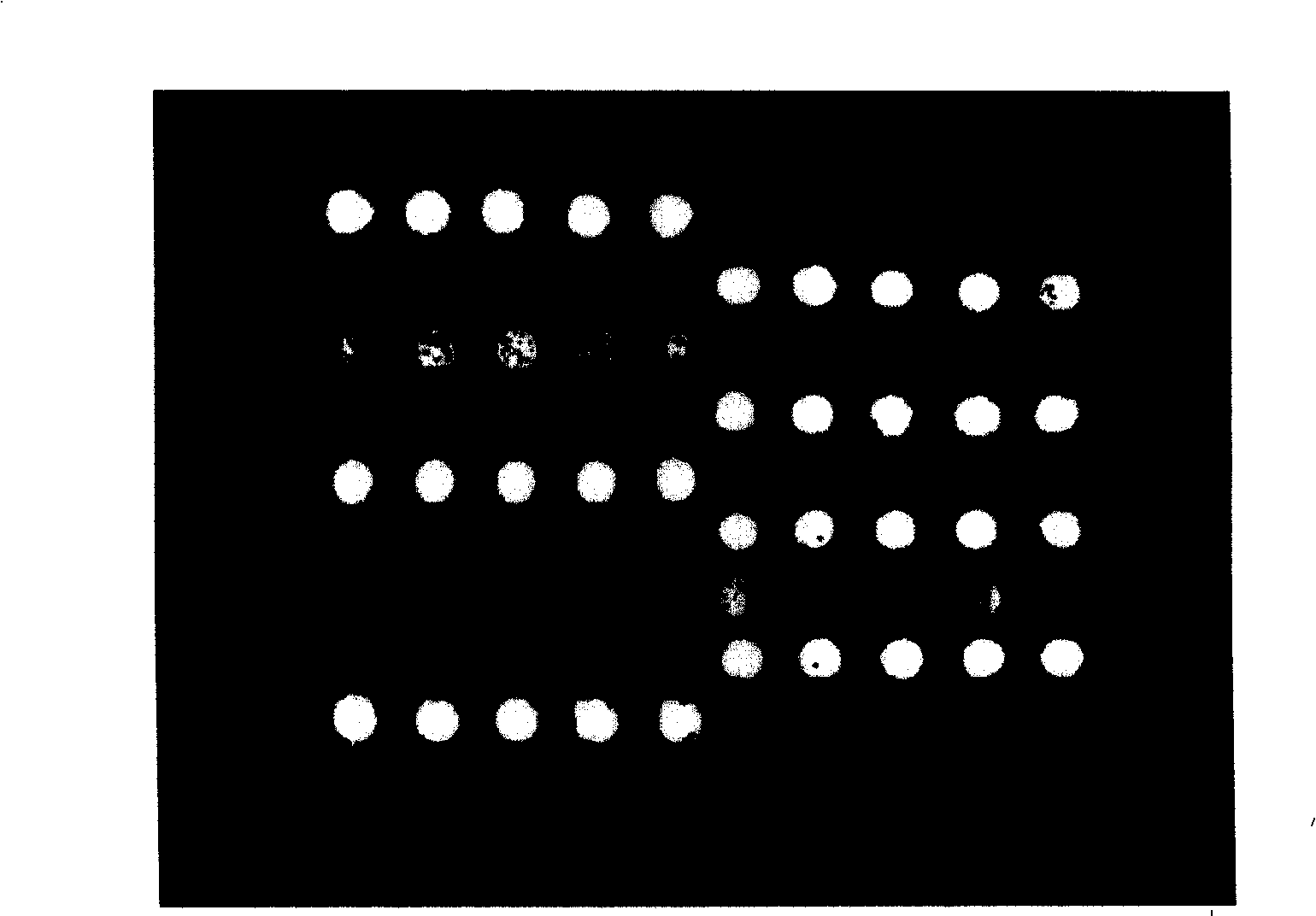Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Drug responsiveness" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
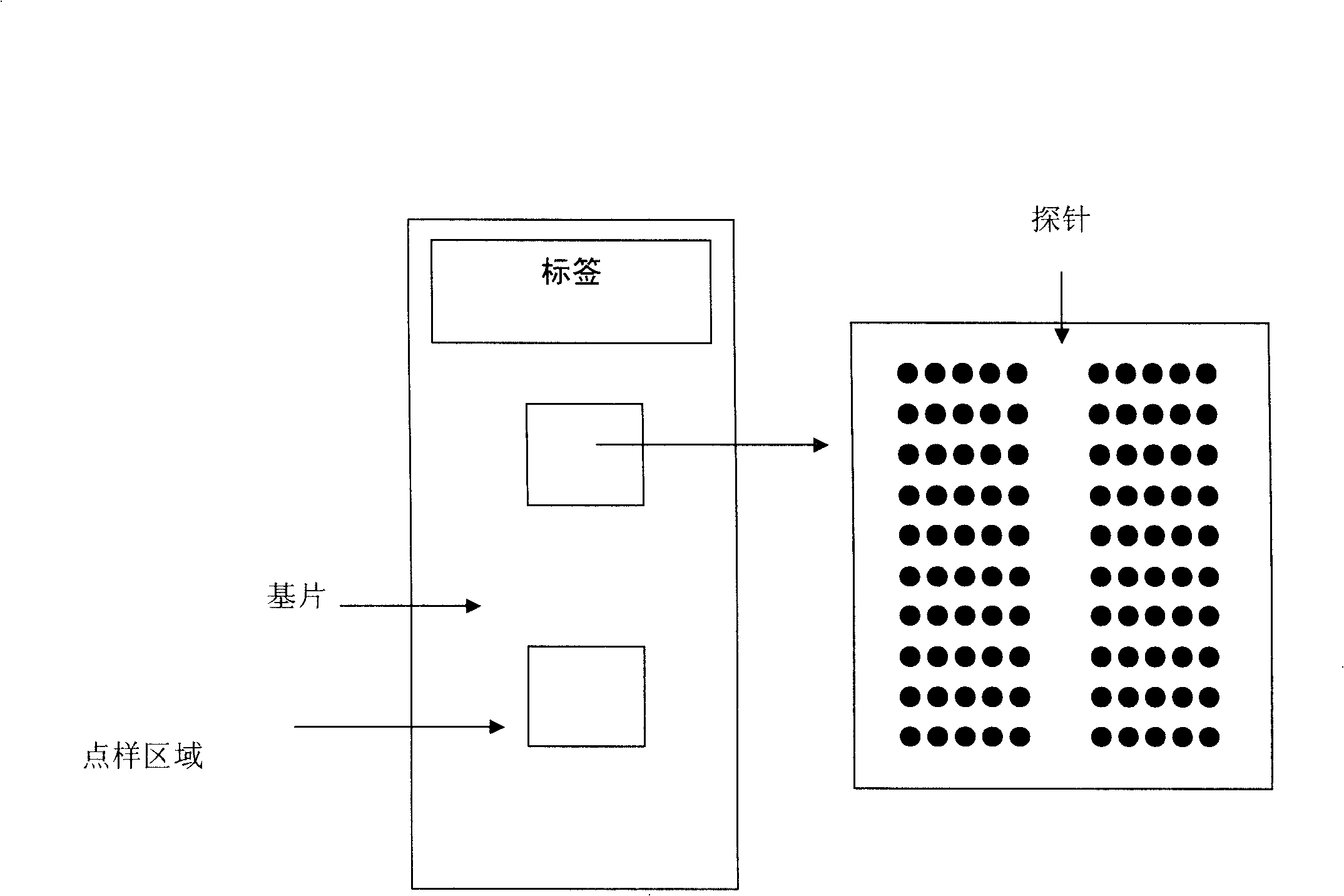
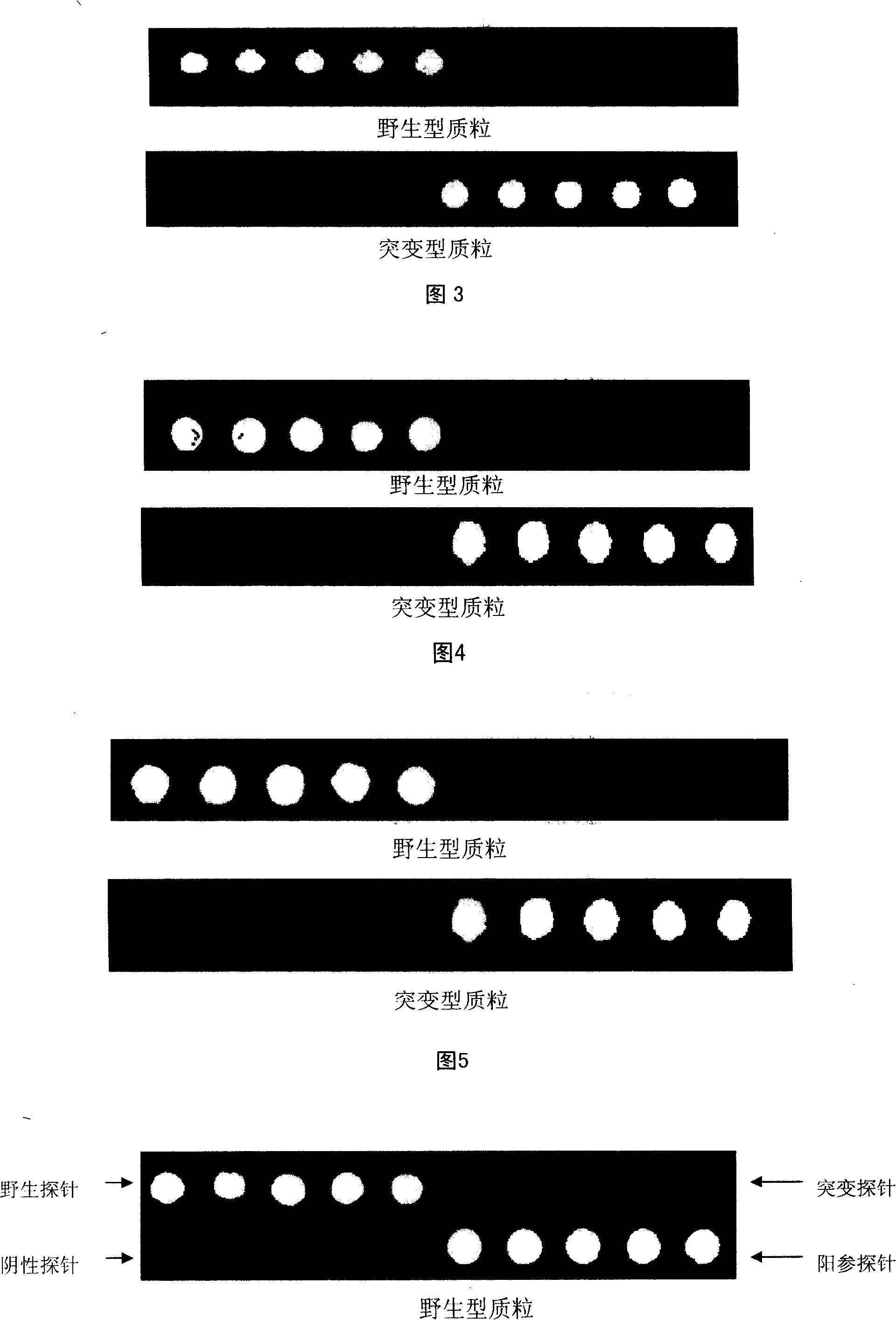
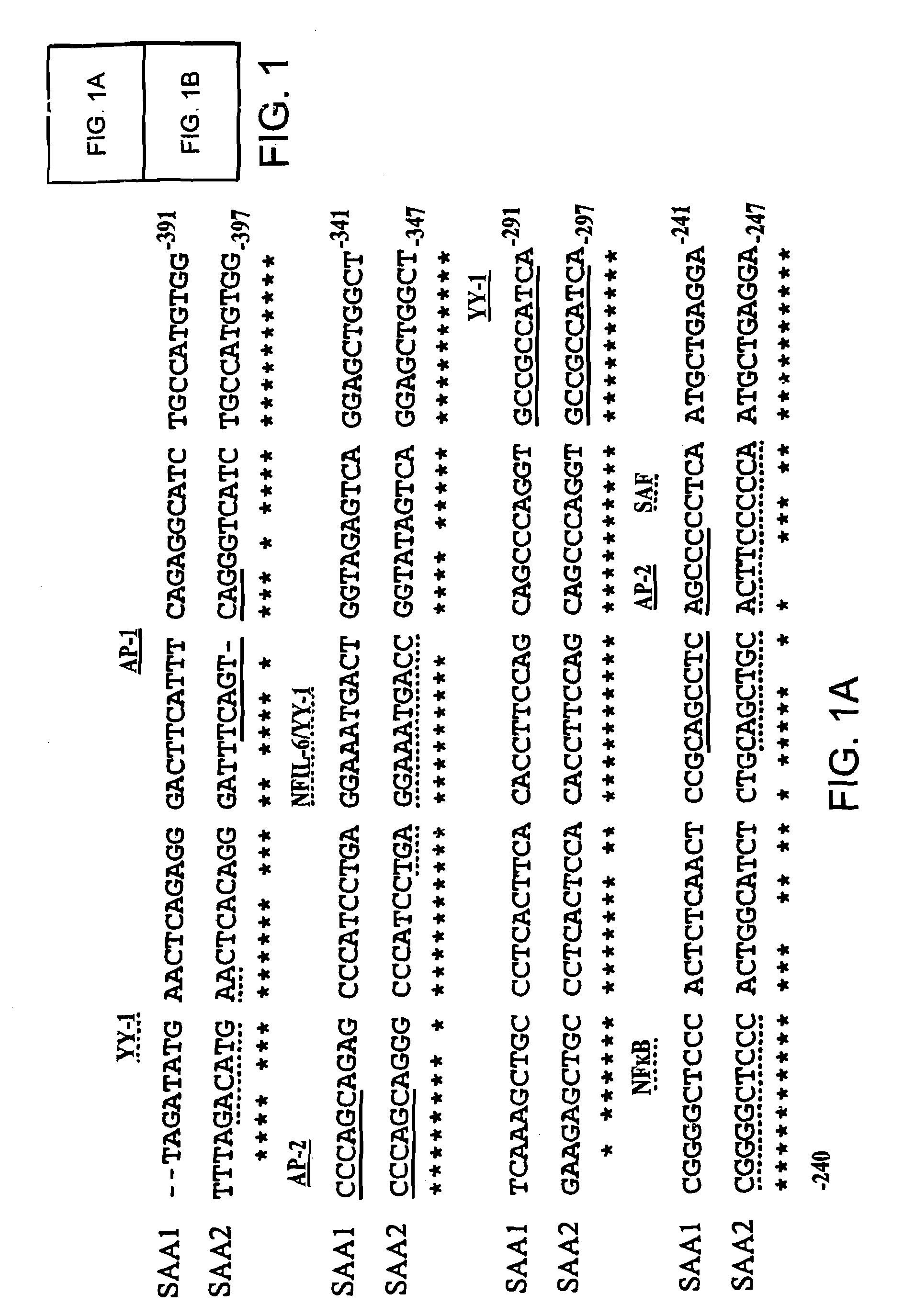
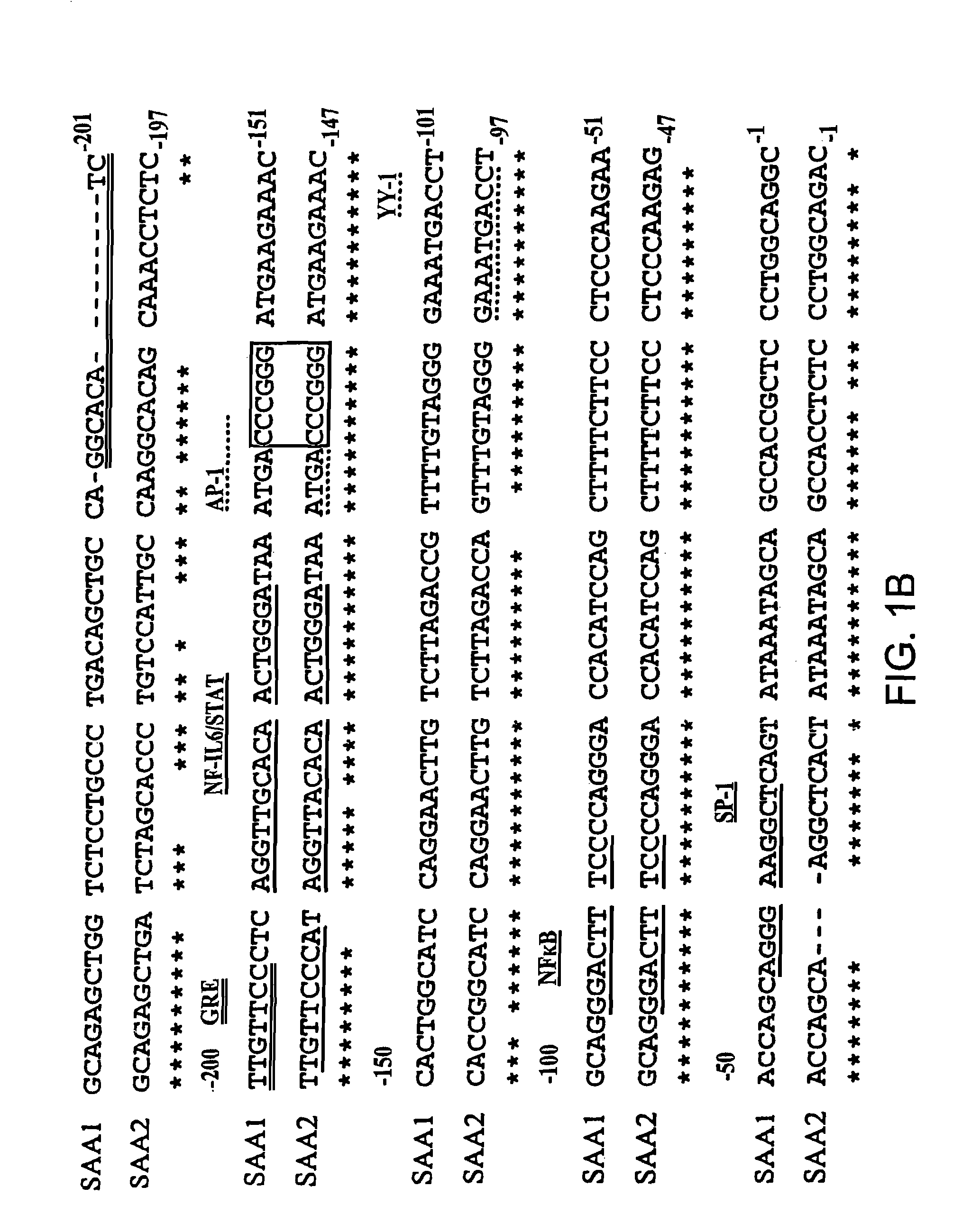
Gene chip for detection of hyperpiesis individual medicine correlated gene mutation and uses thereof
InactiveCN101343658AAccurate detectionSensitive detectionMicrobiological testing/measurementGNB3CYP2C9*3
The invention relates to a gene chip used for detecting gene mutation relating to a high blood pressure personalized medicine. The gene chip for detecting the gene mutation relating to high blood pressure personalized medicine comprises a solid phase support, a gene probe (an oligonueleotide probe) fixed in the solid phase support sequentially and a PCR primer used for amplifying the mutated gene fragment in the sample; the gene probe (the oligonueleotide probe) and the PCR primer are designed aiming at one or two points of the gene mutation in ACE (I / D) and CYP3A5*3 and / or two or more points as follows: CYP2C9*3, CYP2C9*13, AGTR1(A1166C), CYP2D6*10, ADRB1(C1165G), TSC(C1784T), ADRB3(T727C), SCNN1G_rs5729, SCNN1G_rs5723, ENOSA_rs1799983 and GNB3(C825T). The invention provides the gene chip and applications thereof for conveniently, quickly and systematically detecting the gene mutation relating to high blood pressure personalized medicine so as to determine drug reactions.
Owner:湖南宏灏基因生物科技有限公司
Methods for predicting drug responsiveness in cancer patients
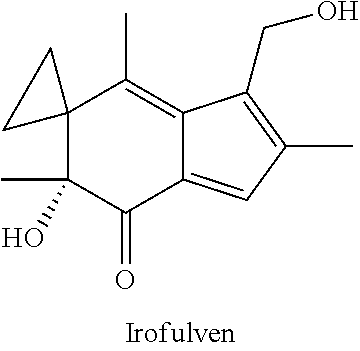
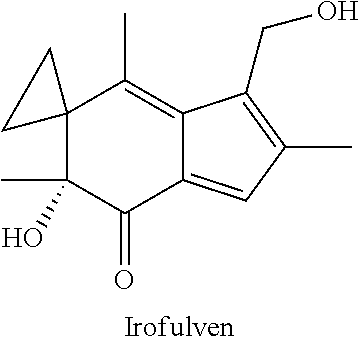
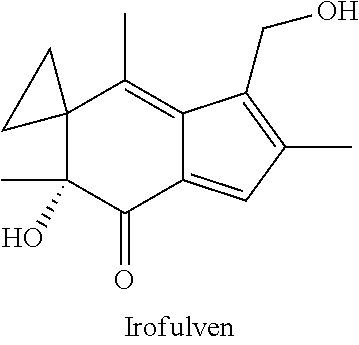
InactiveUS20180100197A1Reduction in tumor massLesser cell growthOrganic active ingredientsMicrobiological testing/measurementIrofulvenSurgery
The present invention features methods, devices, and kits for detecting expression in a patient having cancer or determining responsive of a patient having cancer to a treatment, such as irofulven. The invention further includes methods of treating a patient having cancer by administering, e.g., irofulven.
Owner:ALLARITY THERAPEUTICS EURO APS
Methods for predicting drug responsiveness in cancer patients
ActiveCN108265106AHeavy metal active ingredientsMicrobiological testing/measurementLipofectaminePhospholipase
METHODS FOR PREDICTING DRUG RESPONSIVENESS IN CANCER PATIENTS The present invention features methods, devices, and kits for detecting a level of one or more 5 biomarkers in a patient having cancer ordetermining the responsiveness of a patient having cancer to a treatment, such as treatment with a secretory phospholipase A2 (sPLA2) hydrolysable, cisplatin-containing liposome. The invention furtherincludes methods of treating a patient having cancer by administering, e.g., the liposome.
