumbar spine
ActiveUS9072897B2Recovery functionRelieve back painSpinal electrodesMagnetotherapy using permanent magnetsRadiologyMuscle functions
A system for restoring muscle function to the lumbar spine to treat low back pain is provided. The system may include electrodes coupled to an implantable pulse generator (IPG), a handheld activator configured to transfer a stimulation command to the IPG, and an external programmer configured to transfer programming data to the IPG. The stimulation command directs the programmable controller to stimulate the tissue in accordance with the programming data. The system may include a software-based programming system run on a computer such that the treating physician may program and adjust stimulation parameters.
Owner:MAINSTAY MEDICAL
Medical implant and method of reducing back pain
An expandable medical implant capable of being inserted between adjacent vertebral endplates in a spine. The implant, when in an expanded position, includes a thicker peripheral portion and a thinner interior portion. Also, a method of manufacturing an expandable medical implant having a thicker peripheral portion and a thinner interior portion. Further, a method for reducing back pain that includes placing a medical implant between two adjacent vertebral endplates and positioning the implant such that the implant distributes stresses toward stronger portions of the endplates when pressure is placed on the implant.
Owner:PATEL TUSHAR
Systems and methods for restoring muscle function to the lumbar spine
ActiveUS20150306405A1Recovery functionRelieve back painSpinal electrodesMagnetotherapy using permanent magnetsLumbarMuscle functions
A system for restoring muscle function to the lumbar spine to treat low back pain is provided. The system may include electrodes coupled to an implantable pulse generator (IPG), a handheld activator configured to transfer a stimulation command to the IPG, and an external programmer configured to transfer programming data to the IPG. The stimulation command directs the programmable controller to stimulate the tissue in accordance with the programming data. The system may include a software-based programming system run on a computer such that the treating physician may program and adjust stimulation parameters.
Owner:MAINSTAY MEDICAL
Seat cushion
InactiveUS8696059B2Relieve back painRelieve pressureOperating chairsMusic stoolsBack painEngineering
Owner:CARMICHAEL THRONE
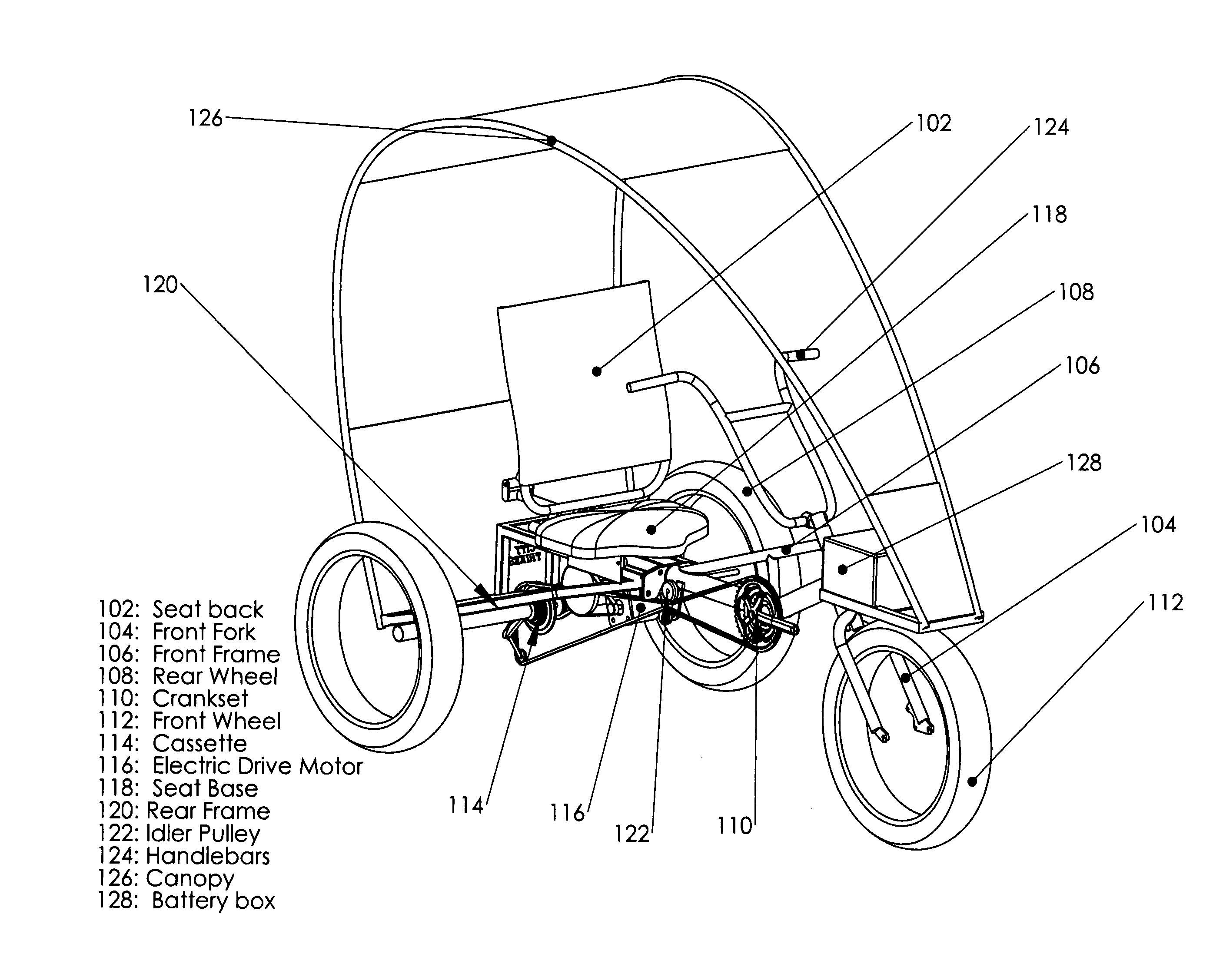
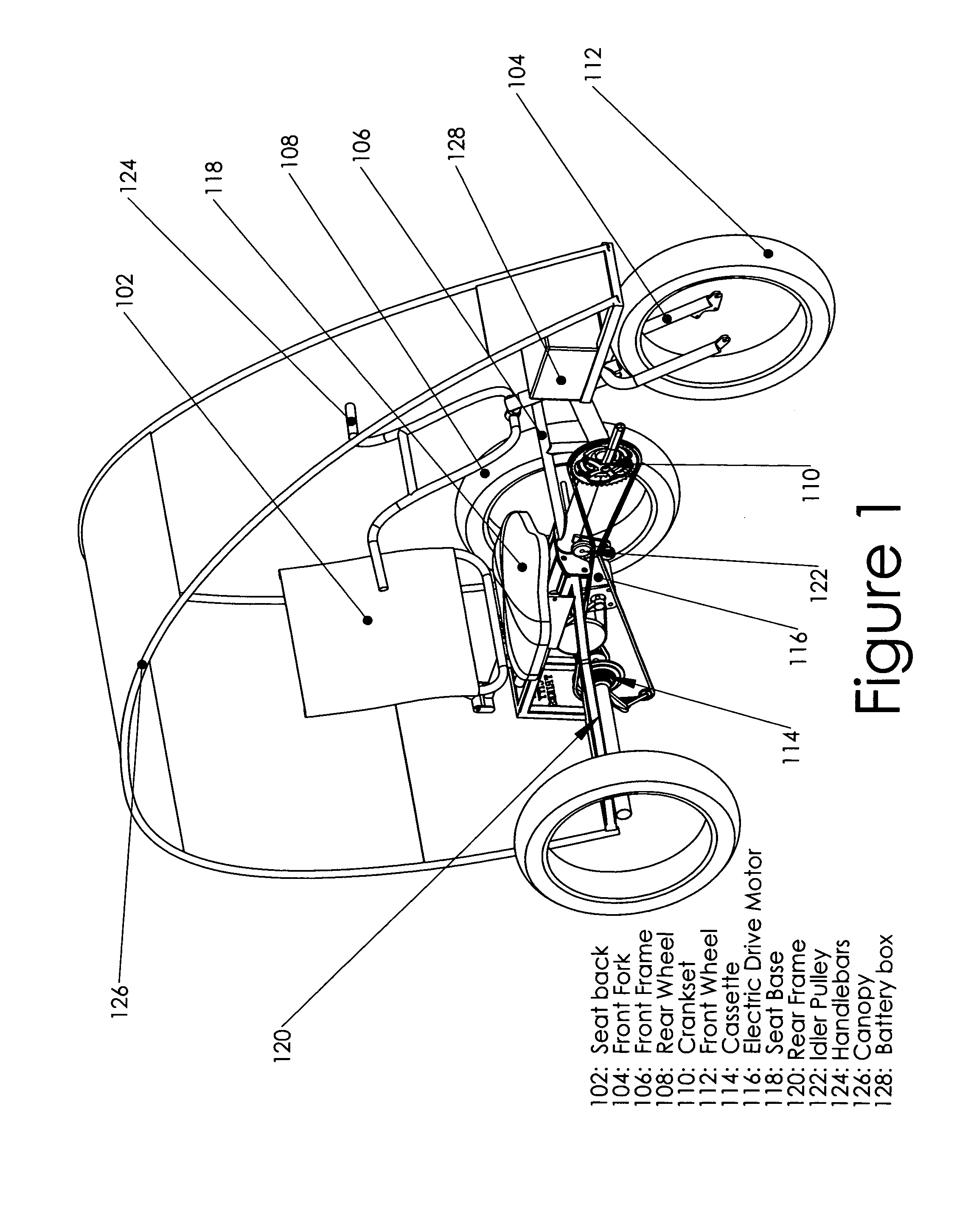
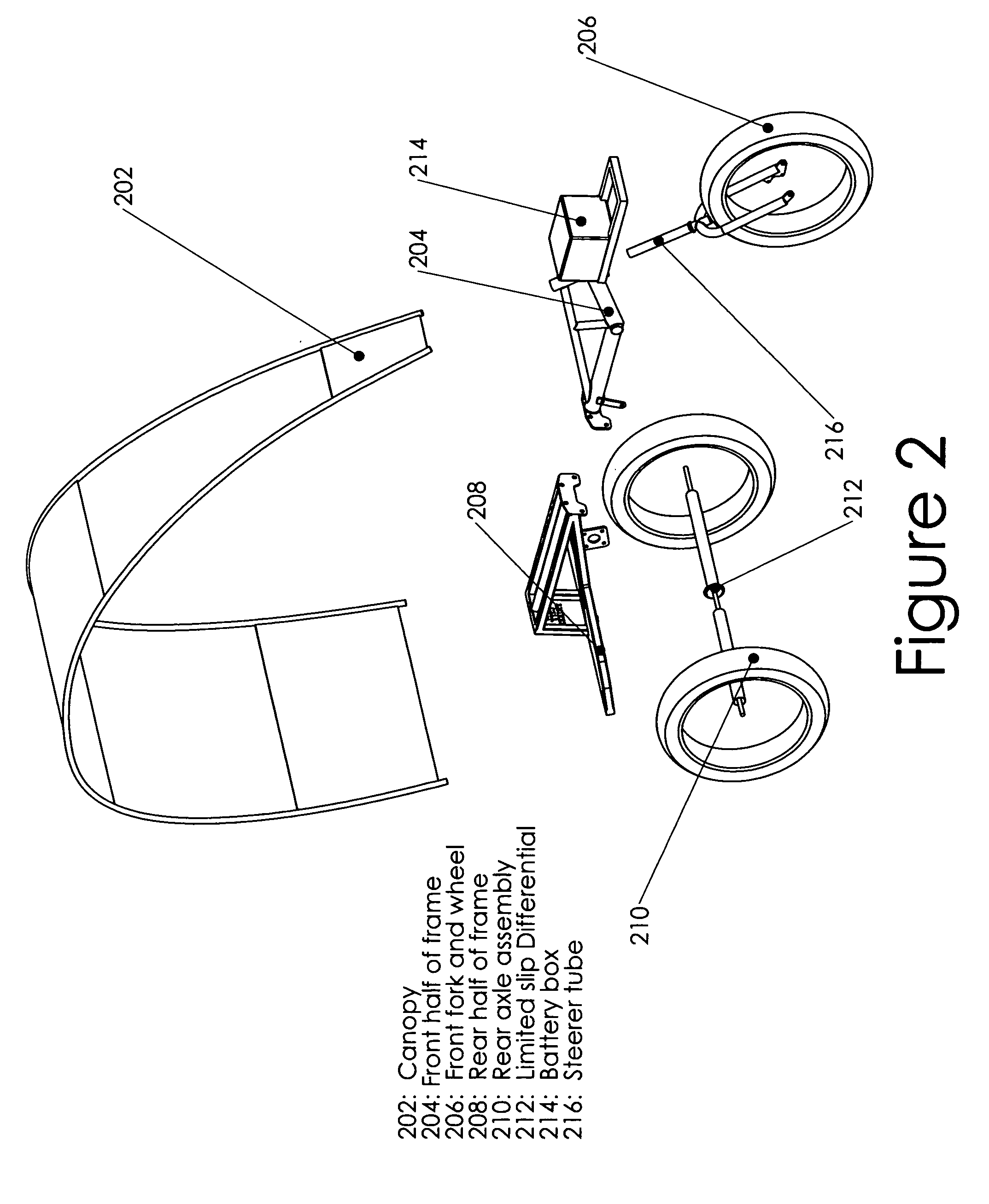
Recreational and utility three or four-wheeled recumbent cycle with on-demand zero emmissions electric motor and multi-geared manual pedal drive
InactiveUS20100314179A1Lower resistanceFresh airAuxillary drivesWeather guardsElectrical batteryGear wheel