Owner:亚拉勒提治疗欧洲私人有限公司
Methods of classifying drug responsiveness using multiparameter analysis
InactiveUS7343247B2Sugar derivativesMicrobiological testing/measurementDrug responsivenessBioinformatics
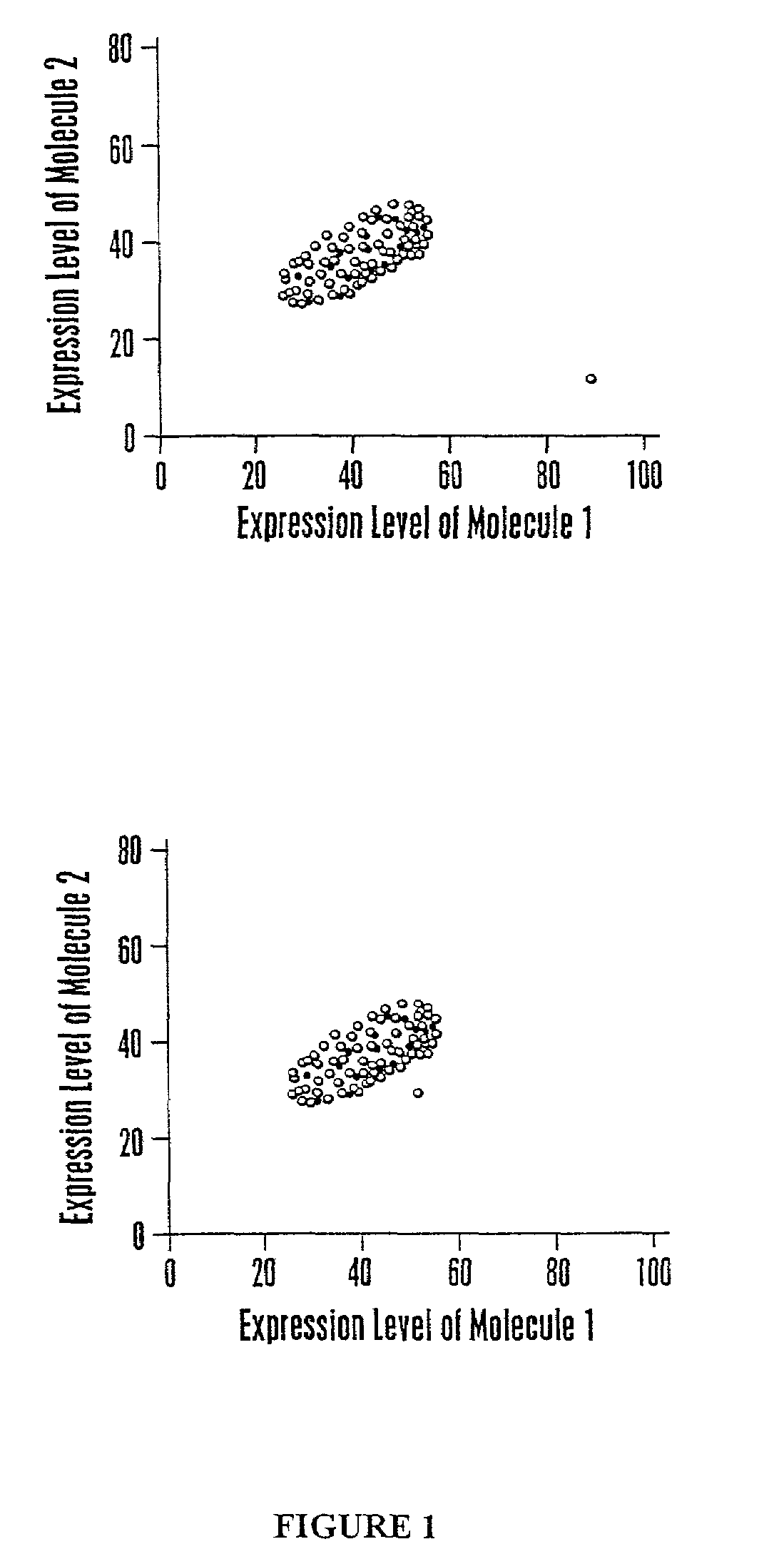
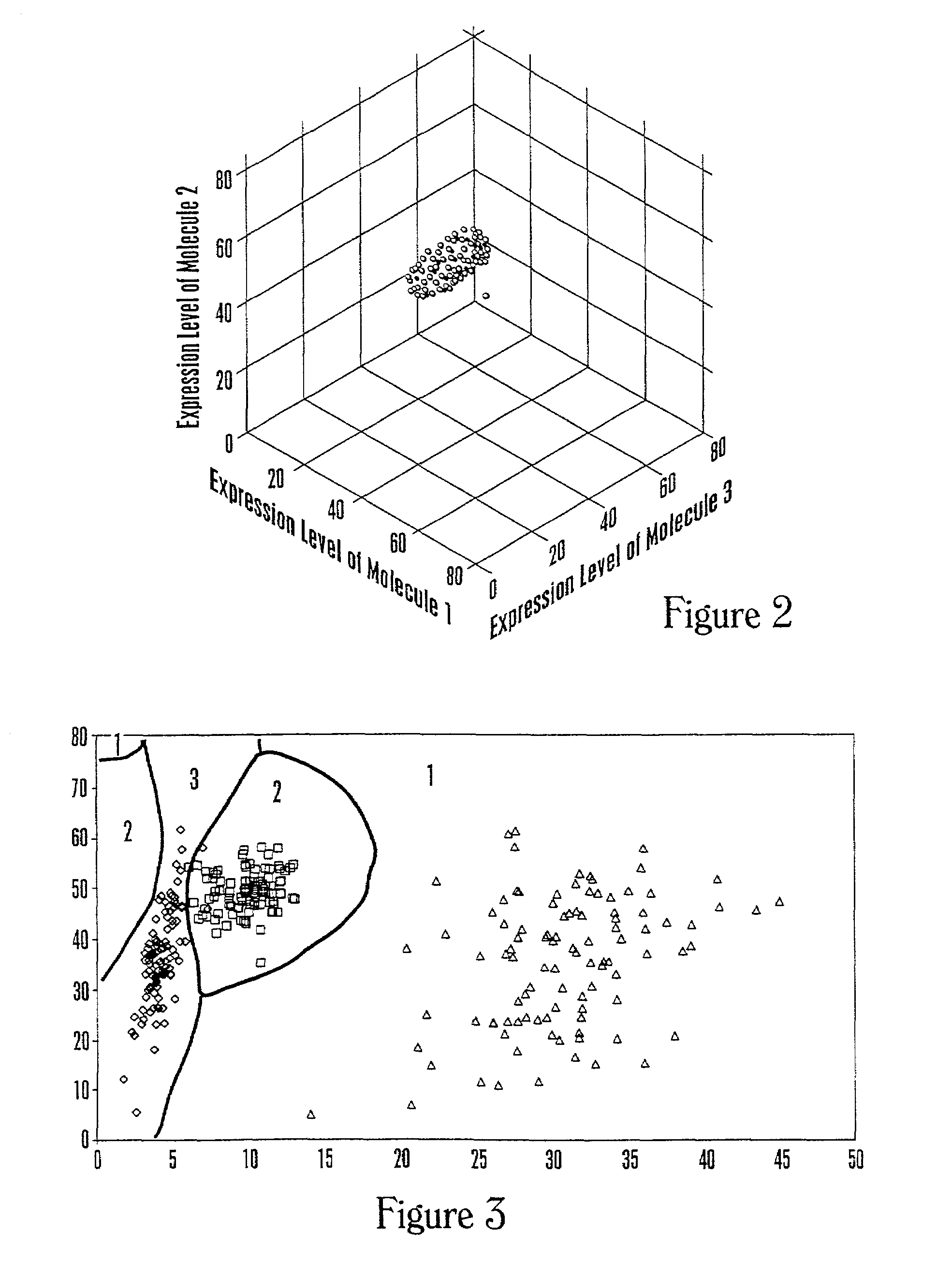
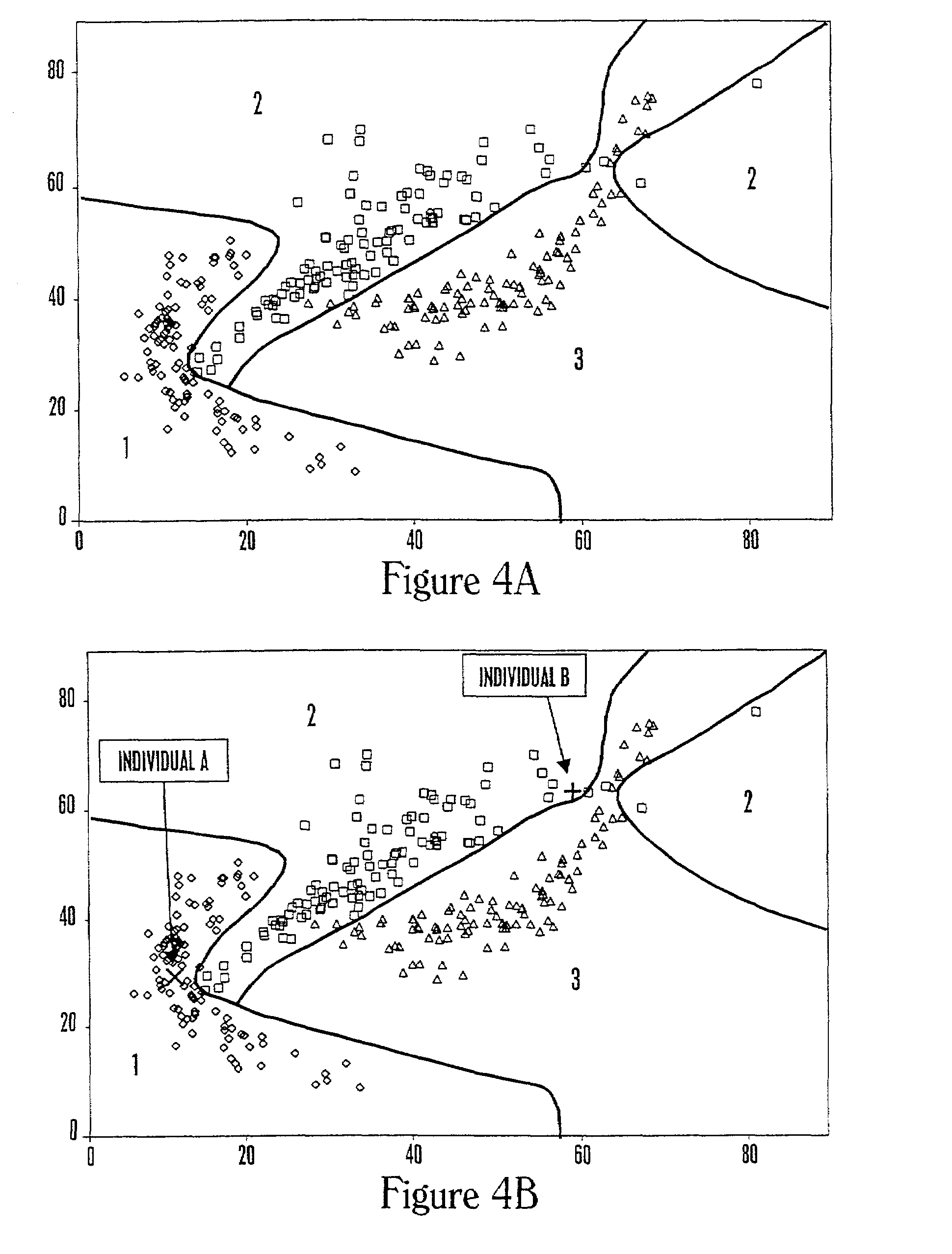
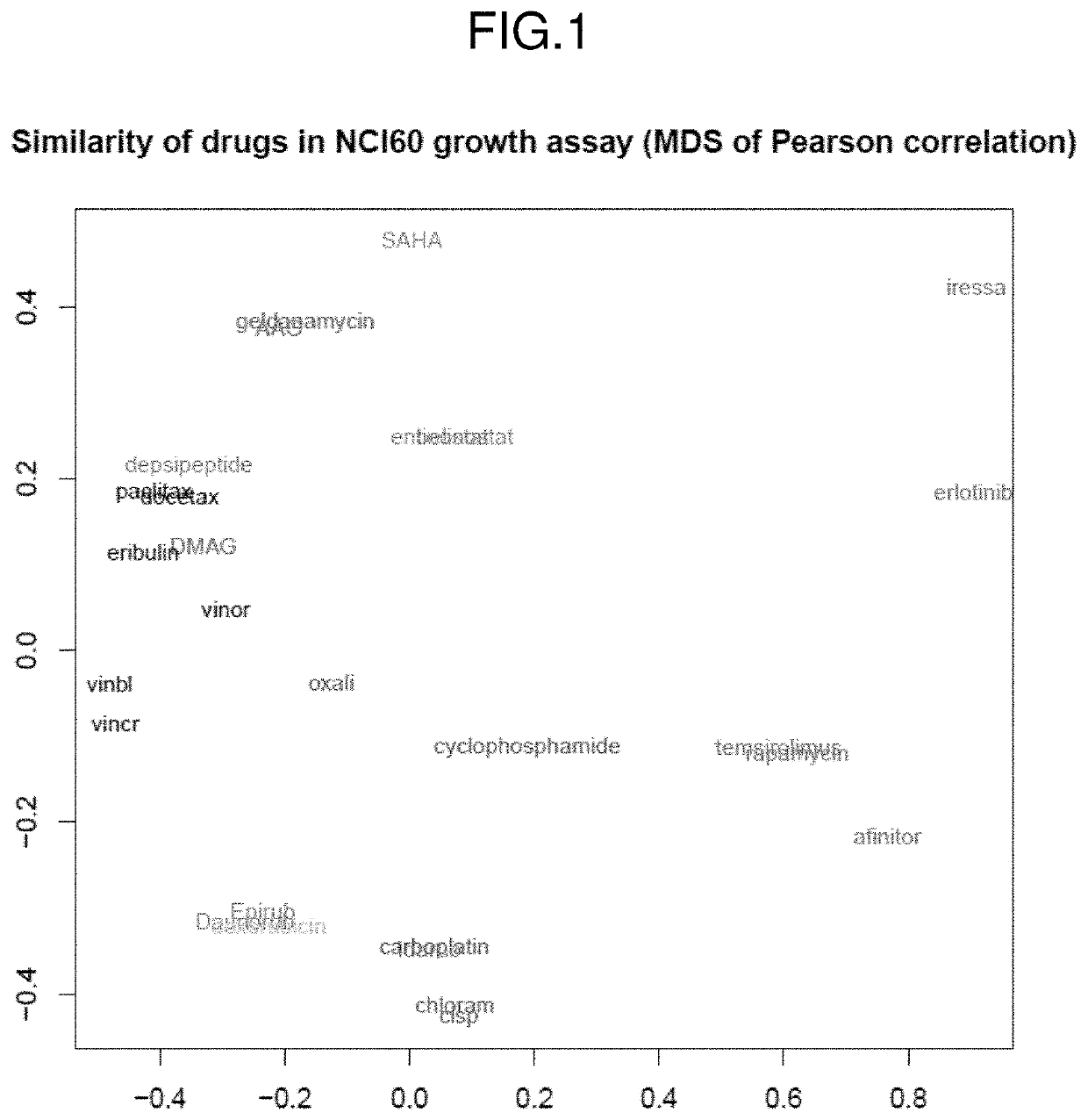
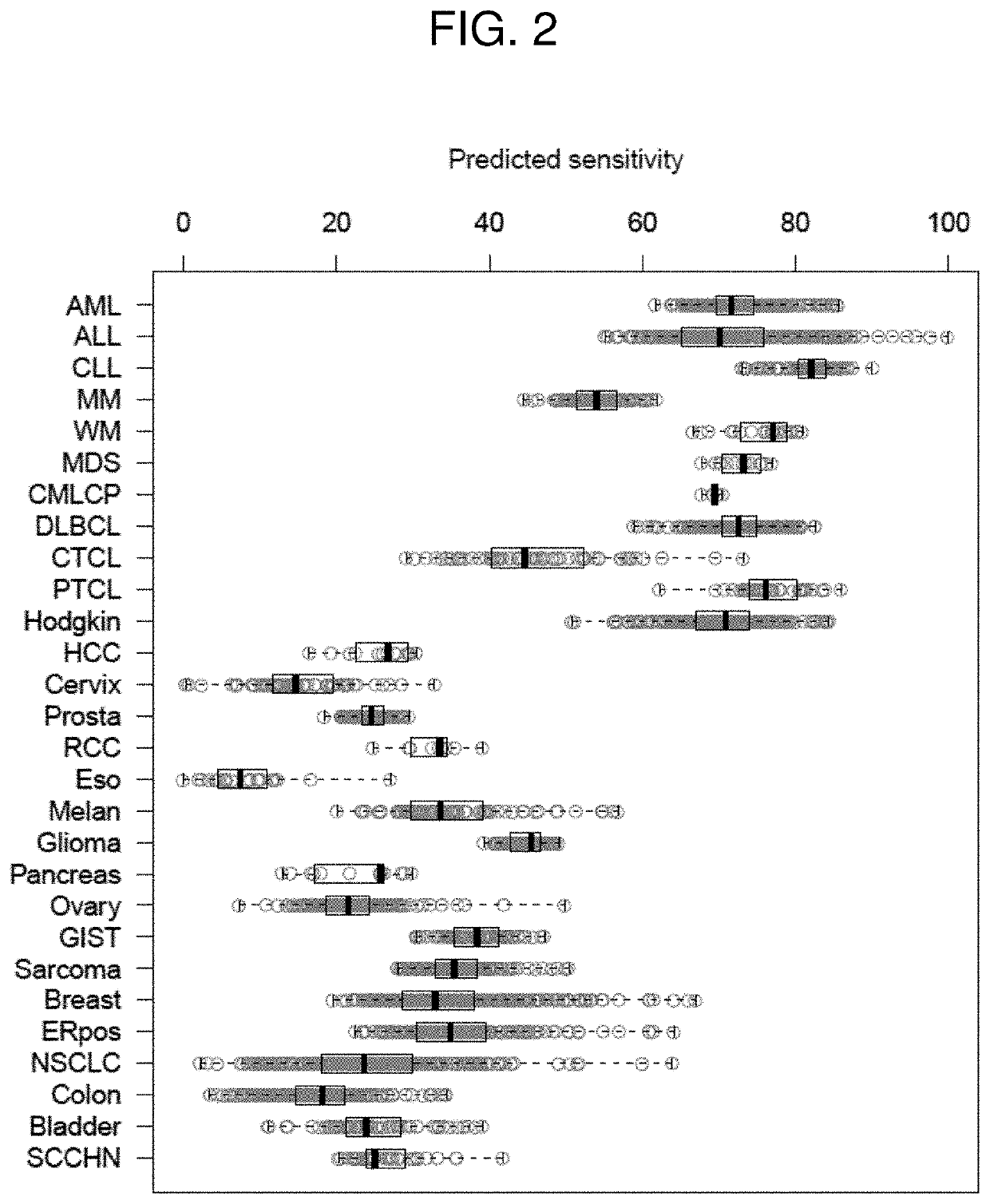
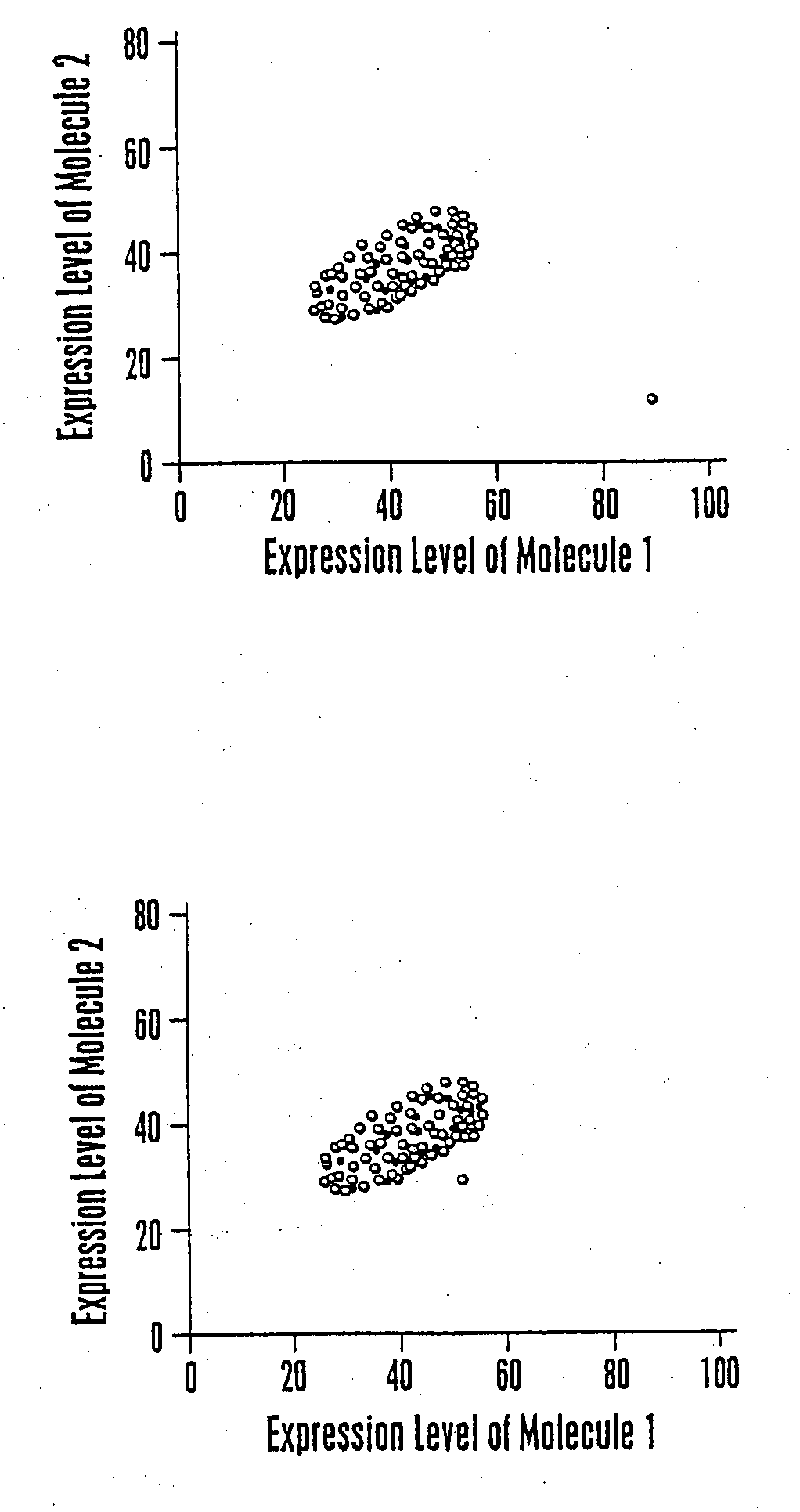
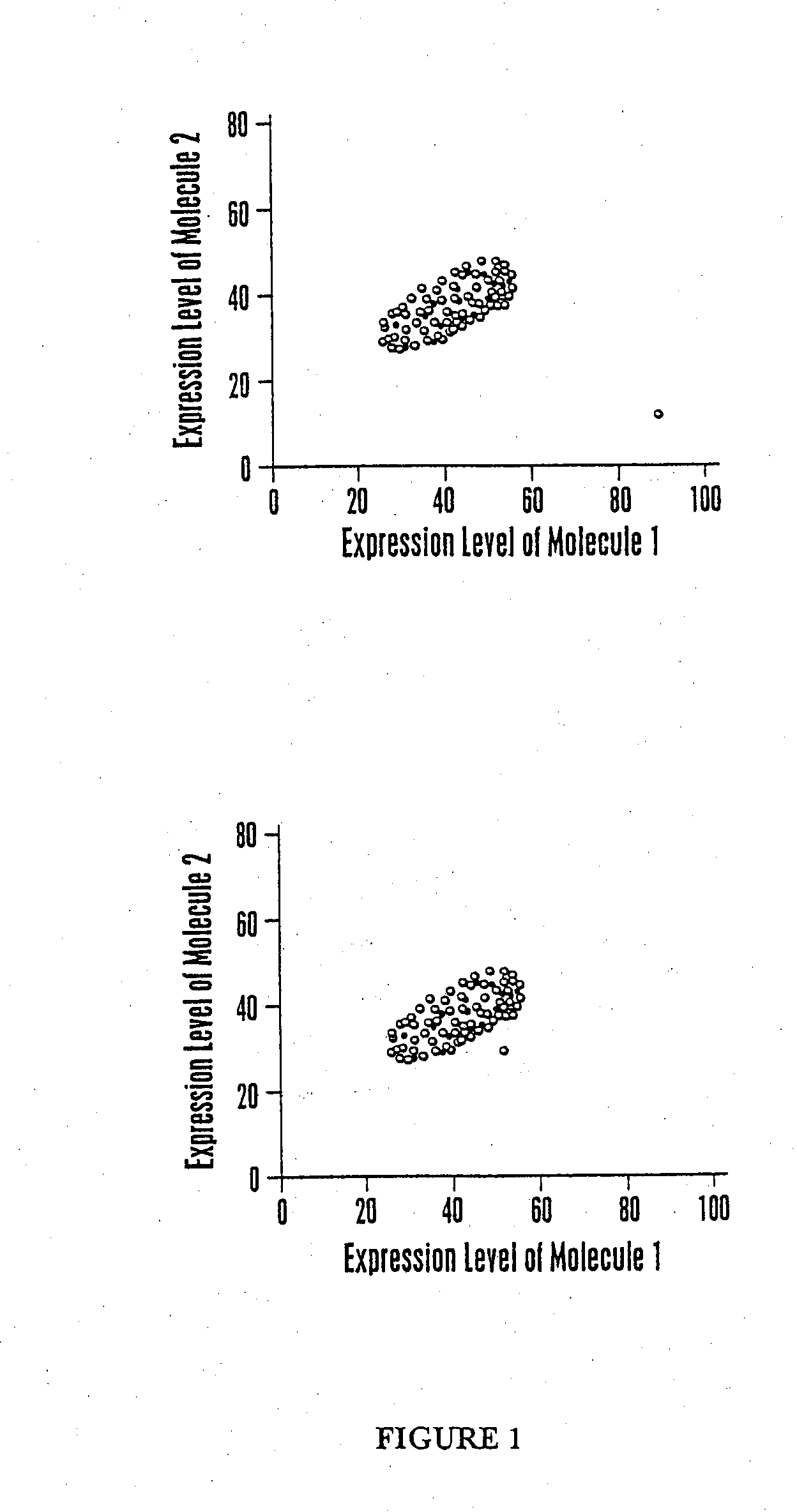
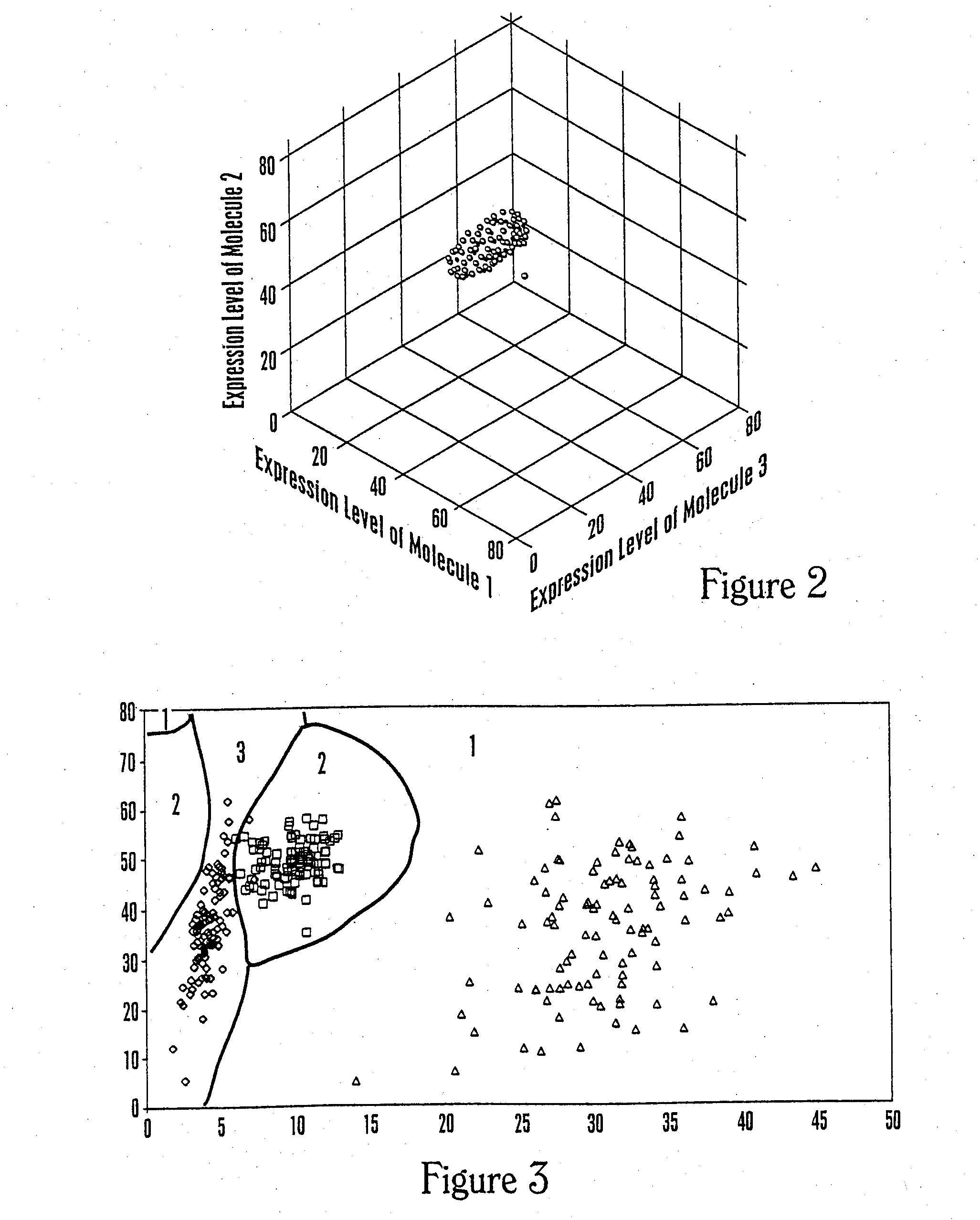
The invention provides a method of determining a comparative expression profile in an individual by comparing the expression levels of a sample of molecules in a population of molecules in a specimen from the individual with a health-associated reference expression region of the sample of molecules, wherein expression levels within the health-associated reference expression region indicate a reference expression profile and wherein expression levels outside the health-associated reference expression region indicate a perturbed expression profile. The invention also provides methods of diagnosing a disease or a health state in an individual by comparing the expression level of a sample of molecules in a specimen from the individual with a health-associated reference expression region of the sample of molecules. The invention additionally provides a method of classifying a population by drug responsiveness.
Owner:INSTITUTE FOR SYSTEMS BIOLOGY
Methods for predicting drug responsiveness in cancer patients
ActiveUS20200340067A1Organic active ingredientsMicrobiological testing/measurementPharmaceutical drugOncology
The present invention features methods, devices, and kits for detecting expression in a patient having cancer or determining responsive of a patient having cancer to a treatment, such as irofulven. The invention further includes methods of treating a patient having cancer by administering, e.g., irofulven.
Owner:ALLARITY THERAPEUTICS EURO APS
Methods for treating cancer and predicting drug responsiveness in cancer patients
InactiveUS20190231795A1Reduce the overall heightPromote cell growthOrganic active ingredientsHeavy metal active ingredientsCisplatinOncology
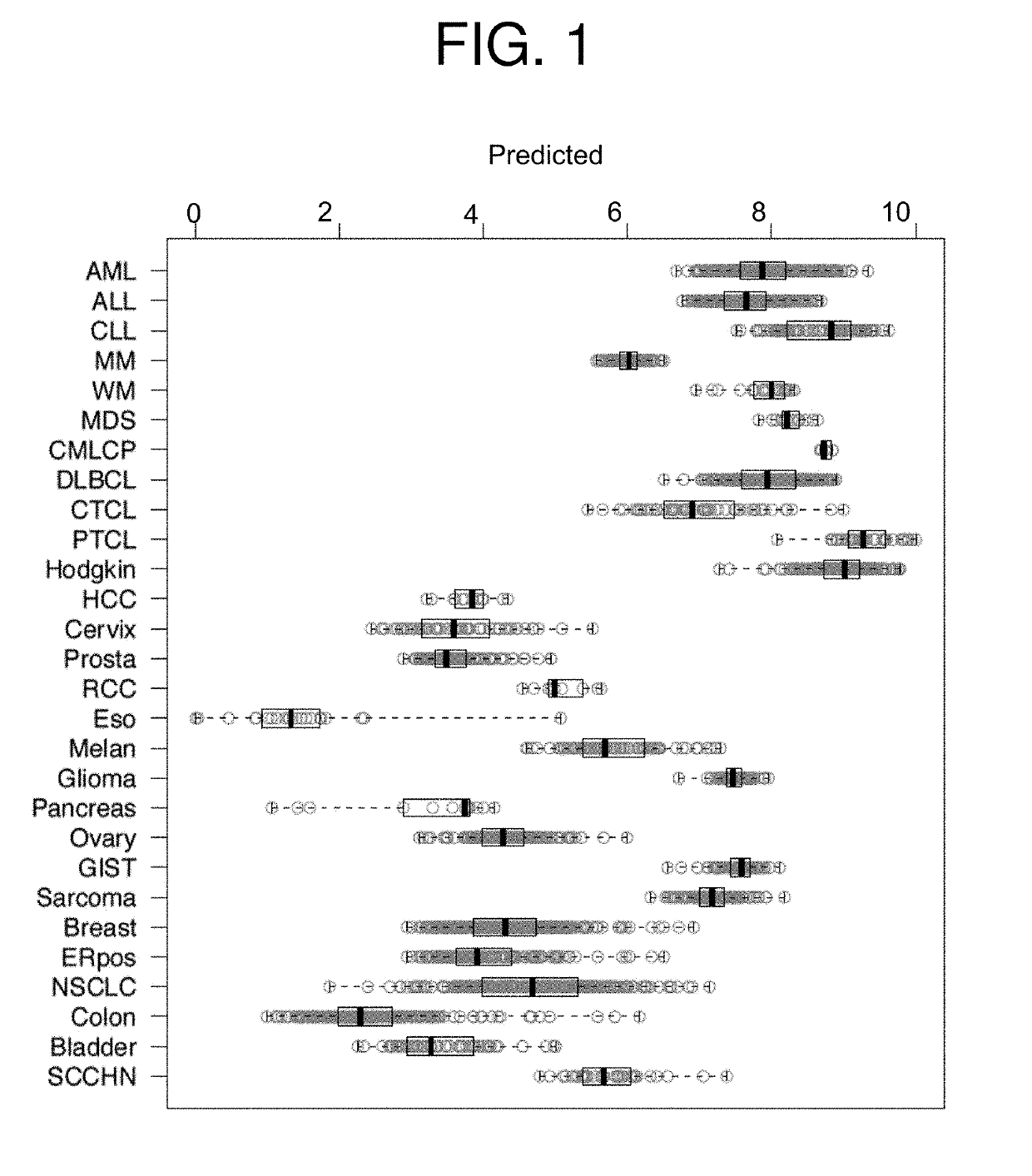
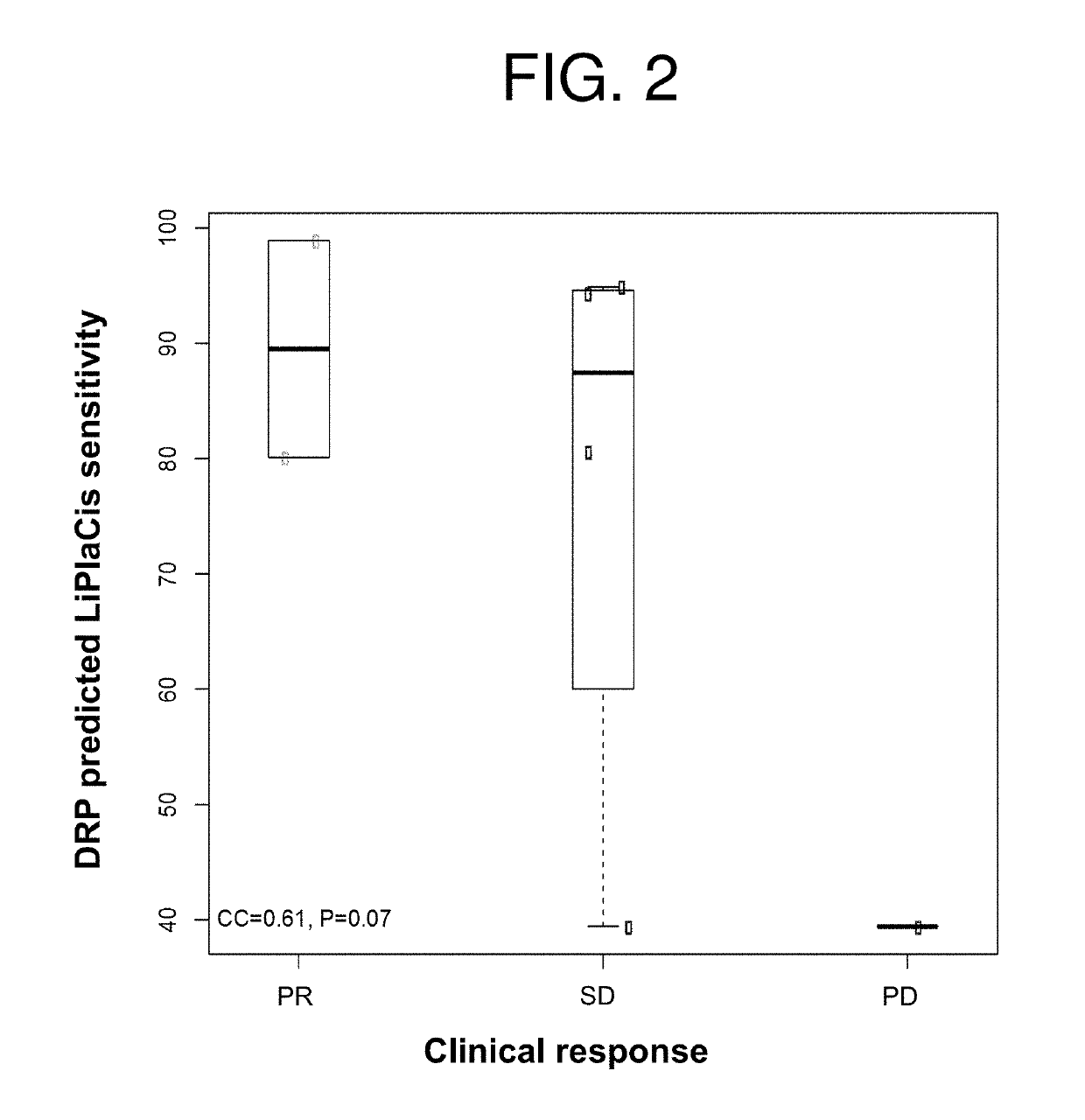
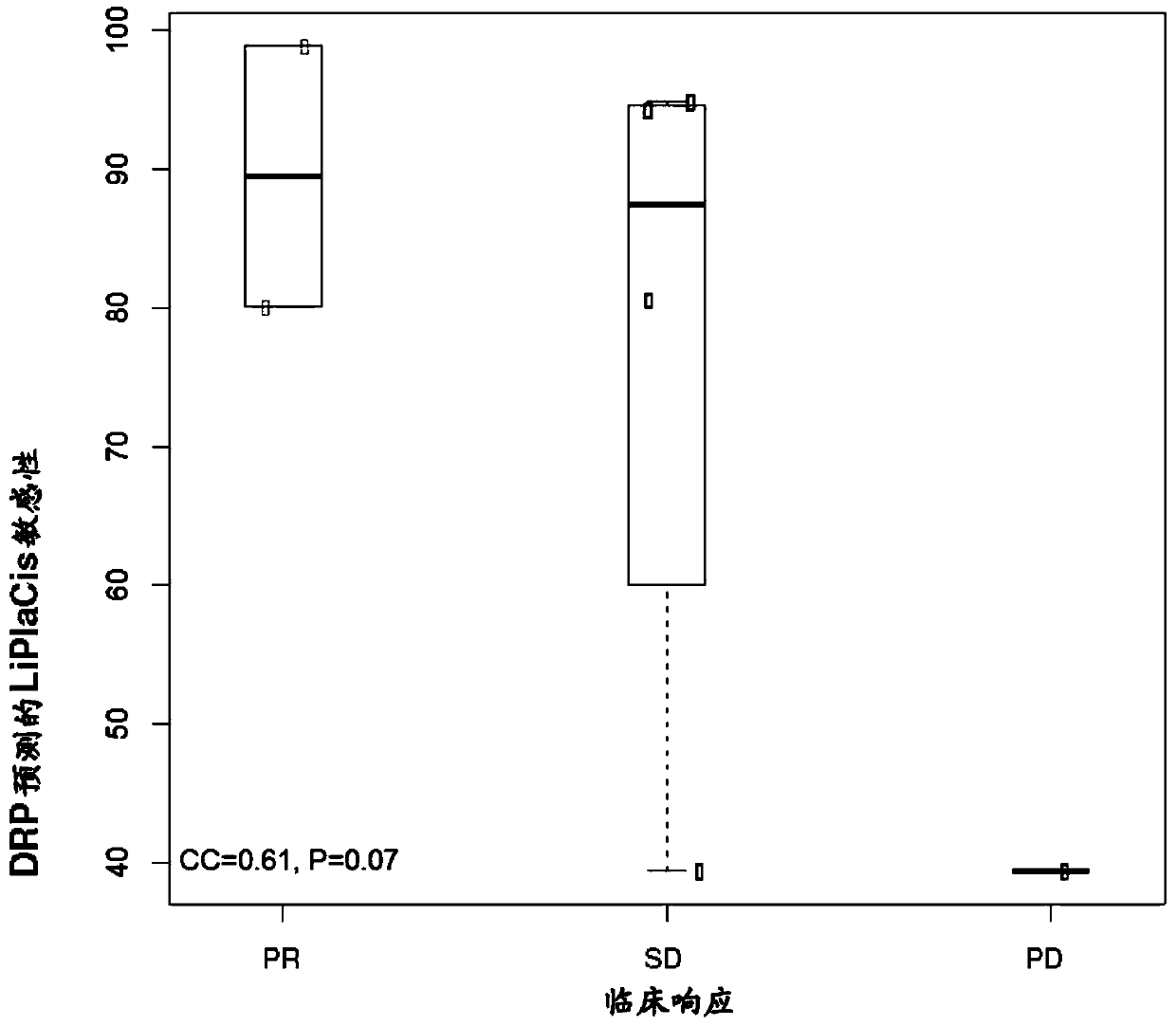
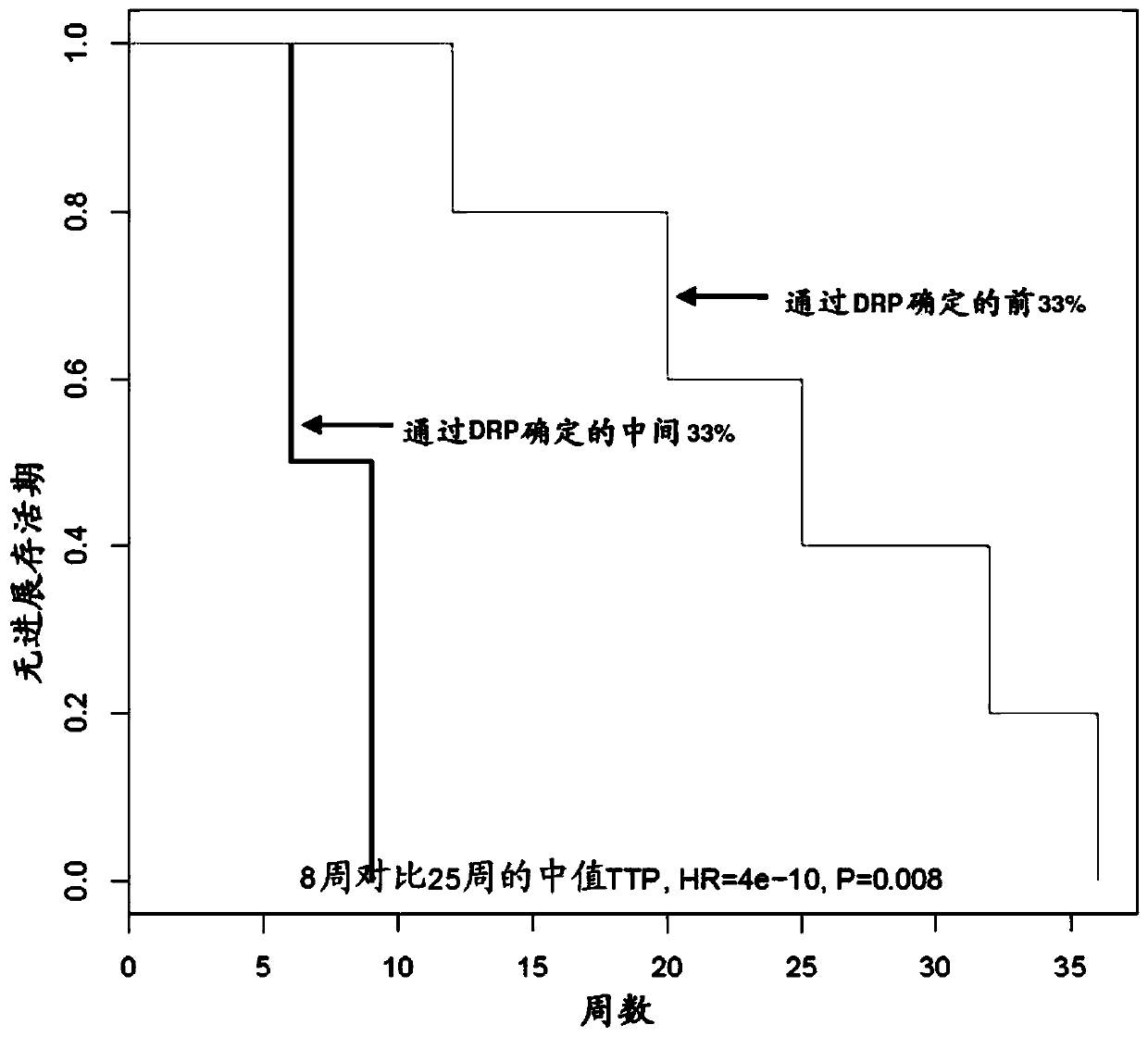
Featured are methods of treating a patient with cancer by administering, e.g., a secretory phospholipase A2 (sPLA2) hydrolysable, cisplatin-containing liposome composition (e.g., LiPlaCis). The patient may be assessed for their responsiveness to the liposomal therapy prior to treatment using the methods, devices, and kits also described herein for detecting a level of one or more biomarkers in a sample from the patient with cancer.