One embodiment of a single person recumbent three or four-wheeled, zero emissions utility and recreational cycle vehicle provided with an electric motor driven from a battery and a multiple geared manual pedal mechanism with rear axle derailleur allowing the user to either select individually, or select simultaneously, electric powered or manual pedaling locomotion; also incorporating a rear axle limited slip differential and extra wide tires; and incorporating modular frame, canopy and rear axle design and construction, front and rear disc brakes, a highly-visible canopy; and incorporating a multi-purpose trailer or accessory hitch or receiver for attaching on-board cargo and material carrying devices or for towing of other vehicles for transport of personnel and materials.
Owner:GIBSON ROBERT DENIS
Ergonomic gobelek chair
ActiveUS20140265495A1Improves strength and endurance and flexibilityFunction increaseOperating chairsDental chairsBack painEngineering
The present invention includes an ergonomic chair that is useful for people who sit for an extended period of time at work, in avoiding work station related back pain and neck pain. The present invention incorporates a hemispherical seat which can be locked in position and comprises a fixed inner hemisphere, a movable outer hemisphere, and a shroud. The support pole where the inner hemisphere is bolted into absorbs the load from the user, and the outer hemisphere equipped with hydraulic or pneumatic resistance devices and attached to the tension springs provides a balanced movement of the chair. Accordingly, while sitting on the chair, the present invention allows no deformation on the rigid hemispherical seat, and keeps the spine of the user in a desirable alignment, further improving the internal function of the user's organs. The present invention can also be used as an office stretch GYM ball.