Owner:LIPLASOME PHARMA AS
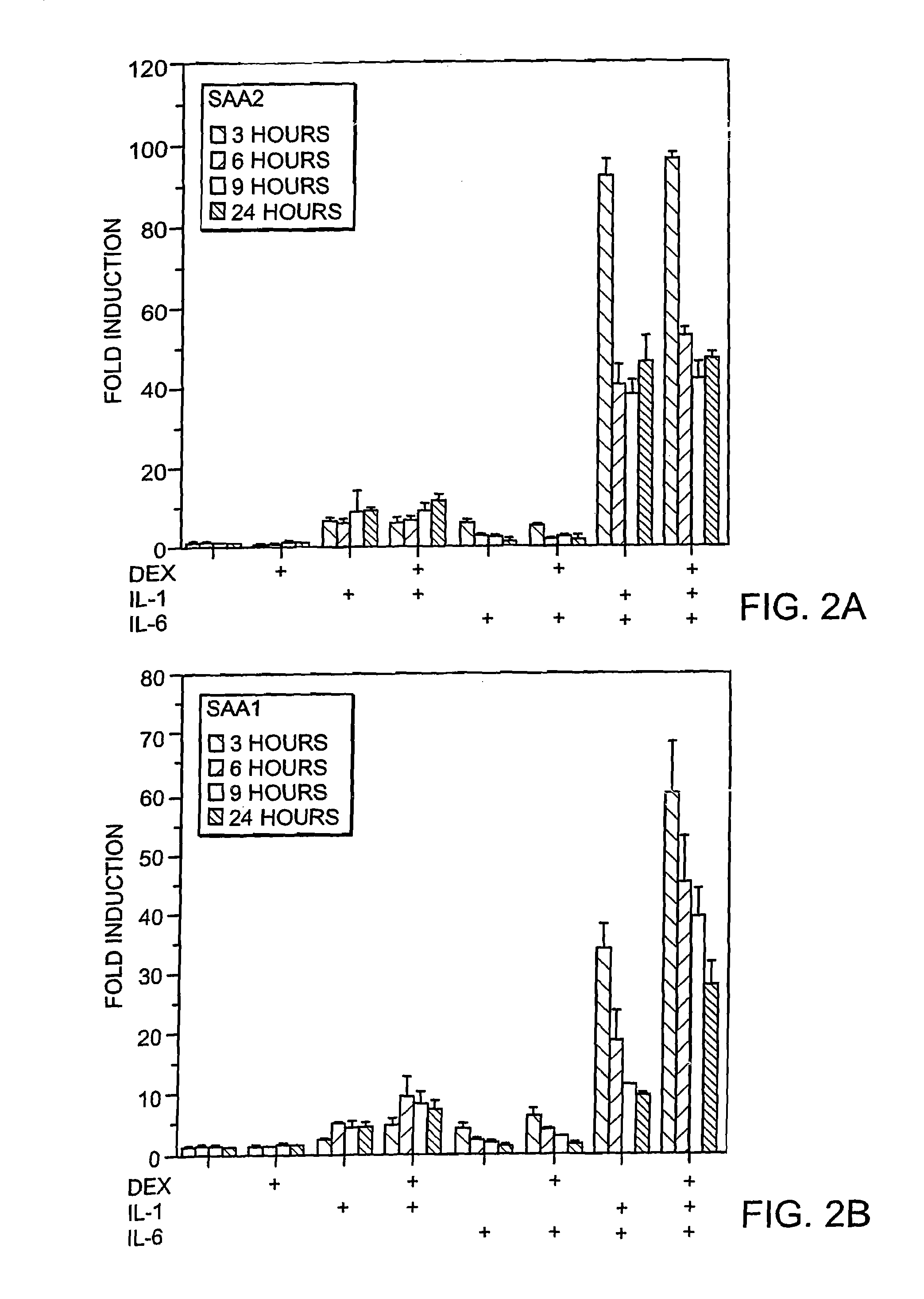
Methods for determining drug responsiveness
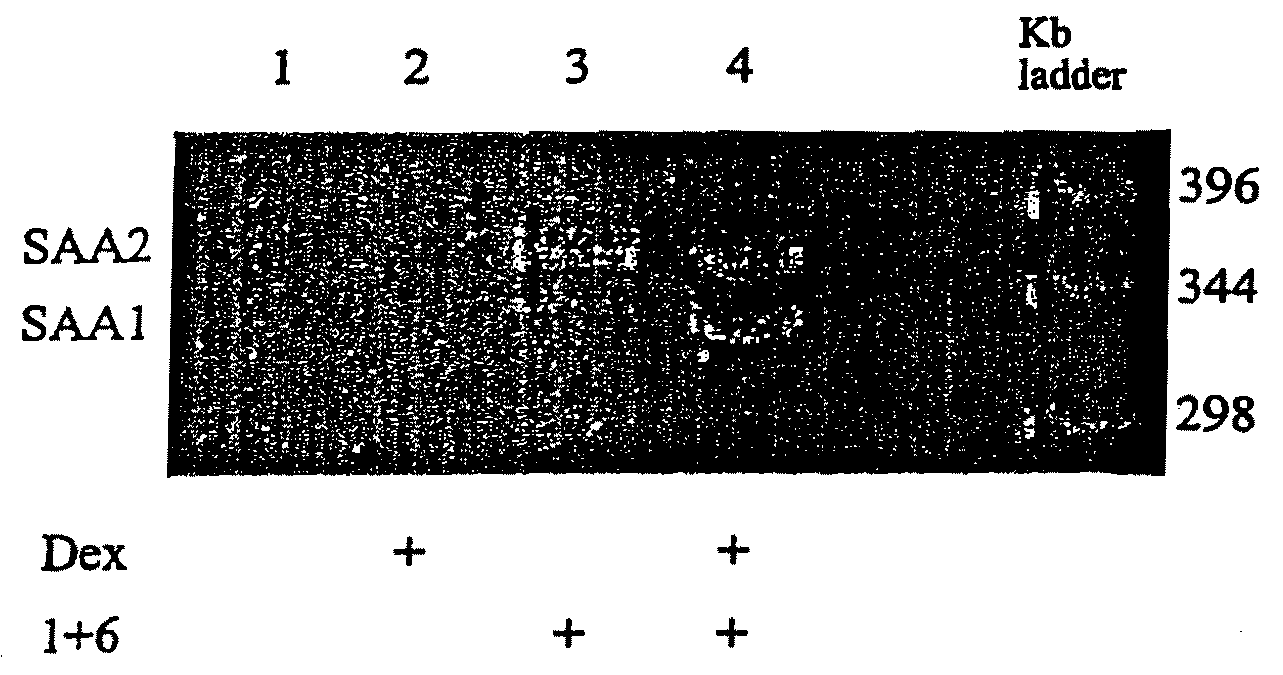
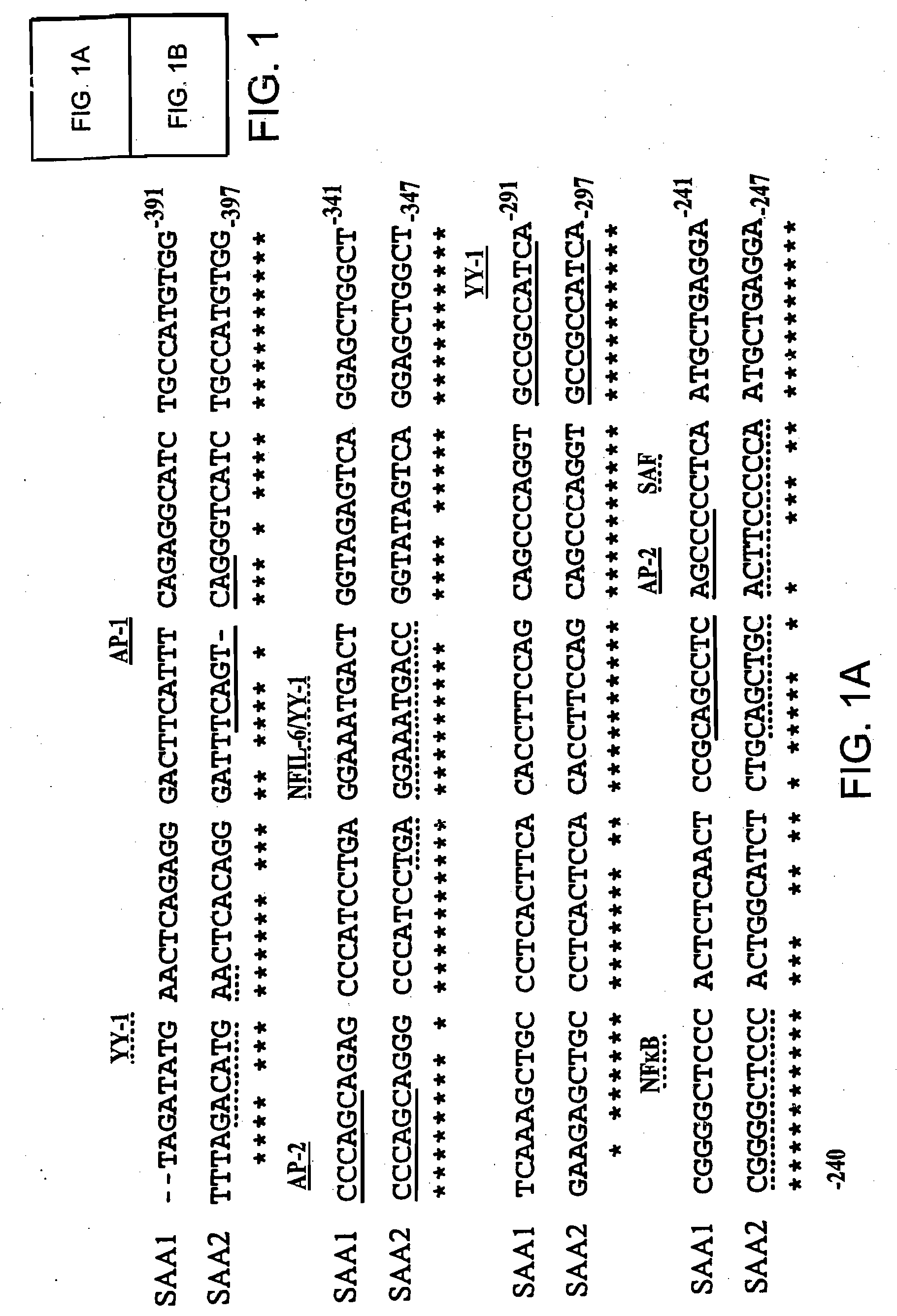
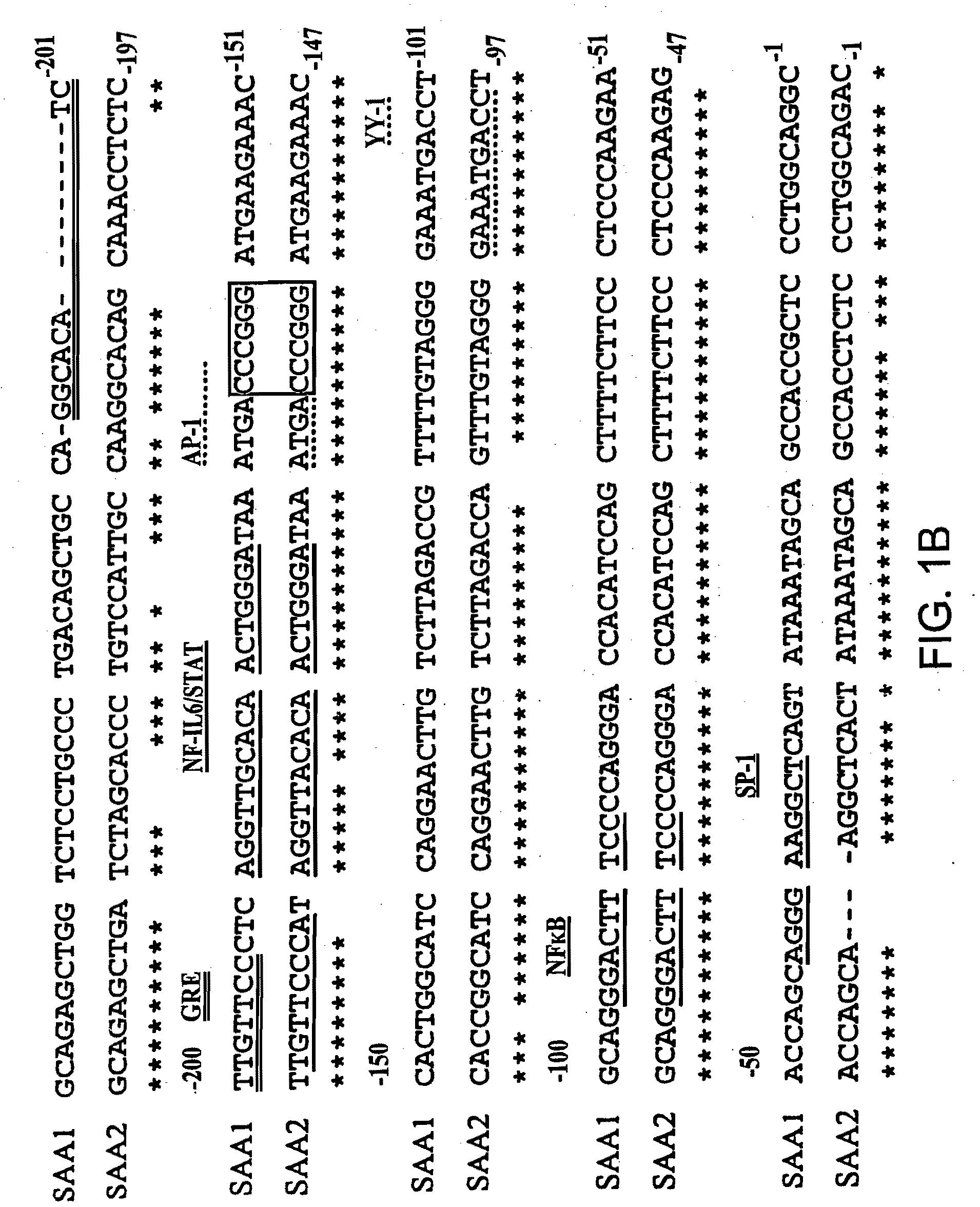
InactiveUS7745138B2Minimize RNA and protein degradationEvaluate steroid responsivenessMicrobiological testing/measurementDisease diagnosisPharmaceutical drugPharmacology
The invention provides a diagnostics assay for measuring the responsiveness to a drug by comparing the mRNA levels of a gene that responds to the drug, such as a steroid, to the mRNA levels of a gene that does not respond to the drug. Methods according to the invention are useful for predicting the ability of a patient (or a tissue, body fluid or cell sample in vitro) to respond to a drug or steroid at any stage of their treatment (i.e., before, during or after), and to monitor the patient (or a tissue, body fluid or cell) over time to assess continued responsiveness to the drug or steroid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods for treating cancer and predicting drug responsiveness in cancer patients
Featured are methods of treating a patient with cancer by administering, e.g., a secretory phospholipase A2 (sPLA2) hydrolysable, cisplatin-containing liposome composition (e.g., LiPlaCis). The patient may be assessed for their responsiveness to the liposomal therapy prior to treatment using the 0 methods, devices, and kits also described herein for detecting a level of one or more biomarkers in asample from the patient with cancer.
Owner:LIPLASOME PHARMA AS
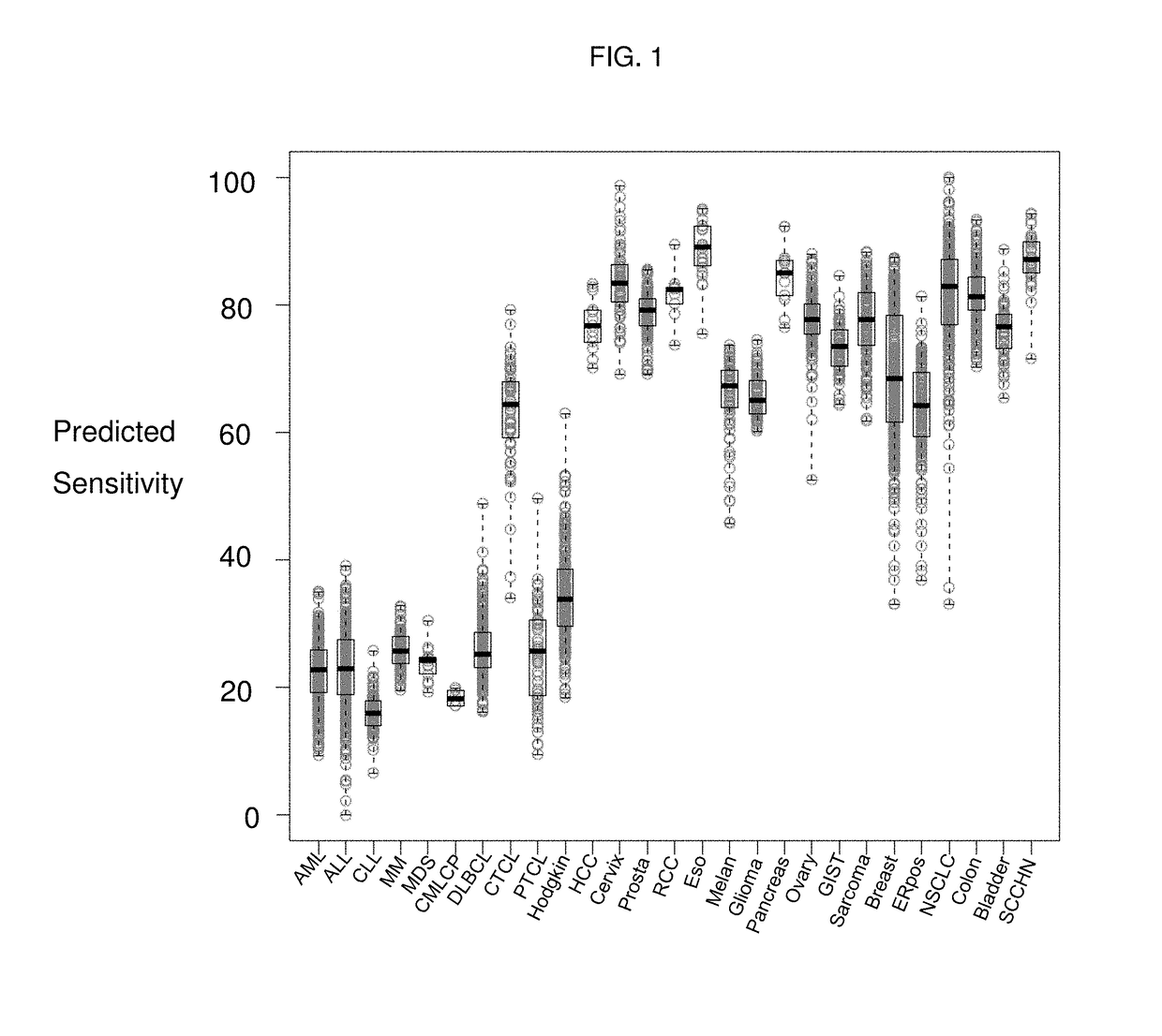
Drug sensitivity prediction method, electronic equipment and computer readable storage medium
PendingCN112951327AEnabling Drug Response PredictionLow costDrug and medicationsProteomicsCancer cellEfficacy
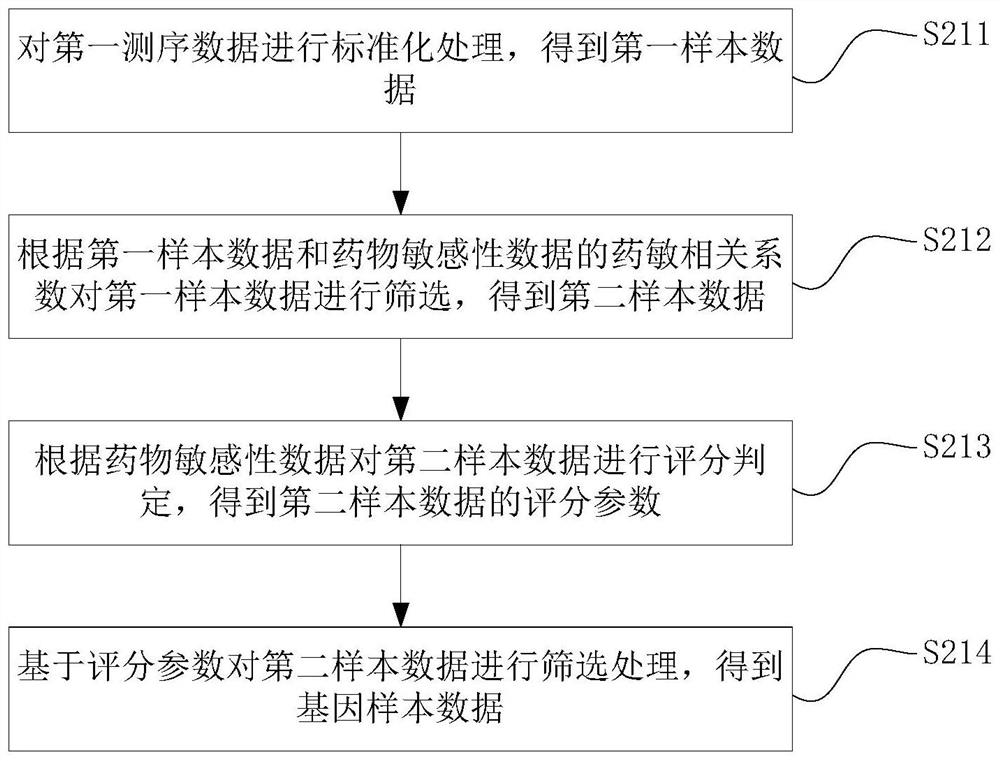
The invention discloses a drug sensitivity prediction method, electronic equipment and a computer readable storage medium, and relates to the technical field of drug detection. The method comprises the steps: acquiring gene sequencing data and drug feature data of cancer cell tissue to be trained, pre-processing the gene sequencing data according to the drug feature data to obtain gene sample data, performing verification processing according to the gene sample data and the drug characteristic data to obtain a prediction model and a gene prediction list, and performing drug sensitivity prediction on the cancer cell tissues to be detected through the gene prediction list and the prediction model. Therefore, drug reactivity prediction on clinical patients can be quickly and accurately realized, the prediction cost and the time cost are reduced, the prediction efficiency is improved. and the efficacy prediction efficiency is improved.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Methods for predicting drug responsiveness in cancer patients
PendingCN112292463AOrganic active ingredientsMicrobiological testing/measurementPharmaceutical drugBiologic marker
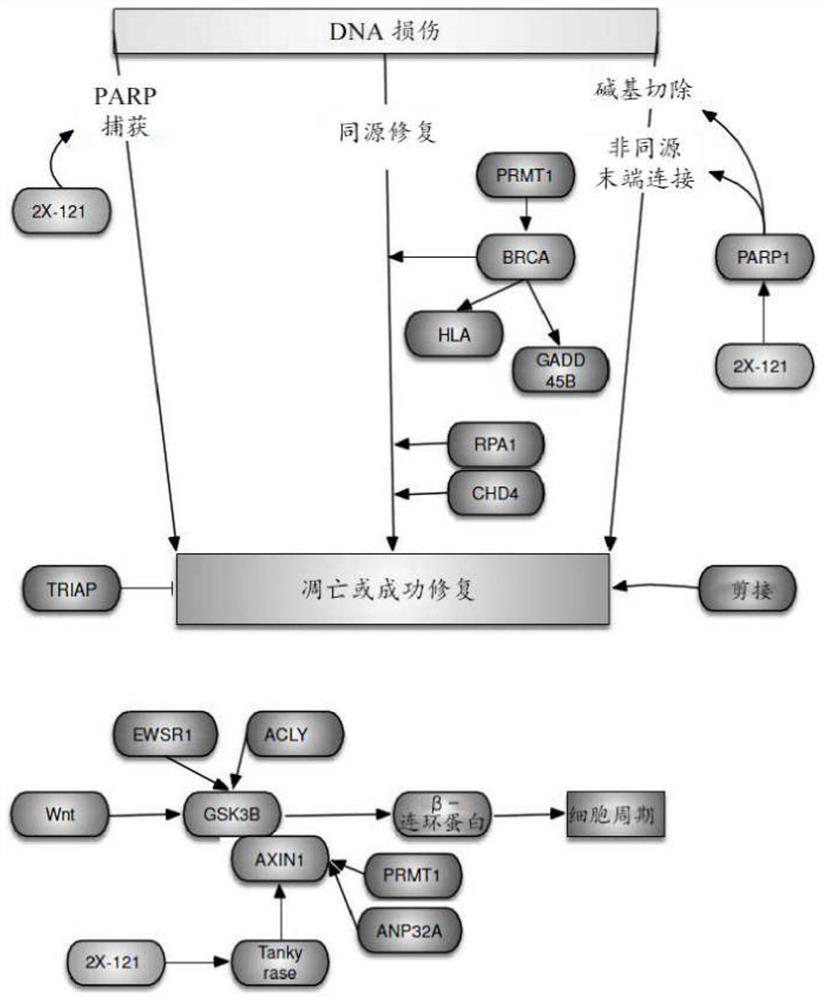
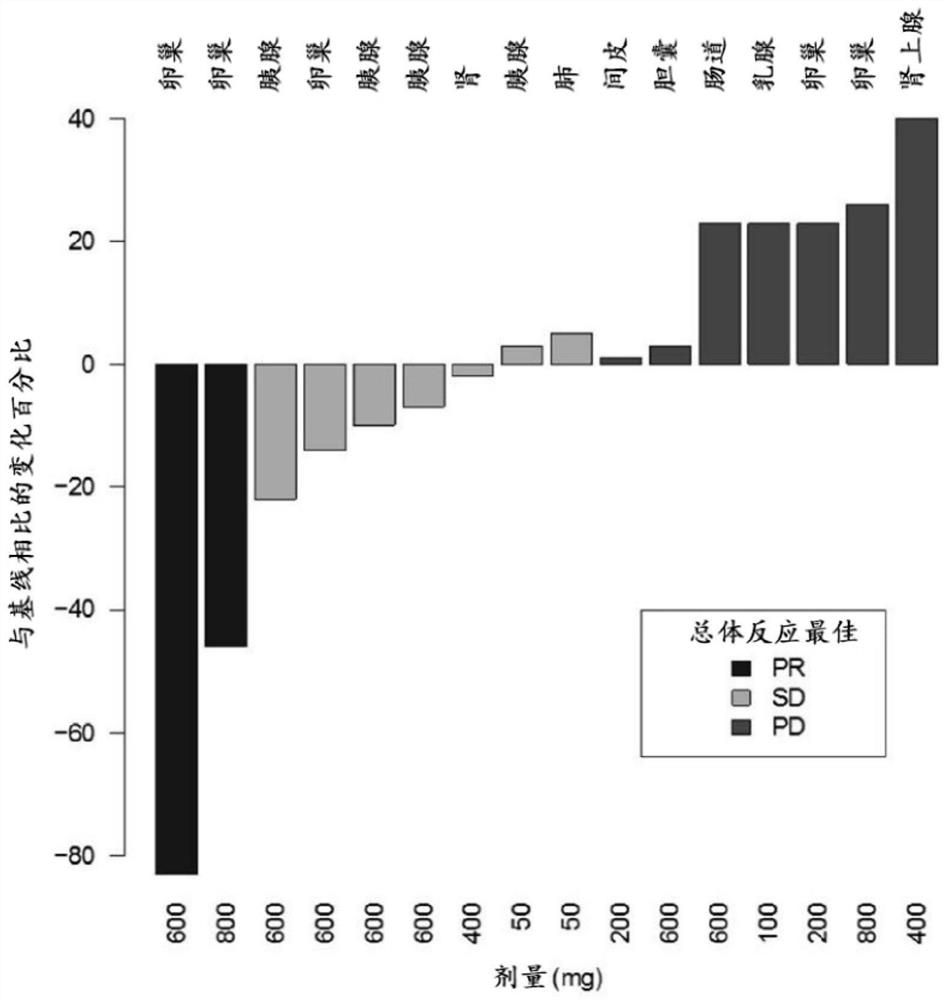
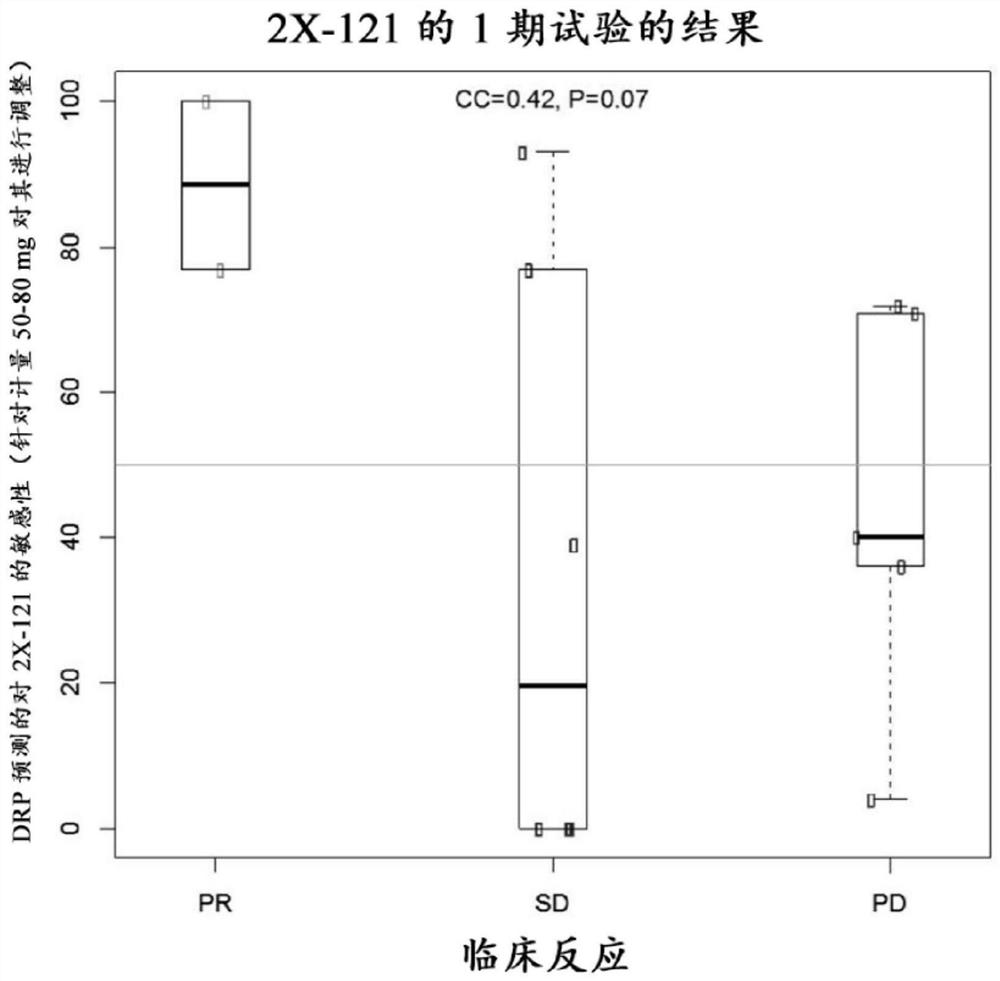
The present invention features methods, devices, and kits for detecting gene expression in a patient with a cancer or determining responsive of a patient with a cancer to a treatment, such as treatment with 2X-121 or a pharmaceutically acceptable salt thereof. The invention further includes methods of treating a patient with a cancer by administering a treatment, e.g., treatment with 2X-121 or a pharmaceutically acceptable salt thereof, in particular when the patient is determined to be responsive to the treatment based on the expression of the biomarkers described herein.
Owner:亚拉勒提治疗欧洲私人有限公司
Drug reactive metabolite mass spectrum detection probe and application thereof
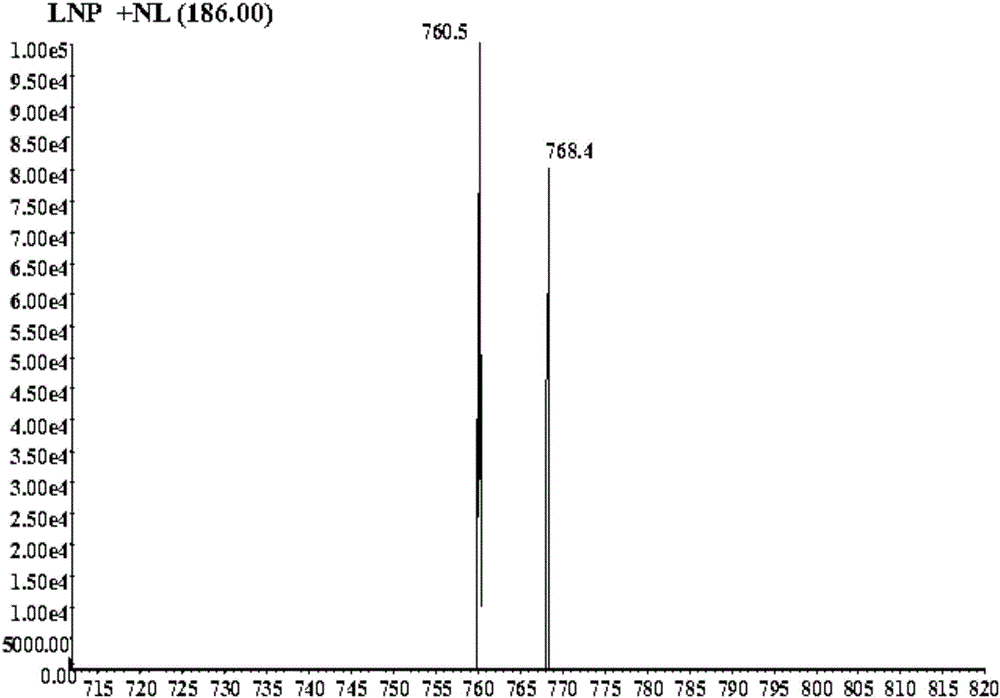
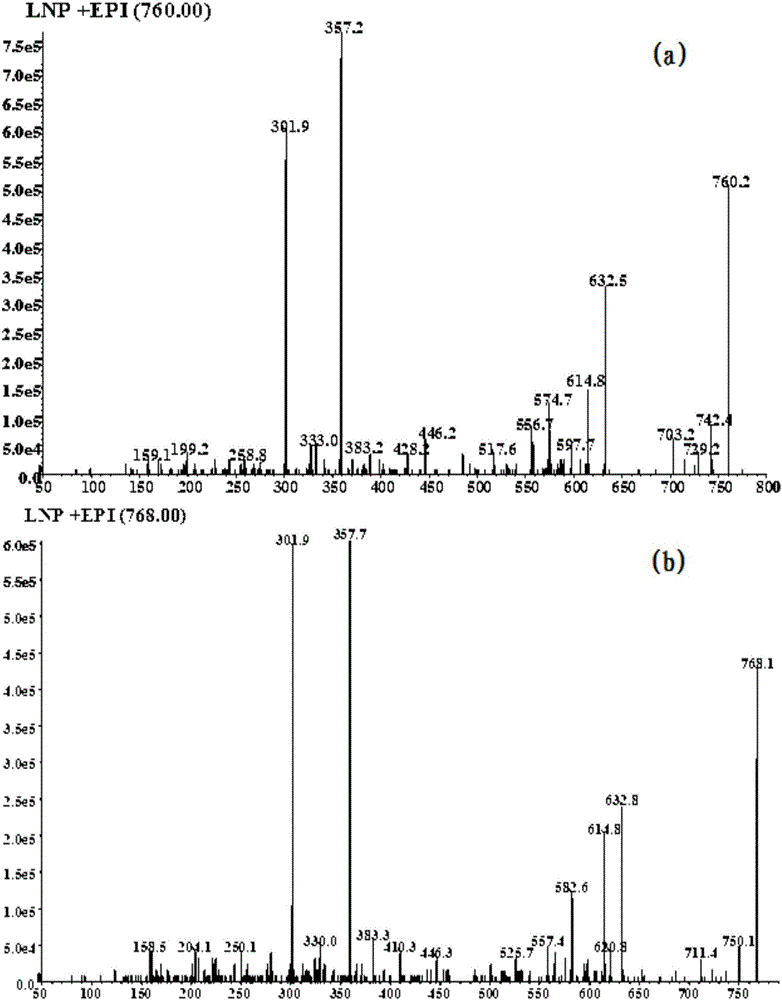
The invention discloses a drug reactive metabolite mass spectrum detection probe and application thereof. The drug reactive metabolite mass spectrum detection probe is oligopeptide composed of 3-6 common amino acids. The oligopeptide comprises at least one cysteine and at least one lysine. Experimental research shows that the oligopeptide formed by the amino acids is used as the drug reactive metabolite mass spectrum detection probe; the drug reactive metabolite mass spectrum detection probe is capable of capturing hard drug reactive metabolite molecules and is also capable of capturing soft drug reactive metabolite molecules, so that the capturing range of the probe is expanded, and then the comprehensive information of drug reactive metabolite products can be obtained; the probe is particularly applied to structural identification of the drug reactive metabolite molecules, high-throughput screening of drug metabolism in the earlier period of research and development of new drugs, latent evaluation of toxic and side effects of the drugs and research of the occurrence mechanism of toxic and side effects of the drugs.
Owner:SHANGHAI UNTANGLED BIOSCIENCES CO LTD +2
Methods for determining drug responsiveness
InactiveUS7666608B2Minimize RNA and protein degradationEvaluate steroid responsivenessSugar derivativesMicrobiological testing/measurementProtein levelBody fluid
The invention provides a diagnostics assay for measuring the responsiveness to a drug by comparing the protein levels of a gene that responds to the drug, such as a steroid, to the protein levels of a gene that does not respond to the drug. Methods according to the invention are useful for predicting the ability of a patient (or a tissue, body fluid or cell sample in vitro) to respond to a drug or steroid at any stage of their treatment (i.e., before, during or after), and to monitor the patient (or a tissue, body fluid or cell) over time to assess continued responsiveness to the drug or steroid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods for predicting drug responsiveness in cancer patients
ActiveUS20180334724A1Reduction in tumor massLesser cell growthOrganic active ingredientsHealth-index calculationPharmaceutical drugBiologic marker
The present invention features methods, devices, and kits for detecting a level of one or more biomarkers in a patient with cancer or determining the responsiveness of a patient with cancer to a treatment, such as treatment with an anthracycline. The invention further includes methods of treating a patient with cancer by administering, e.g., the anthracycline.
Owner:ALLARITY THERAPEUTICS EURO APS
Methods for Determining Drug Responsiveness
InactiveUS20090286267A1Minimize degradationMinimize RNA and protein degradationSugar derivativesMicrobiological testing/measurementBody fluidProtein level
The invention provides a diagnostics assay for measuring the responsiveness to a drug by comparing the protein levels of a gene that responds to the drug, such as a steroid, to the protein levels of a gene that does not respond to the drug. Methods according to the invention are useful for predicting the ability of a patient (or a tissue, body fluid or cell sample in vitro) to respond to a drug or steroid at any stage of their treatment (i.e., before, during or after), and to monitor the patient (or a tissue, body fluid or cell) over time to assess continued responsiveness to the drug or steroid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
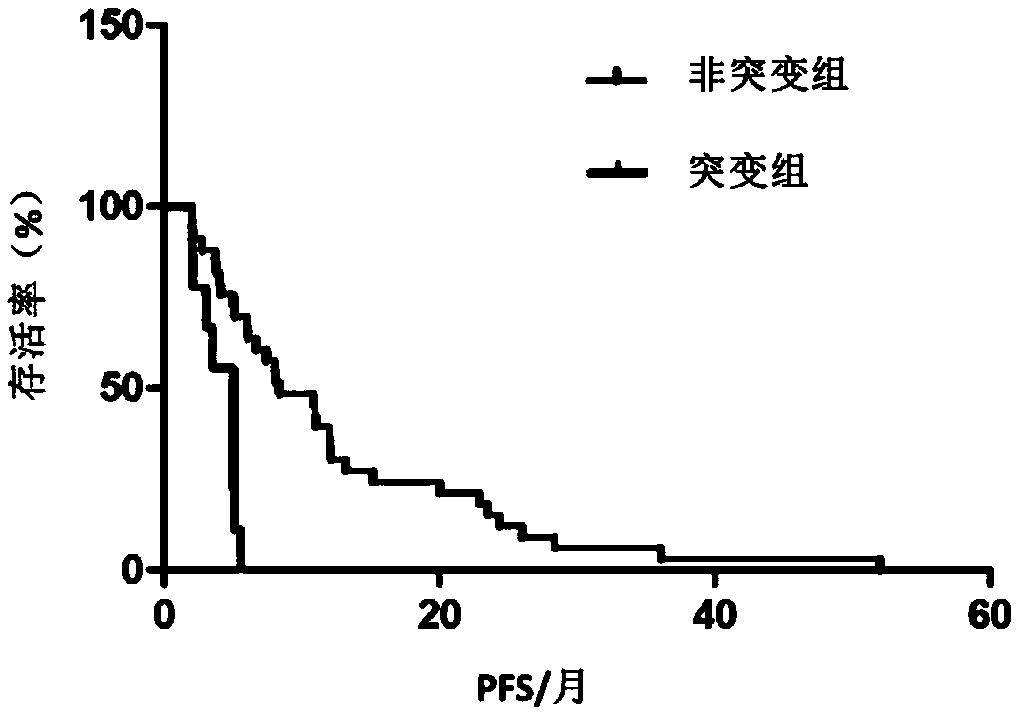
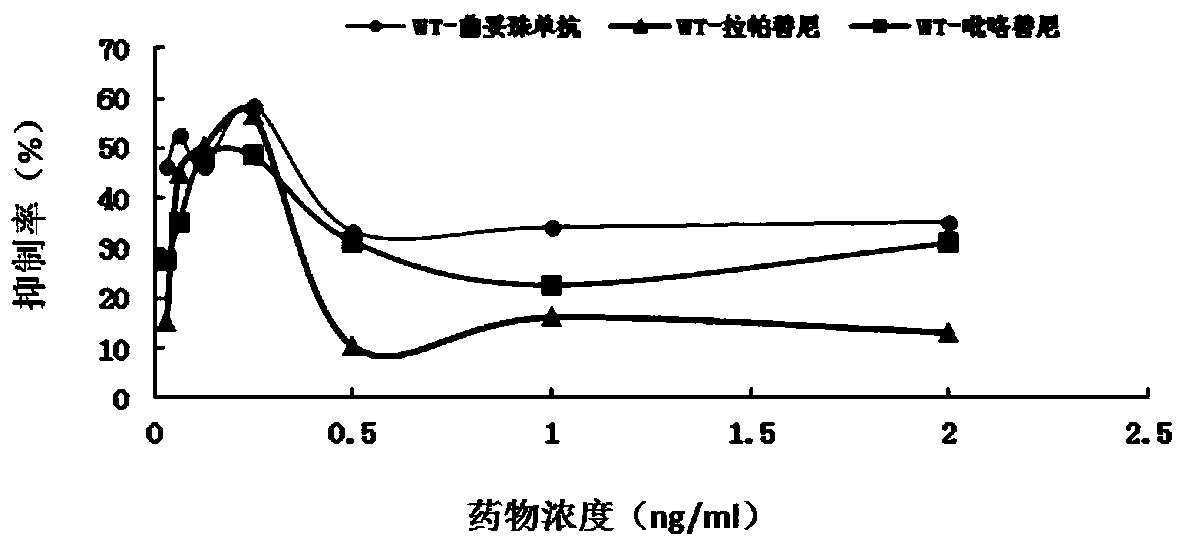
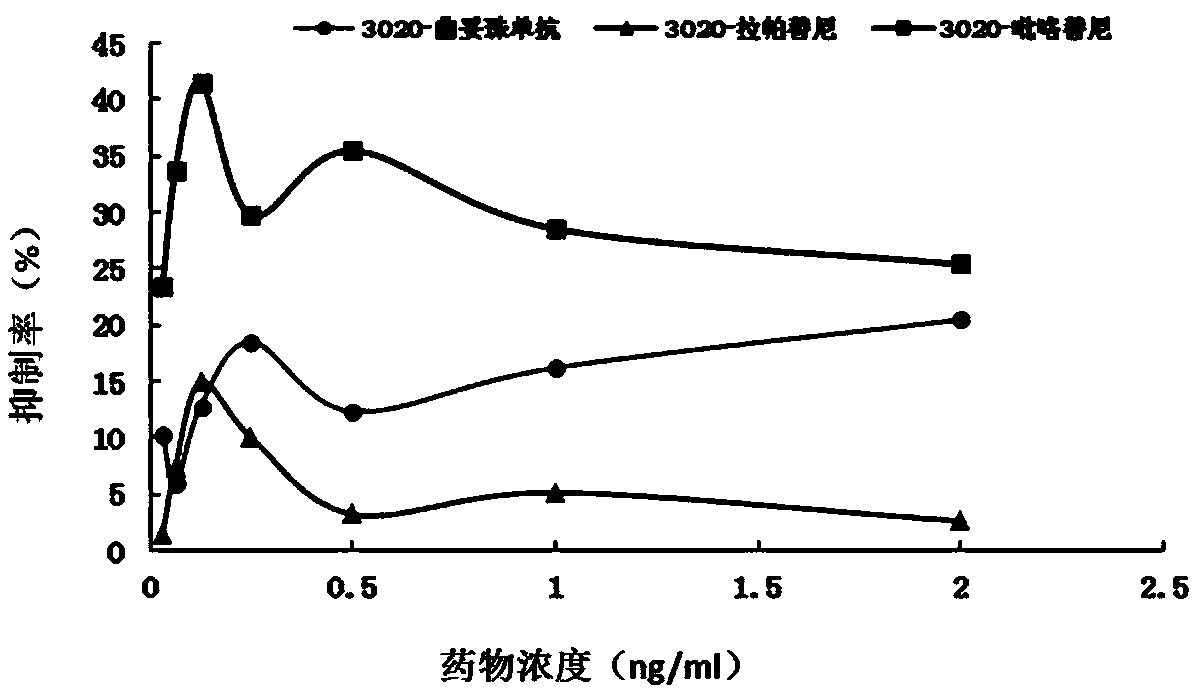
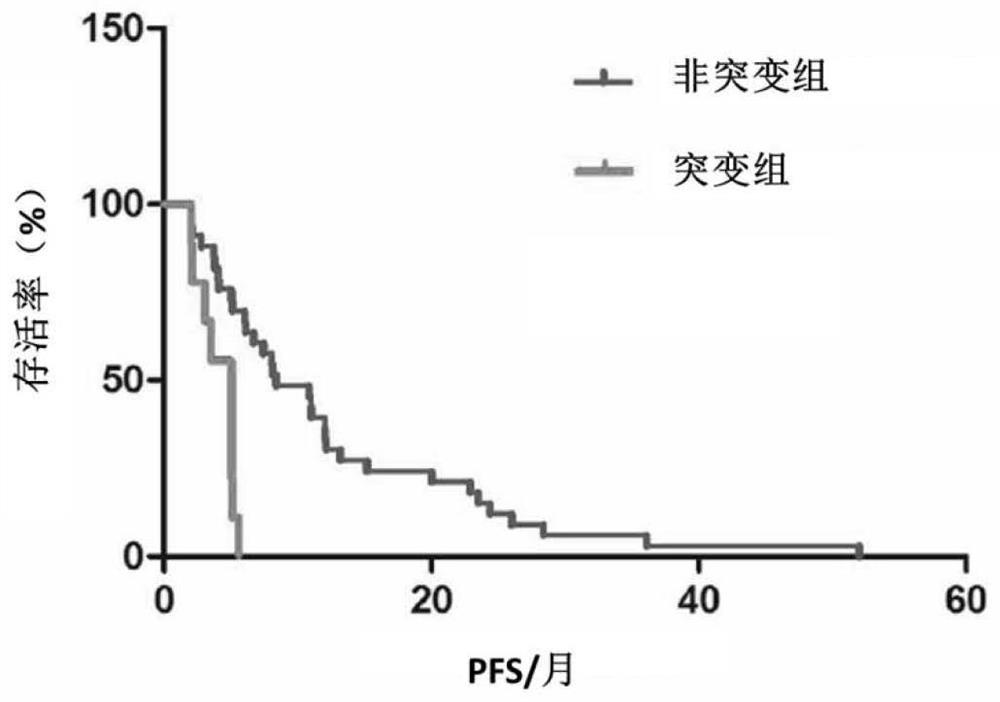
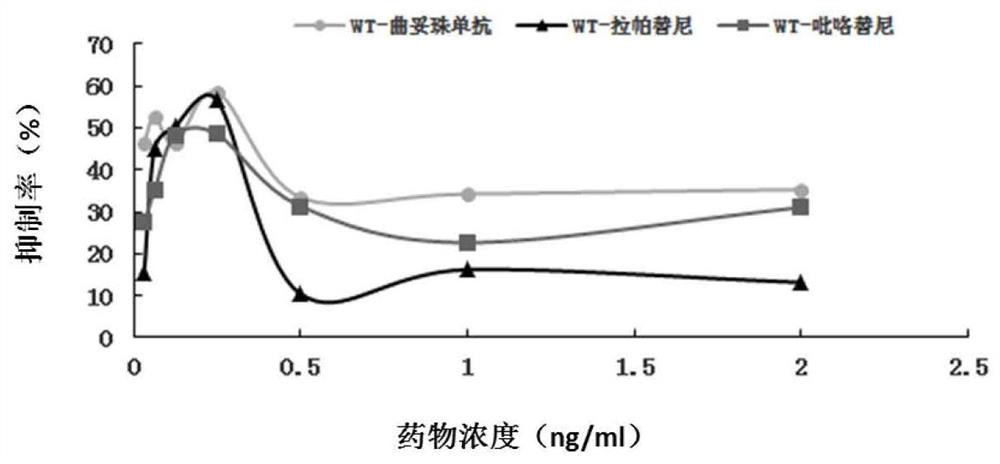
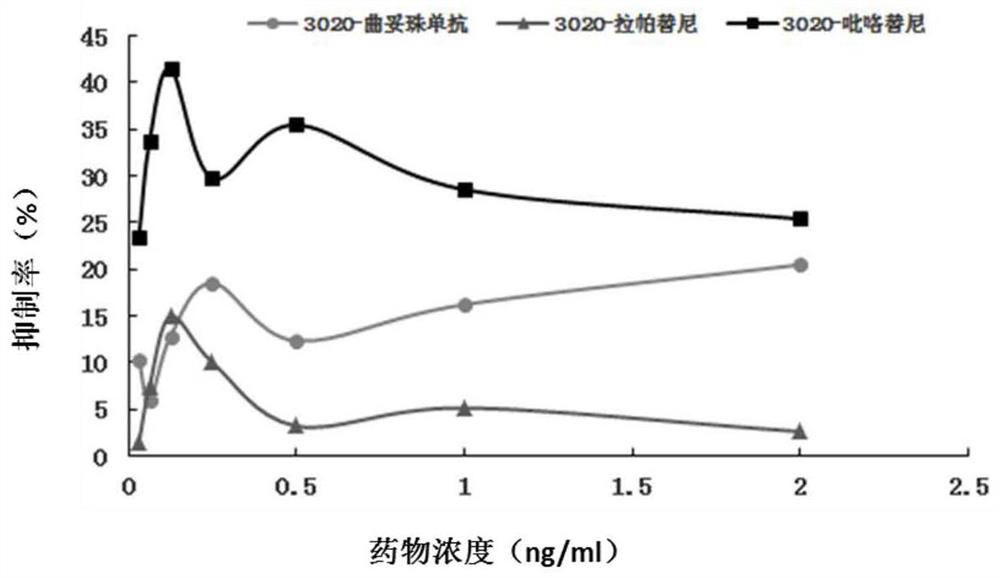
Gene mutation site for predicting anti-HER2 therapeutic drug reactivity of patients with breast cancer and application thereof
ActiveCN108949998ALearn about drug resistanceEffective guidanceMicrobiological testing/measurementFermentationMedicineAnti her2
The invention discloses a gene mutation site for predicting the anti-HER2 therapeutic drug reactivity of patients with breast cancer and application thereof. By a large scale of screening, the inventor discovers for the first time that the 3020 site of the HER2 gene coding sequence is related to the drug reactivity of patients with breast cancer on trastuzumab, lapatinib or pyrotinib. Based on theresearch result, a product for predicting the drug reactivity of a patient with breast cancer on trastuzumab, lapatinib or pyrotinib is developed. The product can be widely applied to clinical application by detecting the function of the 3020 site genome of the HER2 gene coding sequence.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Genetic mutation sites for predicting drug responsiveness to anti-her2 therapy in breast cancer patients and its application
ActiveCN108949998BLearn about drug resistanceEffective guidanceMicrobiological testing/measurementGenetic engineeringTherapy resistantPharmaceutical drug
The invention discloses a gene mutation site for predicting the responsiveness of breast cancer patients to anti-HER2 therapeutic drugs and the application thereof. Specifically, after large-scale screening by the inventors, it was found for the first time that the genotype at position 3020 of the HER2 gene coding sequence is associated with drug responsiveness to trastuzumab, lapatinib or pyrotinib in breast cancer patients. Based on the above research results, the present invention has developed a product that can be used to predict the drug responsiveness of breast cancer patients to trastuzumab, lapatinib or pyrotinib. The above products function by detecting the genotype at the 3020th position of the HER2 gene coding sequence, and can be widely used clinically.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Methods for predicting drug responsiveness in cancer patients
ActiveUS10900089B2Reduce spreadInduces necrosis in a cellOrganic active ingredientsHealth-index calculationPharmaceutical drugBiologic marker
The present invention features methods, devices, and kits for detecting a level of one or more biomarkers in a patient with cancer or determining the responsiveness of a patient with cancer to a treatment, such as treatment with an anthracycline. The invention further includes methods of treating a patient with cancer by administering, e.g., the anthracycline.