Owner:BAY ARAZ
Composition to reduce allodynic back pain and related method of use
InactiveUS20110269727A1Maximize resultReduce back painBiocideNervous disorderOpioid antagonistMedetomidine
The invention relates to a pharmaceutical composition to reduce back pain comprising two compounds: an opioid antagonist and a direct-acting alpha 2 adrenegic agonist. The Opioid antagonist is selected from the group consisting of alvimopan, nalmefene, naloxone, naltrexone, methylnaltrexone, nalorphine, and pharmaceutically acceptable salt. The direct-acting alpha 2 adrenegic agonist is selected from a group consisting of Apraclonidine, Brimonidine, Clonidine, Detomidine, Dexmedetomidine, Guanabenz, Guanfacine, Lofexidine, Medetomidine, Romifidine, Tizanidine, Tolonidine, Xylazine and Fadolmidine. In one embodiment, the composition may include naltrexone (or its pharmaceutically acceptable salt) as the opioid antagonist and clonidine hydrochloride (or its pharmaceutically acceptable salt) as the direct-acting alpha 2 adrenegic agonist. It is preferred naltrexone be administered with a second agent such as an antitussive, expectorant, decongestant, or antihistamine. The dosage of naltrexone may range between 0.25 mg to 15 mg, while the dosage of clonidine hydrochloride ranges between 0.0125 mg and 0.3 mg.