Owner:ALLARITY THERAPEUTICS EURO APS
Multiparameter analysis for drug response and related methods
InactiveUS20080131905A1Microbiological testing/measurementBiostatisticsDrugs responseDrug responsiveness
The invention provides a method of determining a comparative expression profile in an individual by comparing the expression levels of a sample of molecules in a population of molecules in a specimen from the individual with a health-associated reference expression region of the sample of molecules, wherein expression levels within the health-associated reference expression region indicate a reference expression profile and wherein expression levels outside the health-associated reference expression region indicate a perturbed expression profile. The invention also provides methods of diagnosing a disease or a health state in an individual by comparing the expression level of a sample of molecules in a specimen from the individual with a health-associated reference expression region of the sample of molecules. The invention additionally provides a method of classifying a population by drug responsiveness.
Owner:INSTITUTE FOR SYSTEMS BIOLOGY
Methods for Determining Drug Responsiveness
InactiveUS20090286248A1Minimize degradationMinimize RNA and protein degradationMicrobiological testing/measurementDisease diagnosisBody fluidPhases of clinical research
The invention provides a diagnostics assay for measuring the responsiveness to a drug by comparing the mRNA levels of a gene that responds to the drug, such as a steroid, to the MRNA levels of a gene that does not respond to the drug. Methods according to the invention are useful for predicting the ability of a patient (or a tissue, body fluid or cell sample in vitro) to respond to a drug or steroid at any stage of their treatment (i.e., before, during or after), and to monitor the patient (or a tissue, body fluid or cell) over time to assess continued responsiveness to the drug or steroid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
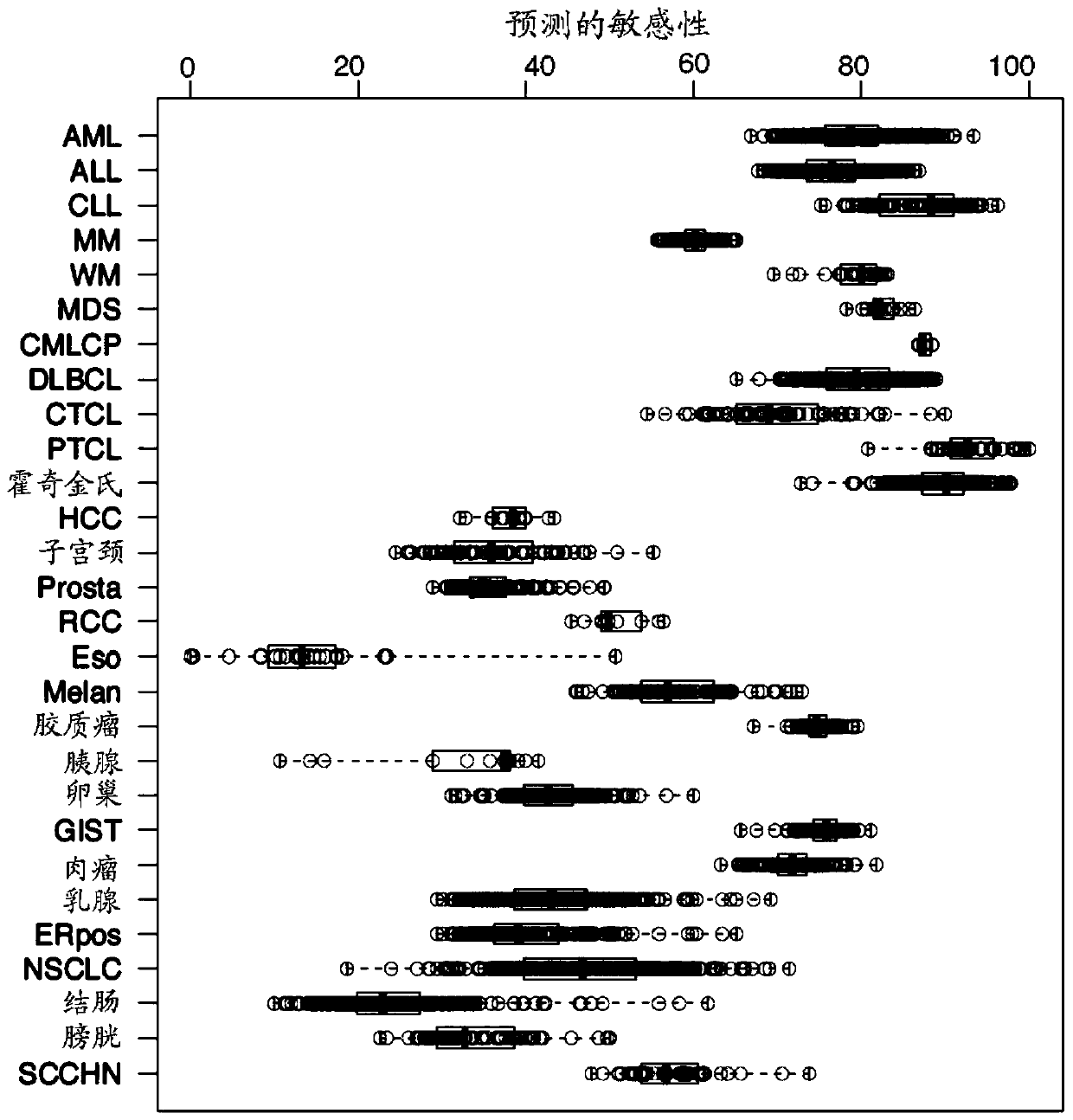
Methods for predicting drug responsiveness in samples from cancer subjects
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Early Diagnosis of Autoimmune and Inflammatory Disorders
The disclosure relates to methods and assay that assists in the diagnosis of autoimmune and chronic inflammatory disorders such as systemic lupus erythematosus and rheumatoid arthritis by analyzing drug-responsiveness of an interferon signal in a hematological sample (e.g., blood) obtained from a human subject. The assay involves comparing the interferon signal in a control aliquot of the sample with the same interferon sample in an aliquot that has been exposed to a therapeutic modality (e.g., combined with a drug) that is known to be efficacious to treat the disorder. A significant difference between the interferon signals of the control and treated aliquots that corresponds to a characteristic interferon signature for the disorder indicates that the subject is afflicted with, or is likely to develop, the disorder.
Owner:P3 INNOVATIONS LLC
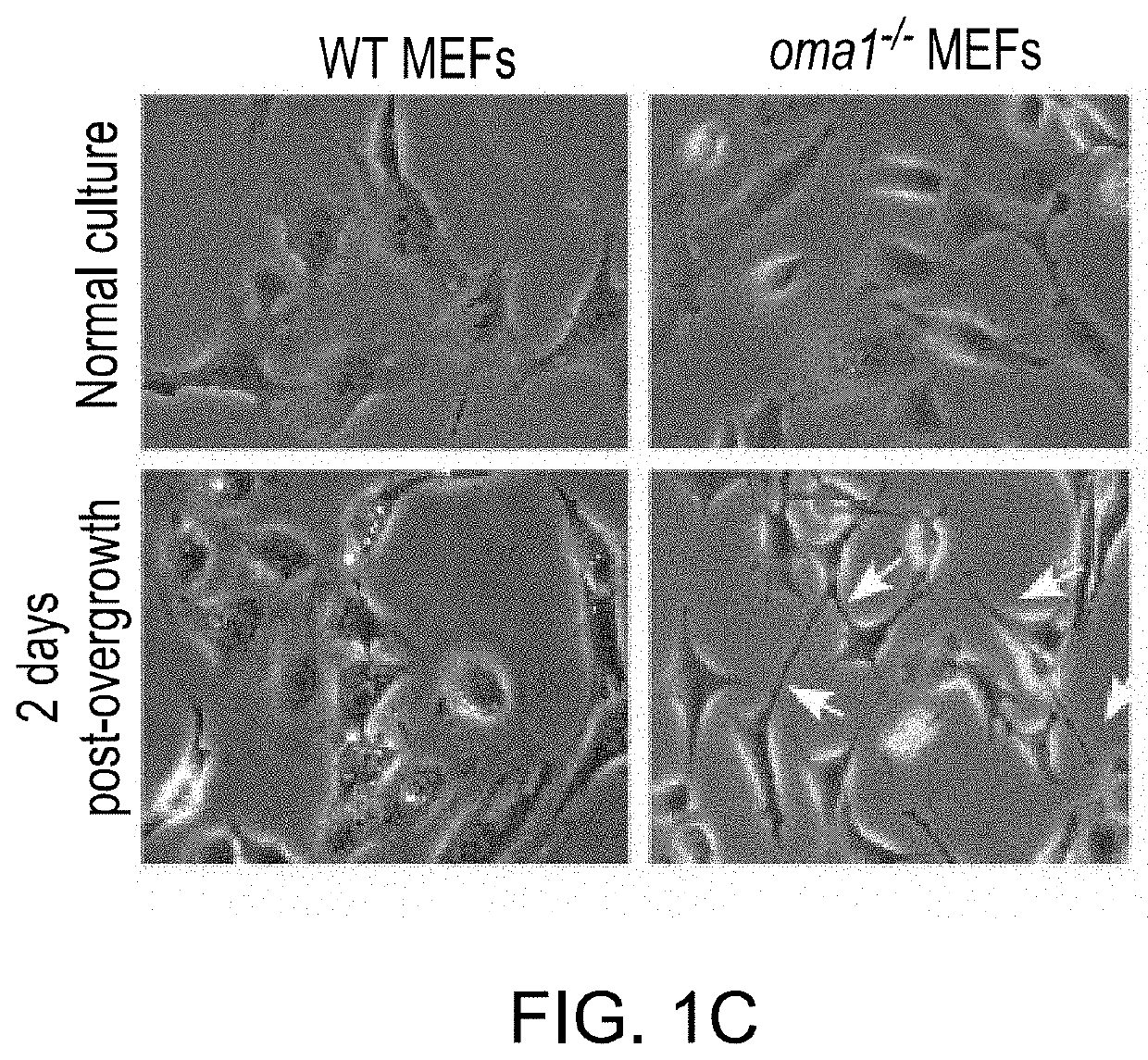
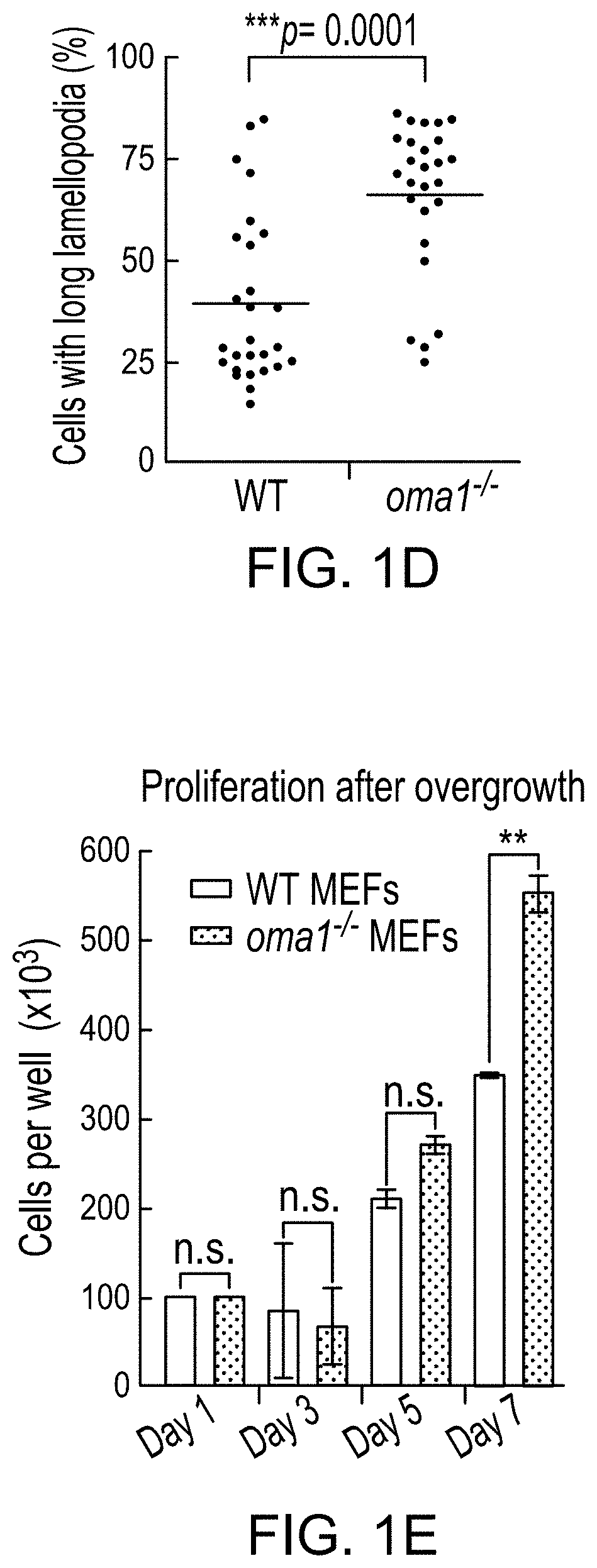
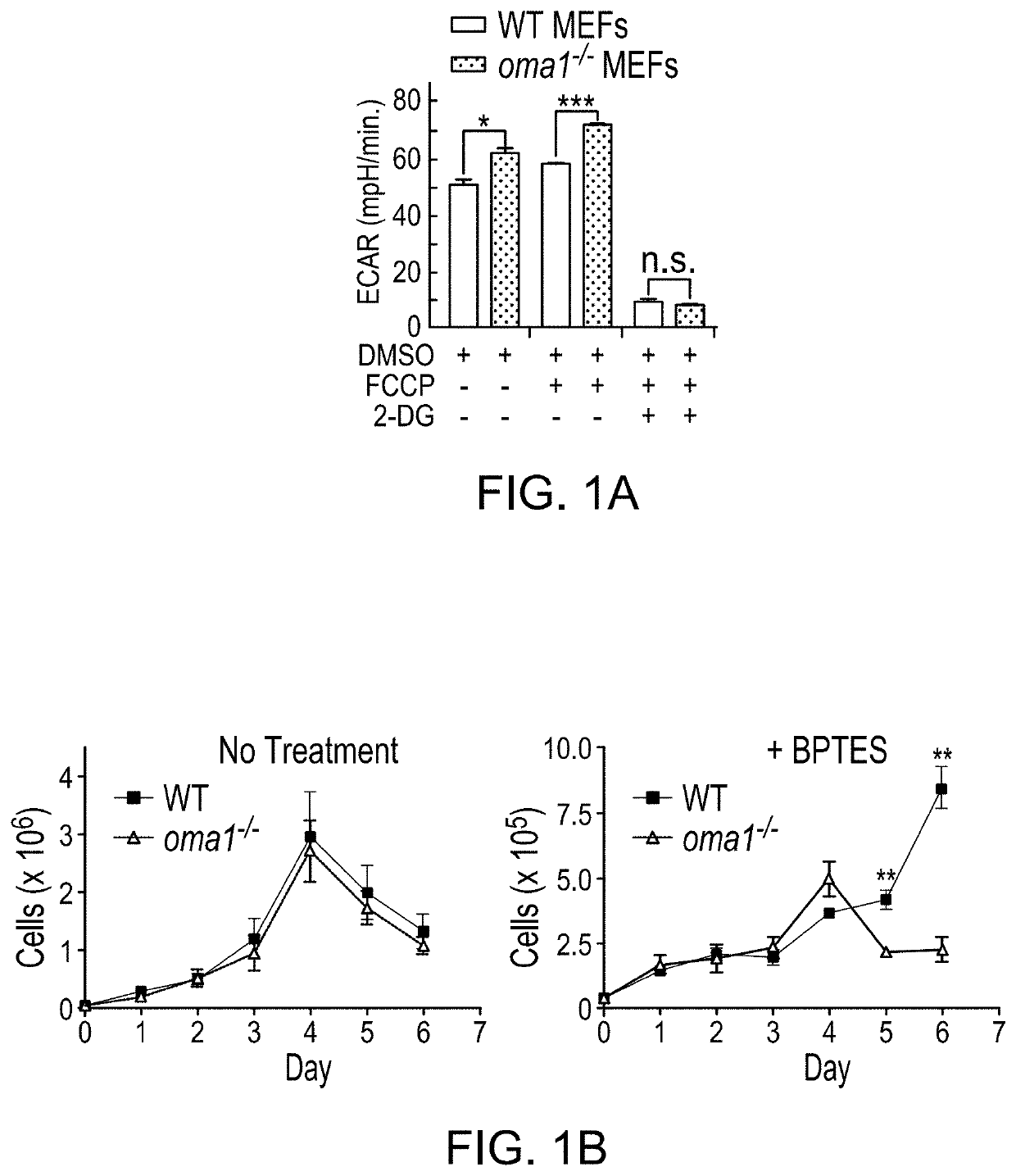
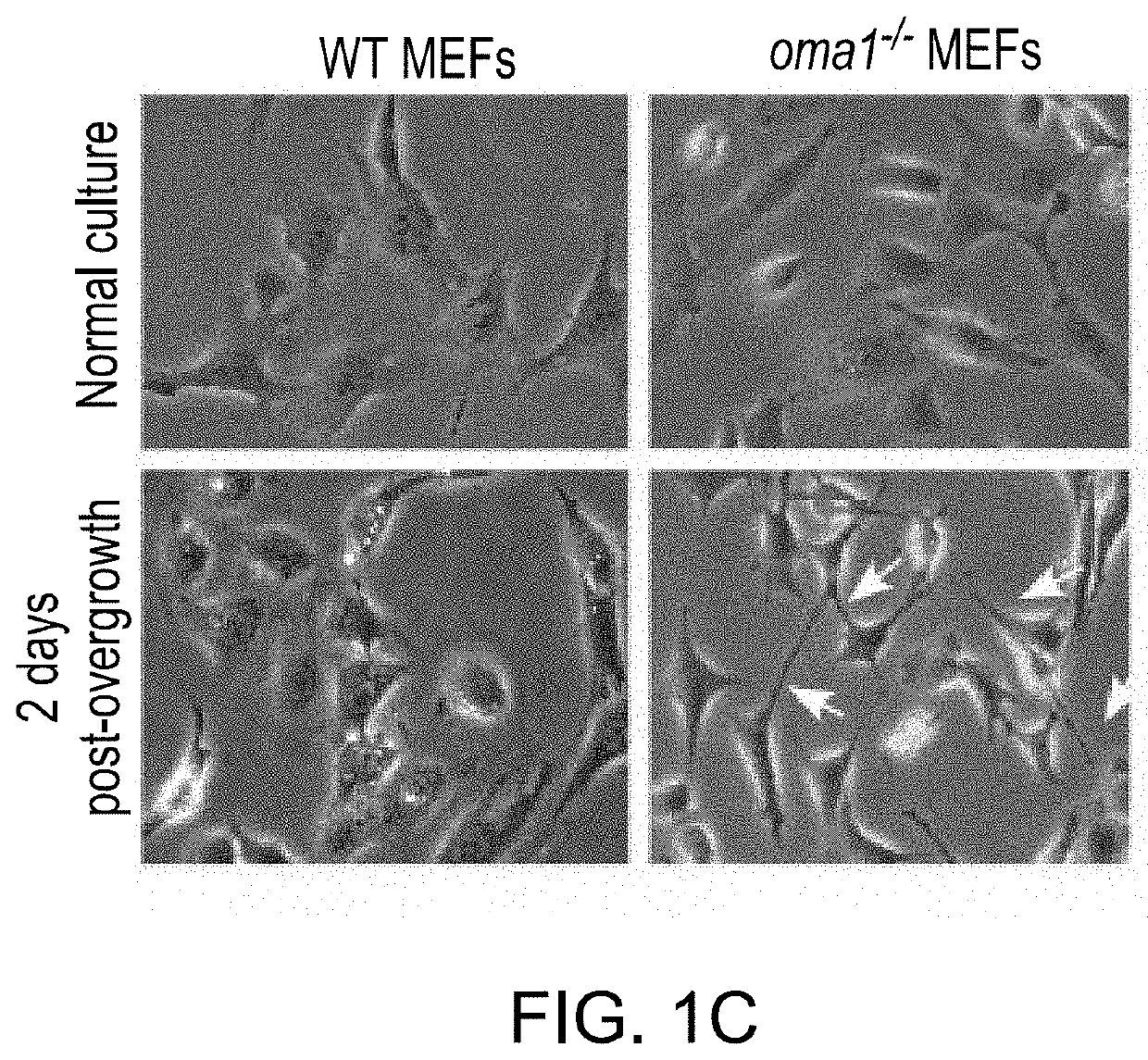
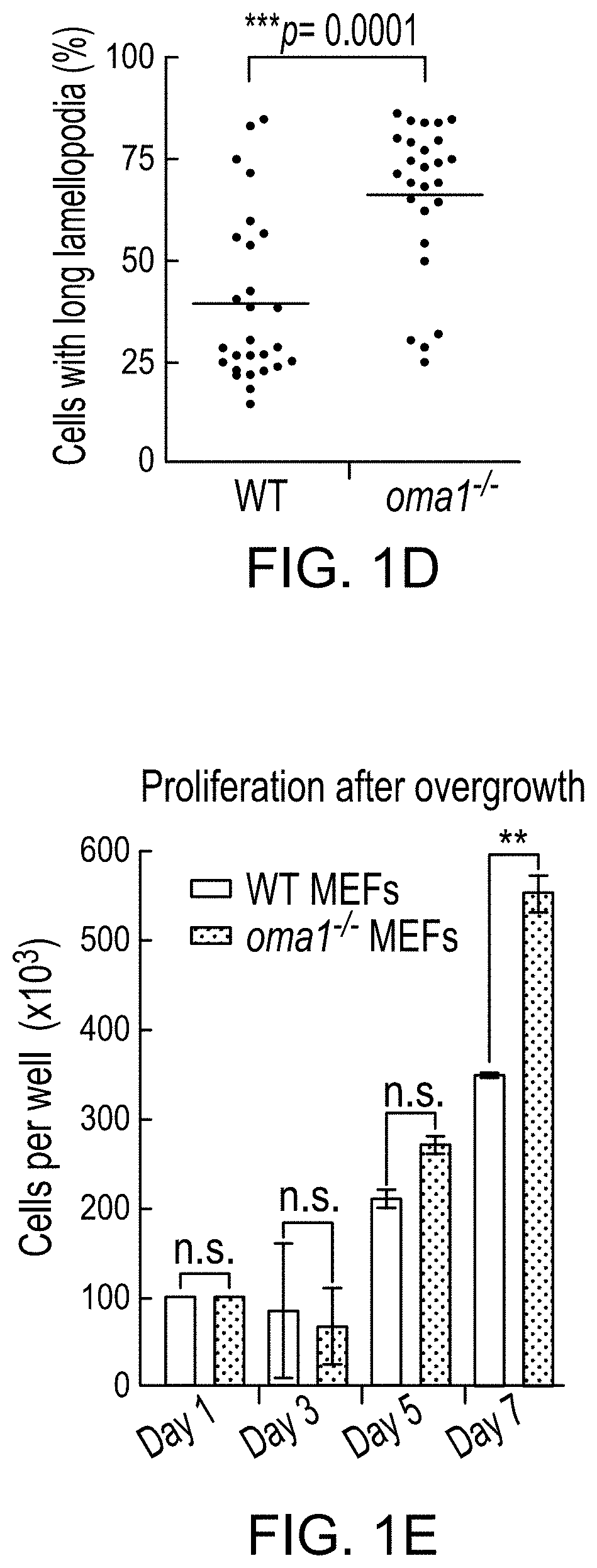
Mitochondrial protease oma1 as a marker for breast cancer
PendingUS20210208144A1HydrolasesImmunoglobulins against cell receptors/antigens/surface-determinantsPharmaceutical drugTumor progression
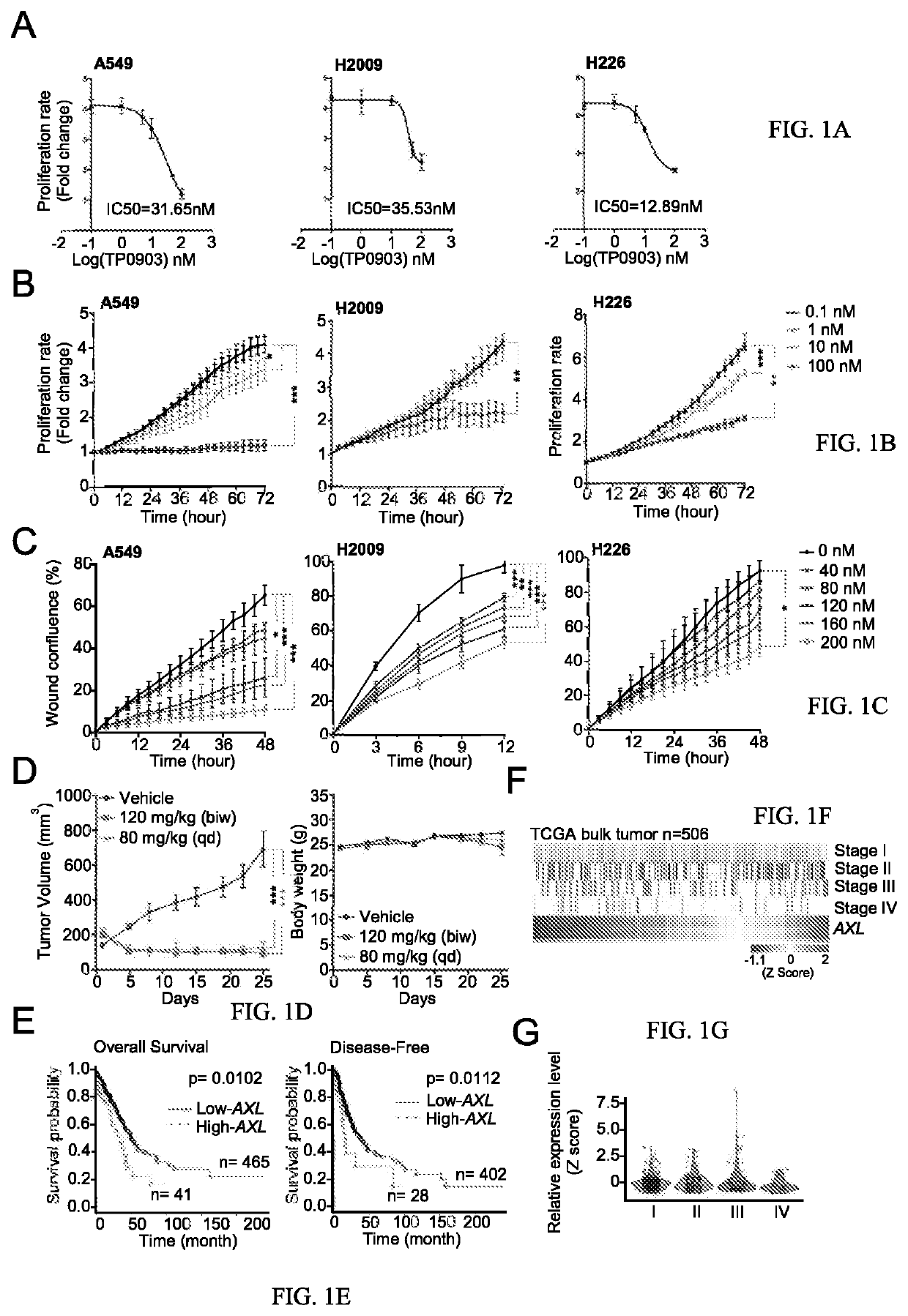
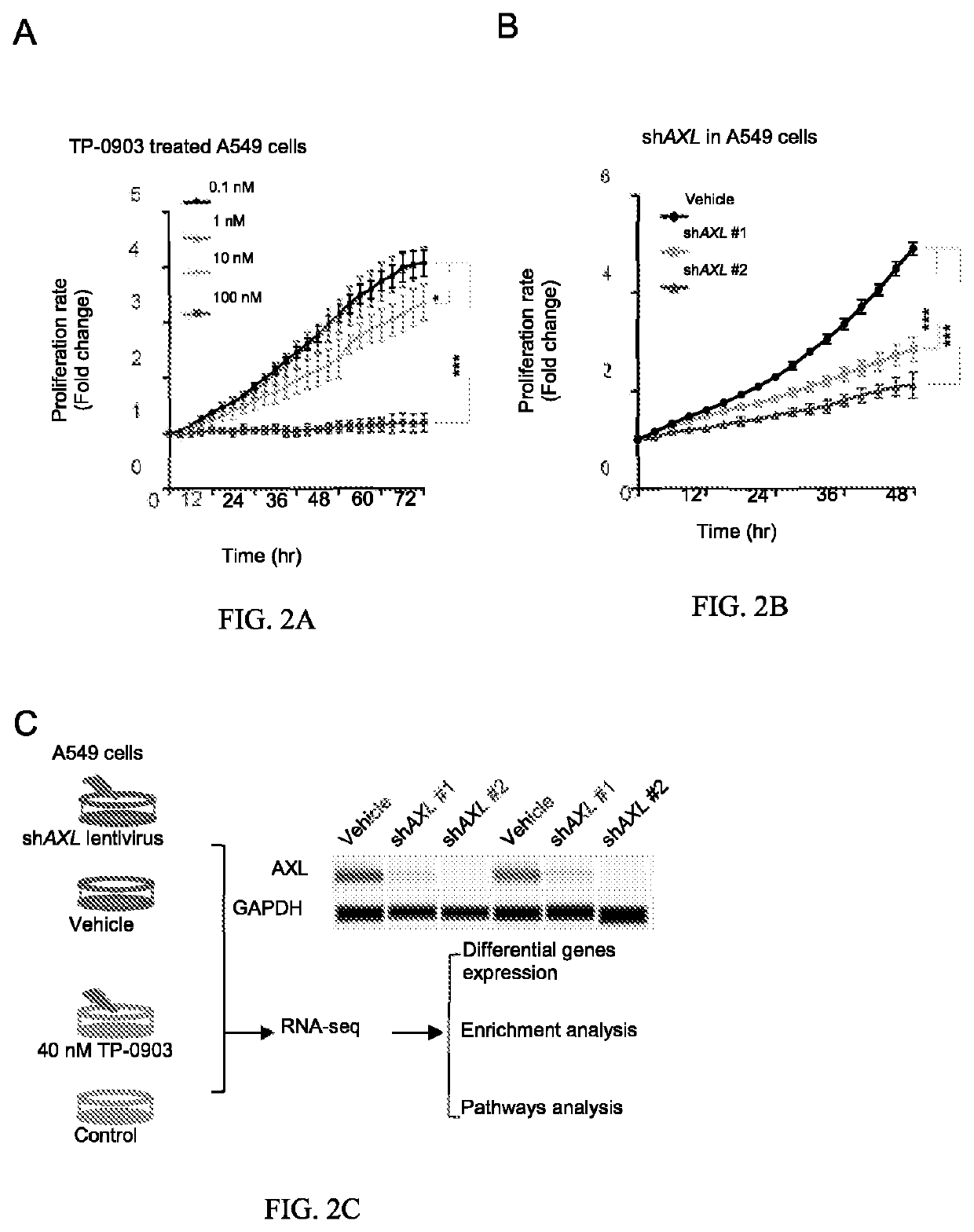
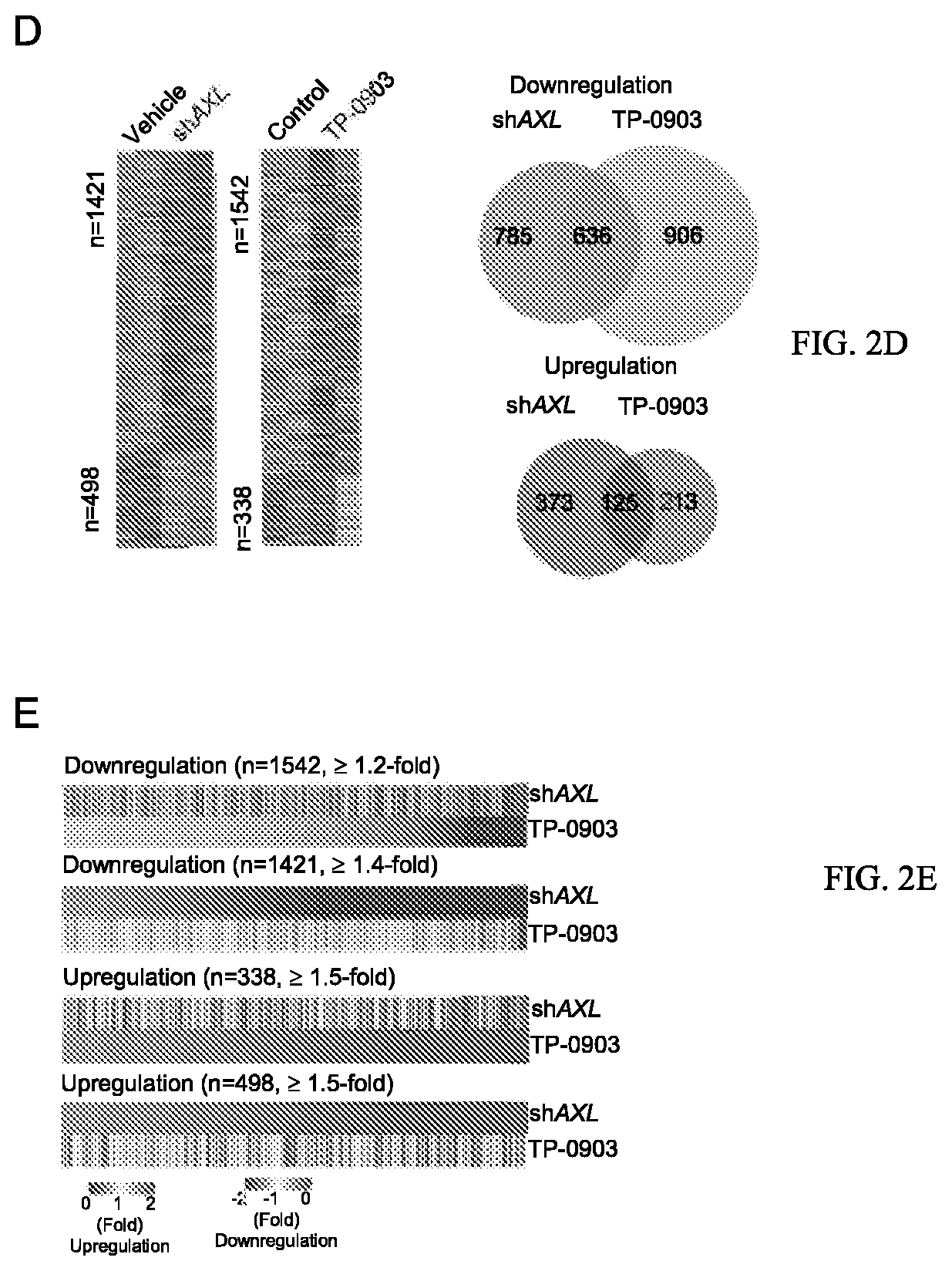
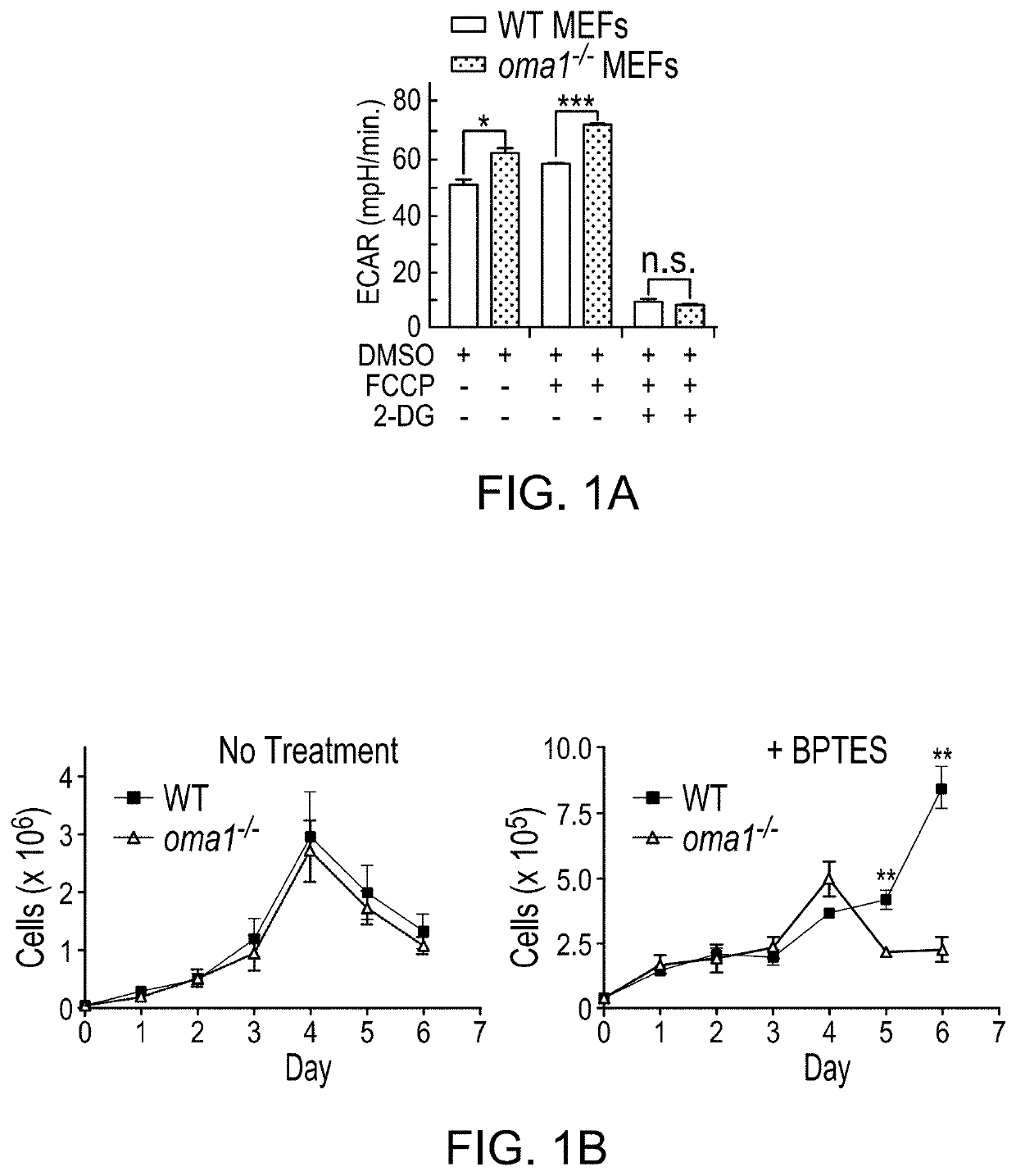
The use of mitochondrial protease OMA1 as a theranostic marker for breast cancer, tumor progression, metastatis and drug responsiveness is disclosed herein.
Owner:NUTECH VENTURES
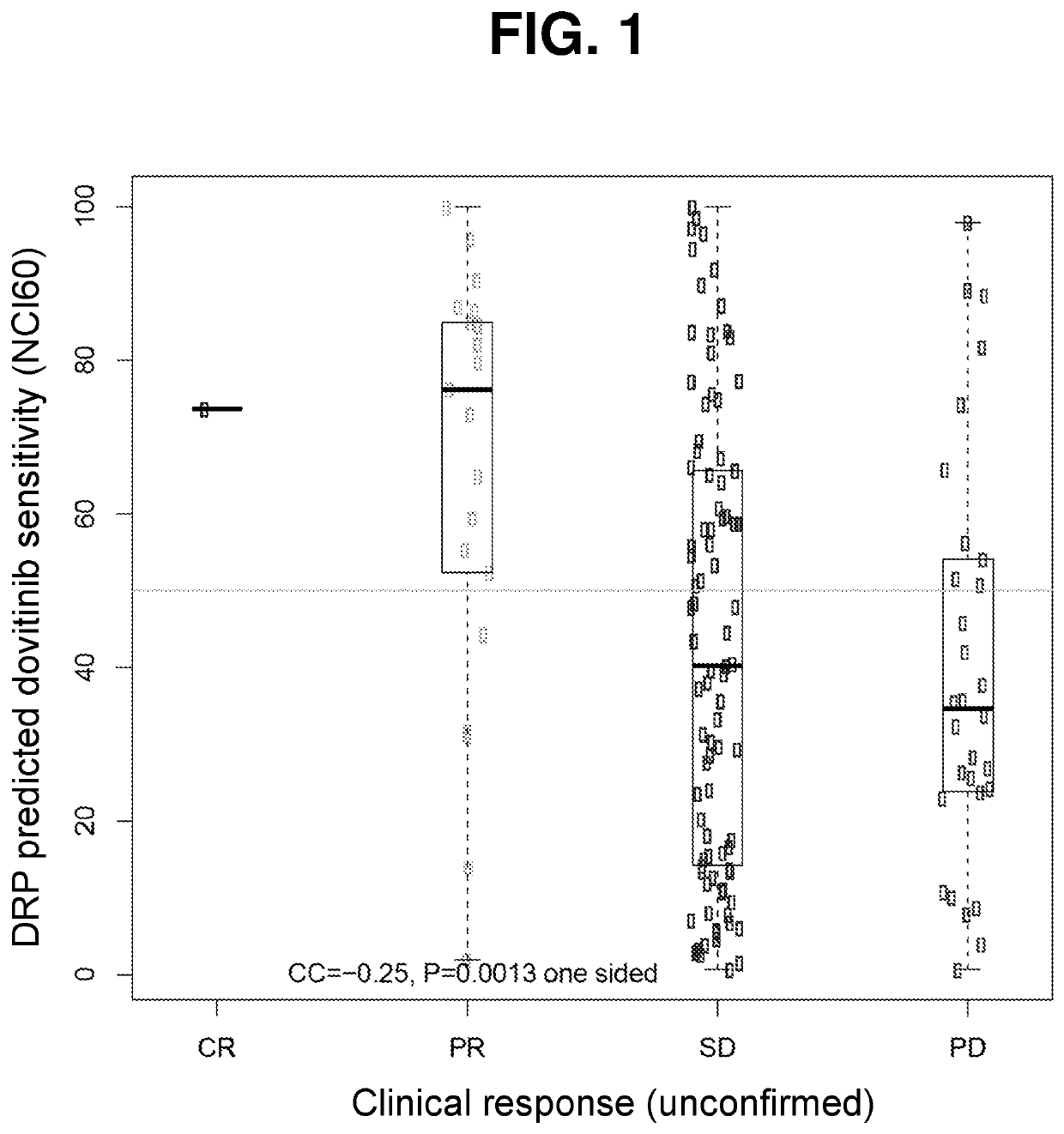
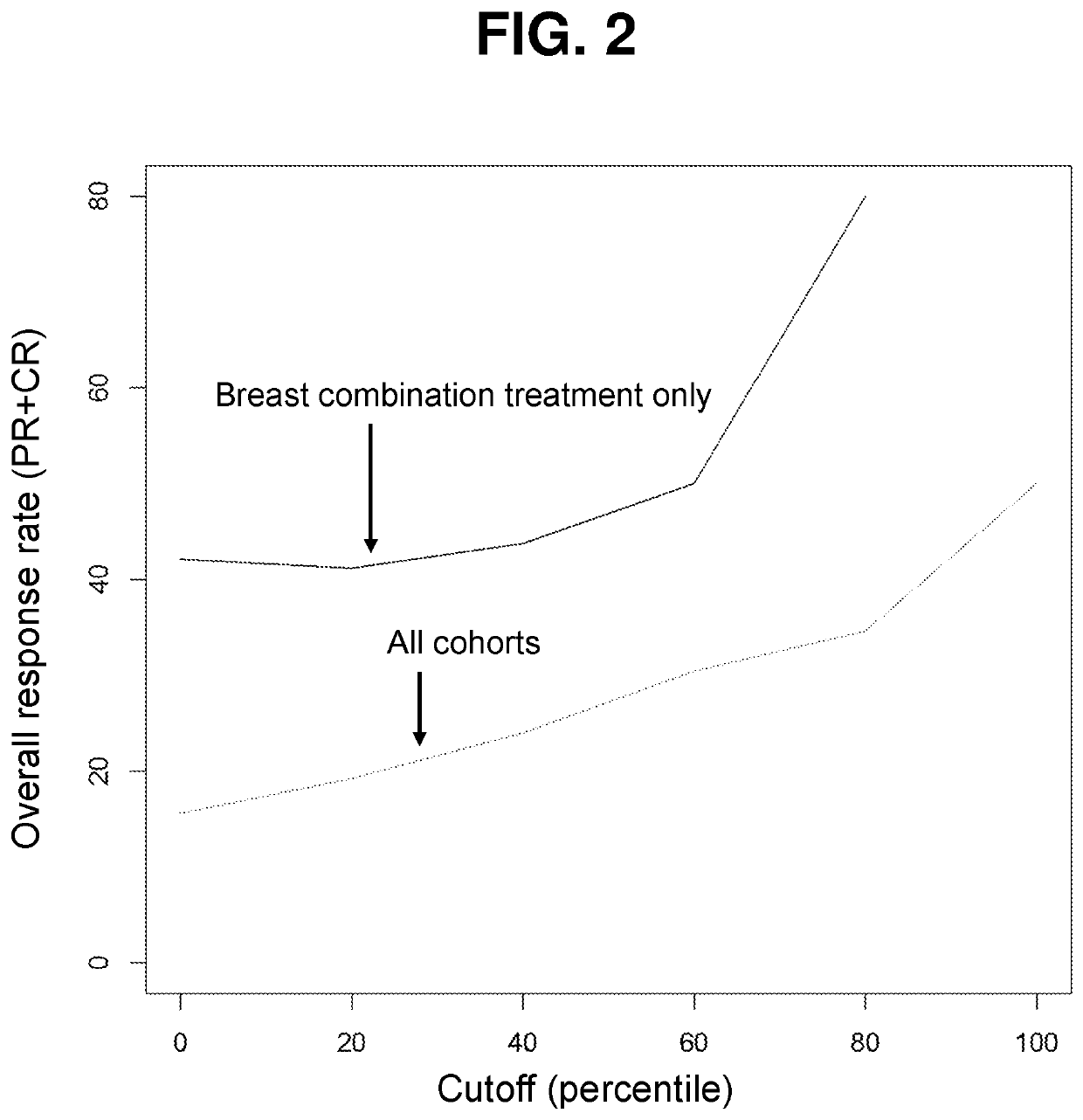
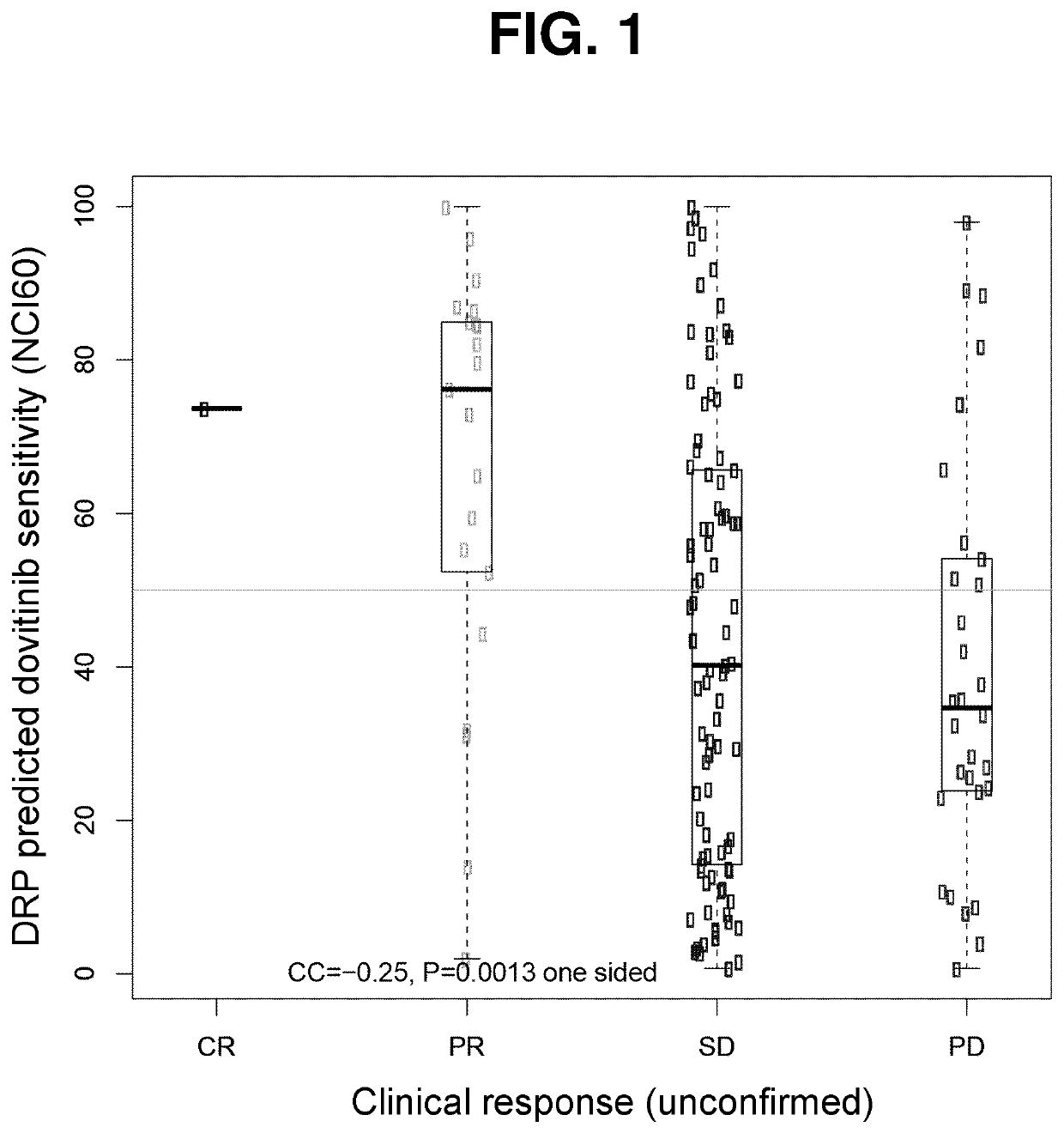
Methods for predicting drug responsiveness in cancer patients
ActiveUS10835531B1Minimize exposureOrganic active ingredientsHealth-index calculationPharmaceutical drugPharmaceutical medicine
The present invention features methods, devices, and kits for detecting gene expression in a patient with a cancer or determining responsive of a patient with a cancer to a treatment, such as treatment with dovitinib or a pharmaceutically acceptable salt thereof. The invention further includes methods of treating a patient with a cancer by administering a treatment, e.g., treatment with dovitinib or a pharmaceutically acceptable salt thereof, in particular when the patient is determined to be responsive to the treatment based on the expression of the biomarkers described herein.