Owner:ALLODYNIC THERAPEUTICS
Supine spinal column flexing fixture and method
InactiveUS6071253AEasy to separateReduce accumulationOperating chairsChiropractic devicesSpinal columnTherapeutic exercise
There is disclosed a therapeutic exercise in which the patient lies in a supine position on a flat surface, with a long, smooth cylindrical roller having a constant compressed diameter from about 2 to about 4 inches throughout its length beneath the patient's back. The roller should be of sufficient length to span laterally across the entire patient's back and shoulders. The patient's slowly rolls his body over the roller from the sacrum along the lumbar and thoracic vertebrae by the pushing against the support surface with his feet and hands, and while shifting or rocking his body, side-to-side. The roller fixture includes a pair of end brackets which rotationally support a pair of end shafts of the roller and includes a frictional brake to restrain the rotation of the roller.
Owner:RIVERA ARNOLD
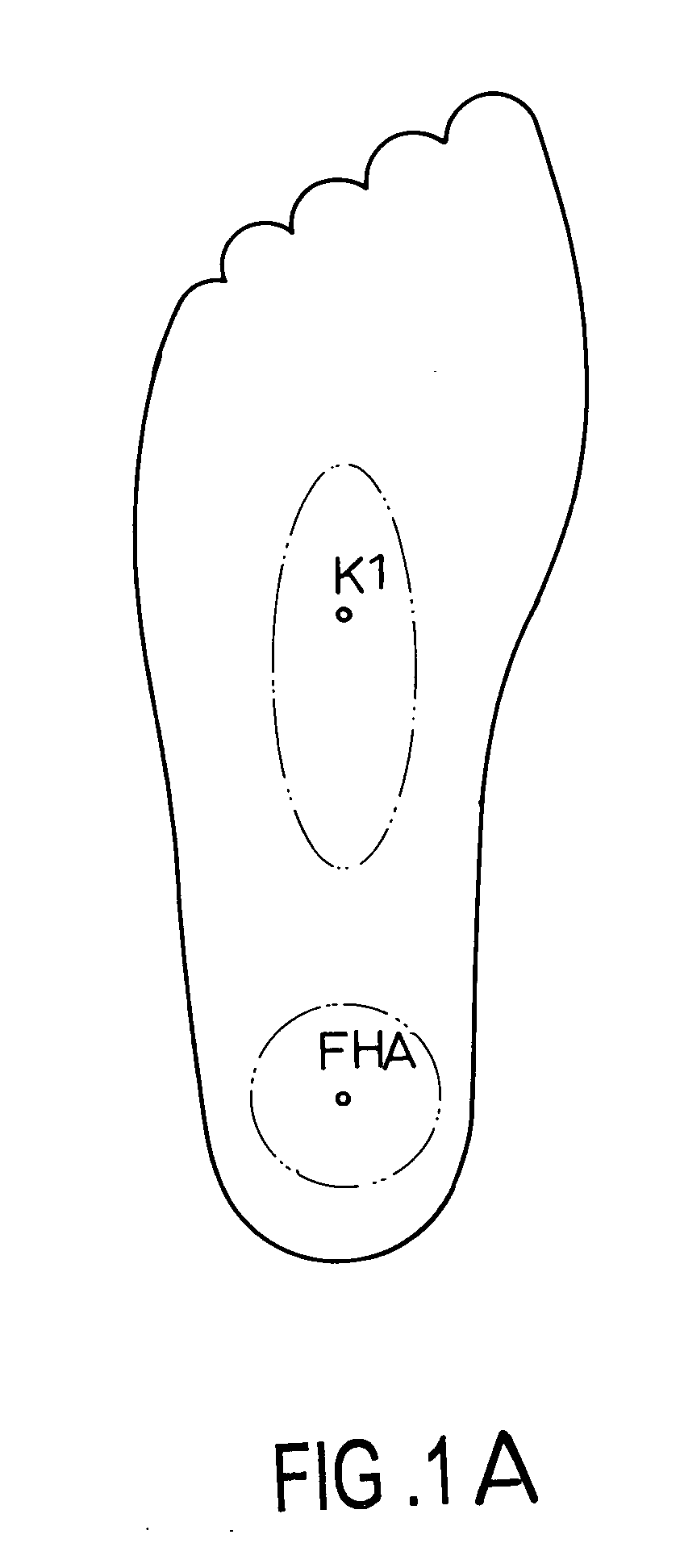
Method for moderation of back pain
InactiveUS20050187601A1Relieve back painLow back painDevices for pressing relfex pointsInternal electrodesBack painNon invasive
A method for moderation lower back pain includes the steps of mounting a non-invasive stimulation device onto the group of stimulation points surrounding K1 and FHA acupuncture points, generating a stimulation signal and stimulating the group of stimulation points surrounding K1 and FHA acupuncture points.
Owner:WANG WEI CHENG
Systems and methods for restoring muscle function to the lumbar spine and kits for implanting the same
ActiveUS20160310732A1Relieve back painRestoring muscle functionSpinal electrodesMagnetotherapy using permanent magnetsSubcutaneous implantationRadiology
A system for restoring muscle function to the lumbar spine to treat low back pain is provided. The system may include one or more electrode leads coupled to an implantable pulse generator (IPG) and a tunneler system for subcutaneously implanting a proximal portion of the lead(s). The system may also include a handheld activator configured to transfer a stimulation command to the IPG, and an external programmer configured to transfer programming data to the IPG. The stimulation command directs the programmable controller to stimulate the tissue in accordance with the programming data. The system may include a software-based programming system run on a computer such that the treating physician may program and adjust stimulation parameters.
Owner:MAINSTAY MEDICAL
Combination abdominal crunch and gravity inversion exercise machine
InactiveUS7857741B1Save floor spaceSession efficientChiropractic devicesStiltsEngineeringExercise machine
A combination gravity inversion exercise machine and abdominal crunch exercise machine which enables a user to perform both exercises in one machine. The machine enables a user to save exercise floor space and perform the two different exercises on the same machine to thereby make an exercise session more efficient. The machine combines the features of a gravity inversion machine which enables a user to lie inverted for a period of time to take pressure off the back muscles and also has the features of an abdominal crunch which enables a user to exercise and tighten stomach muscles.
Owner:BODY FLEX SPORTS
Lumbar support for law enforcement officers
InactiveUS7270377B2Eliminates excessive lumbar pressureReduce Workman 's Compensation paymentBack restsDismountable chairsEngineeringLumbar
Many law enforcement officers have lower back problems arising from the habitual wearing of what is generally known as a “Sam Browne” duty belt. Workmen's Compensation now presumes any lower back ailment suffered by a law enforcement officer was caused by a duty belt; and consequently, automatically covers such back problem. A duty belt seat accessory for minimizing excessive pressure on the lower back of a law enforcement officer when he wears a duty belt is disclosed. The seat accessory comprises: a lower back portion having a minimal thickness; an upper back portion having a thickness generally exceeding the thickness of the lower back portion and the equipped duty belt; and, a strap to maintain the back portion in an upright position adjacent to a seat. When the officer leans back his back is supported largely by the upper back portion of the seat accessory and there is no excessive back pressure on his lower back as a result of the equipped duty belt he is wearing.
Owner:SCHMITZ JACK D +1
System and Methods for Diagnosis and Treatment of Discogenic Lower Back Pain
ActiveUS20160067494A1Relieve back painAvoid stimulationSpinal electrodesVibration massageClosed loopElectrical impulse
Methods and devices to treat discogenic lumbar back pain are disclosed. Electrodes are implanted within the anterior epidural space of the patient. A pulse generator that is connected to the electrodes delivers electrical impulses to sympathetic nerves located within the posterior longitudinal ligament (PLL) of the lumbar spine and outer posterior annulus fibrosus of the intervertebral disc. In alternate embodiments, energy directed to nerves in the PLL may be from light or mechanical vibrations, or the nerves may be cooled. The electrodes may also be used diagnostically to correlate spontaneous nerve activity with spinal movement, fluctuations in autonomic tone and the patient's experience of pain. The electrodes may also be used to generate diagnostic evoked potentials. The diagnostic data are used to devise parameters for the therapeutic nerve stimulation. Automatic analysis of the data may be incorporated into a closed-loop system that performs the nerve stimulation automatically.
Owner:LIPANI JOHN D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com