Owner:ALLARITY THERAPEUTICS EURO APS
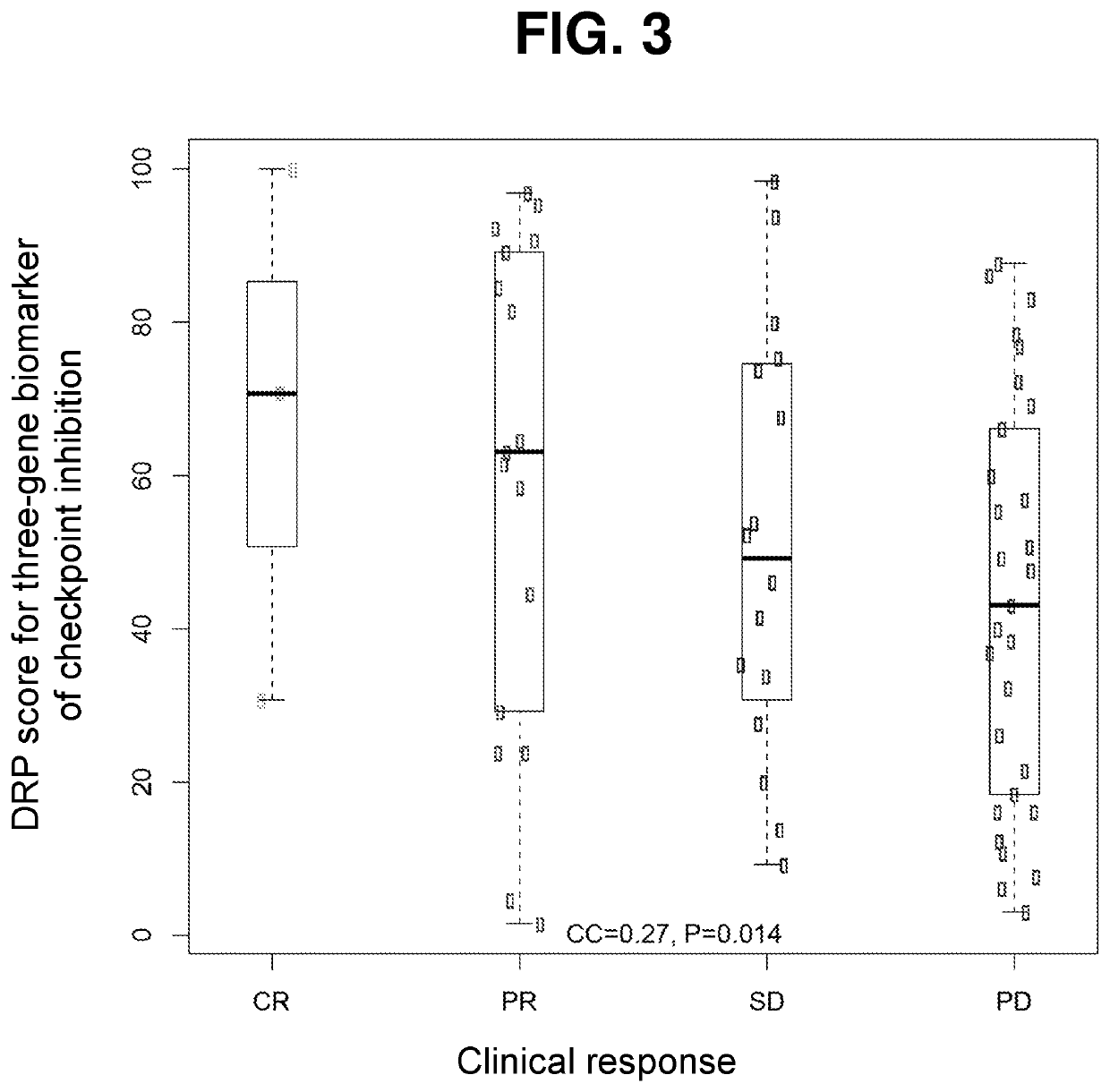
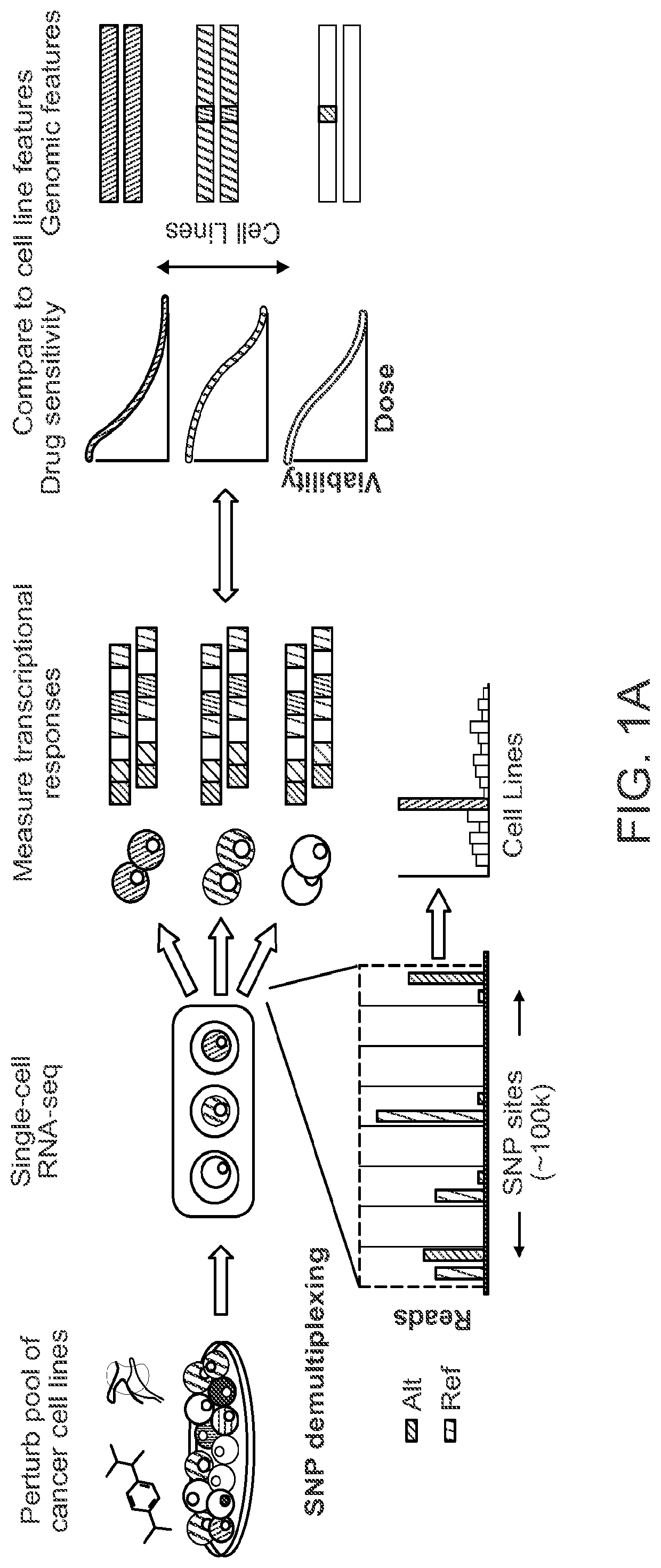
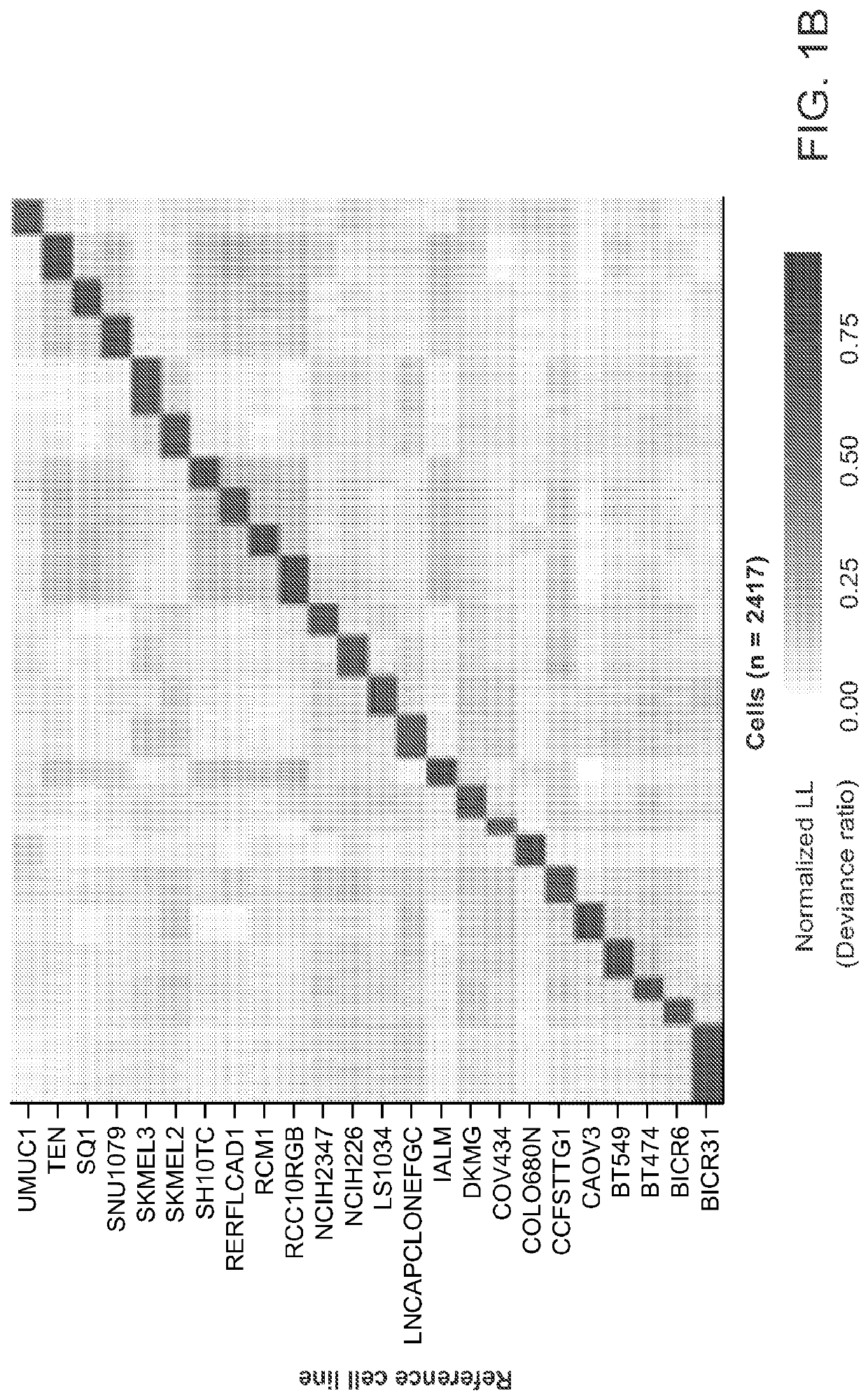
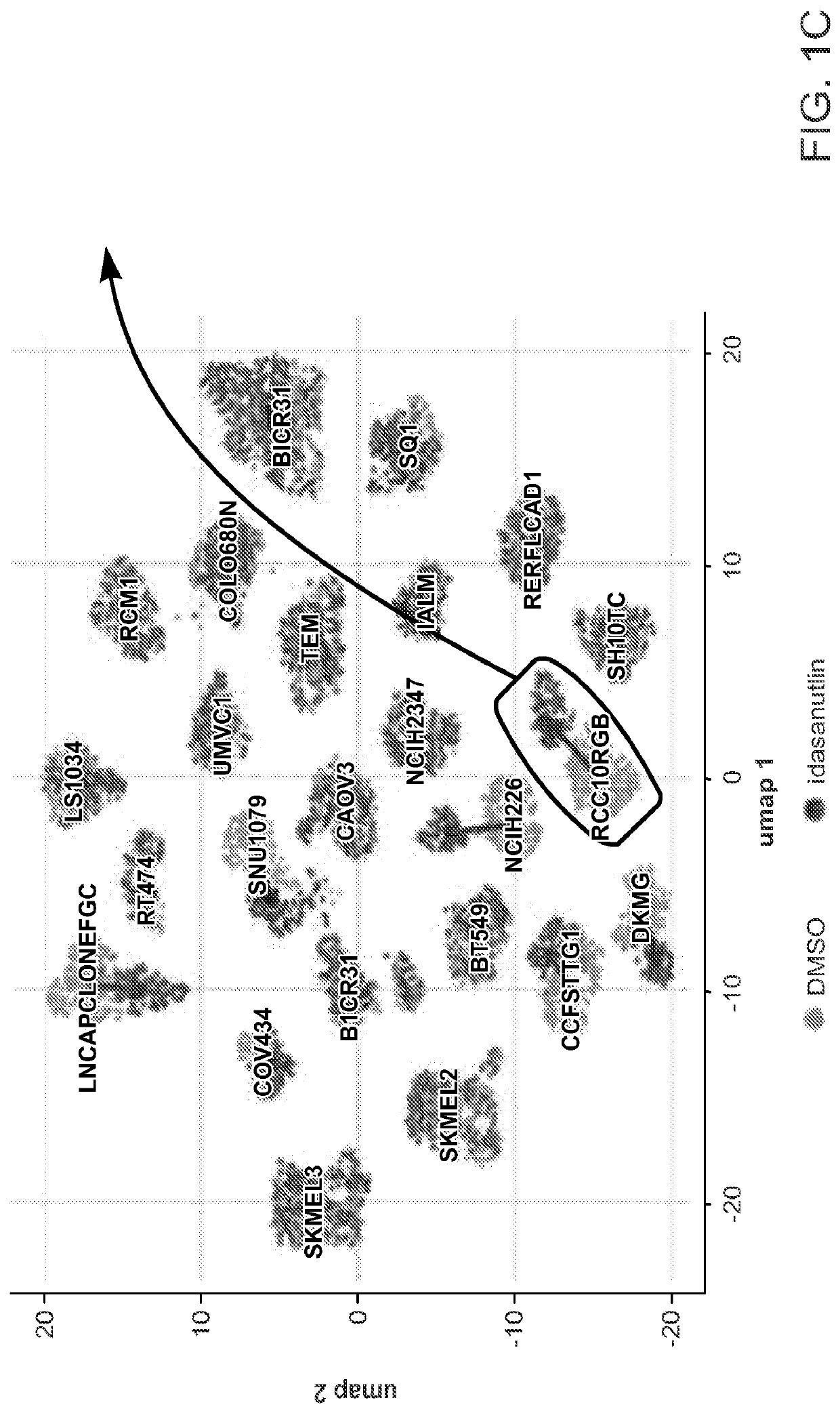
Rapid prediction of drug responsiveness
PendingUS20220340976A1Rapid and reliable drug-response profilingAccurate predictionCompound screeningApoptosis detectionSingle cell transcriptomeBiochemistry
Owner:THE BROAD INST INC +1
Method for evaluating drug responsiveness and drug responsiveness evaluation system
PendingUS20200386743A1Difficult to evaluateShort timeDisease diagnosisEnzymology/microbiology apparatusPharmaceutical drugMyocyte
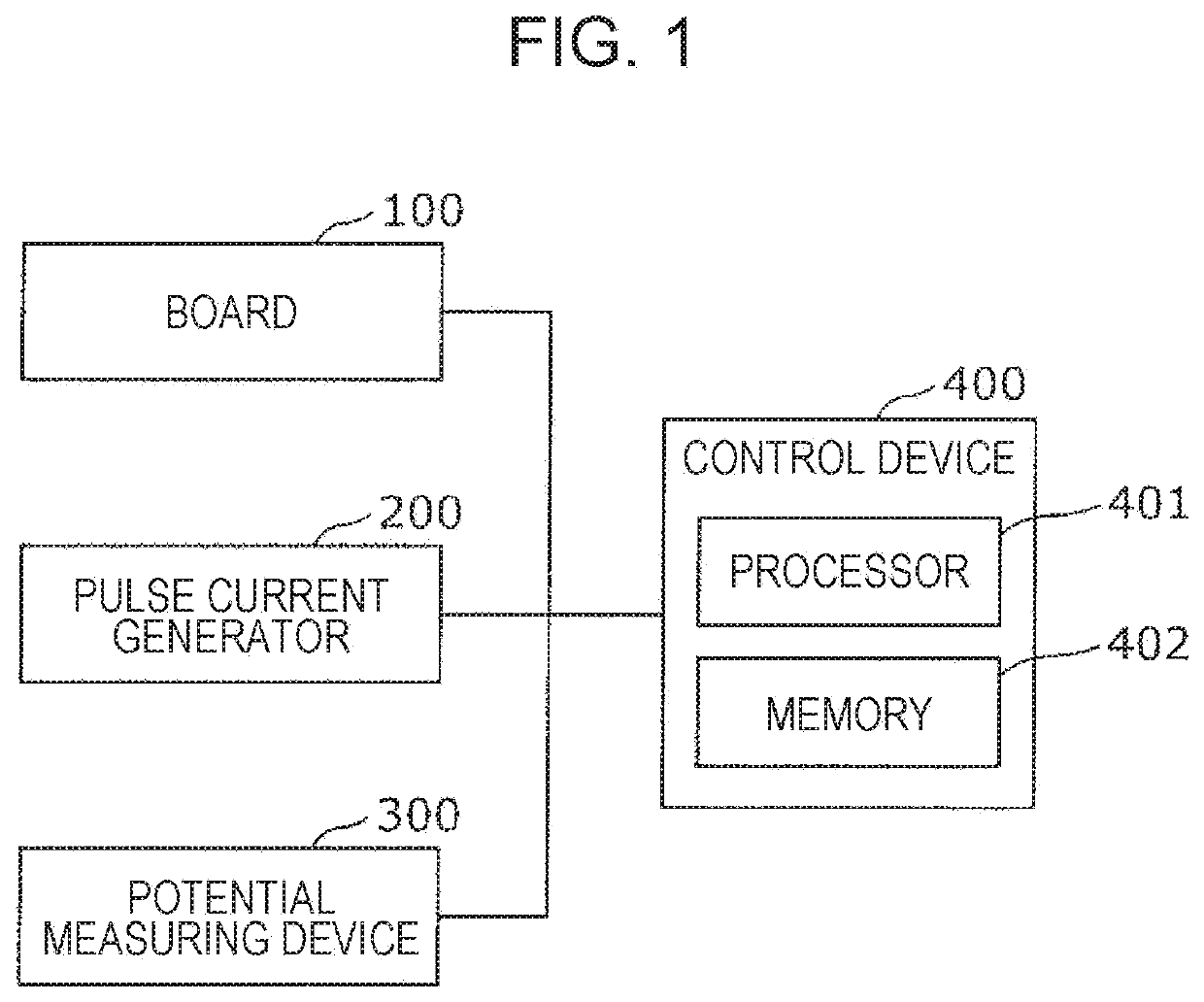
A method for evaluating drug responsiveness includes disposing a myocardial cell produced through differential induction onto a board including an electrode, administering a drug to the myocardial cell, continuously applying, after the drug is administered, a pulse current or a pulse voltage to the myocardial cell through the electrode for a certain period of time, measuring, after the certain period of time has elapsed, a pulsation characteristic of the myocardial cell, and evaluating responsiveness of the myocardial cell to the drug on a basis of the pulsation characteristic.
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
A biodegradable and sugar-responsive Y-shaped polymer drug delivery material and its preparation
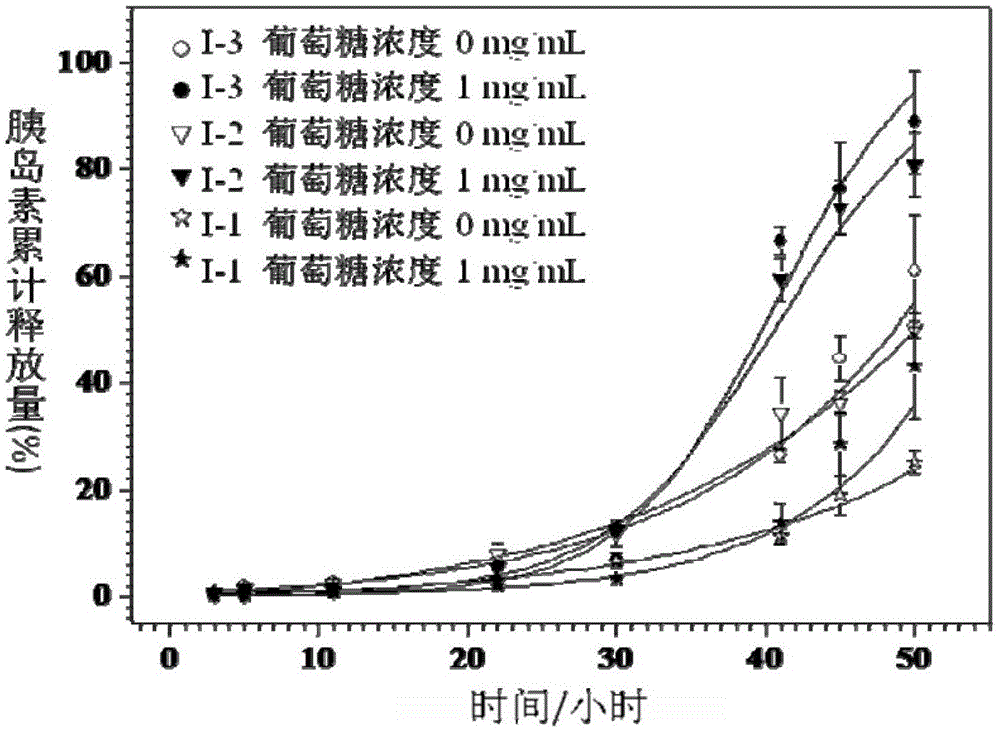
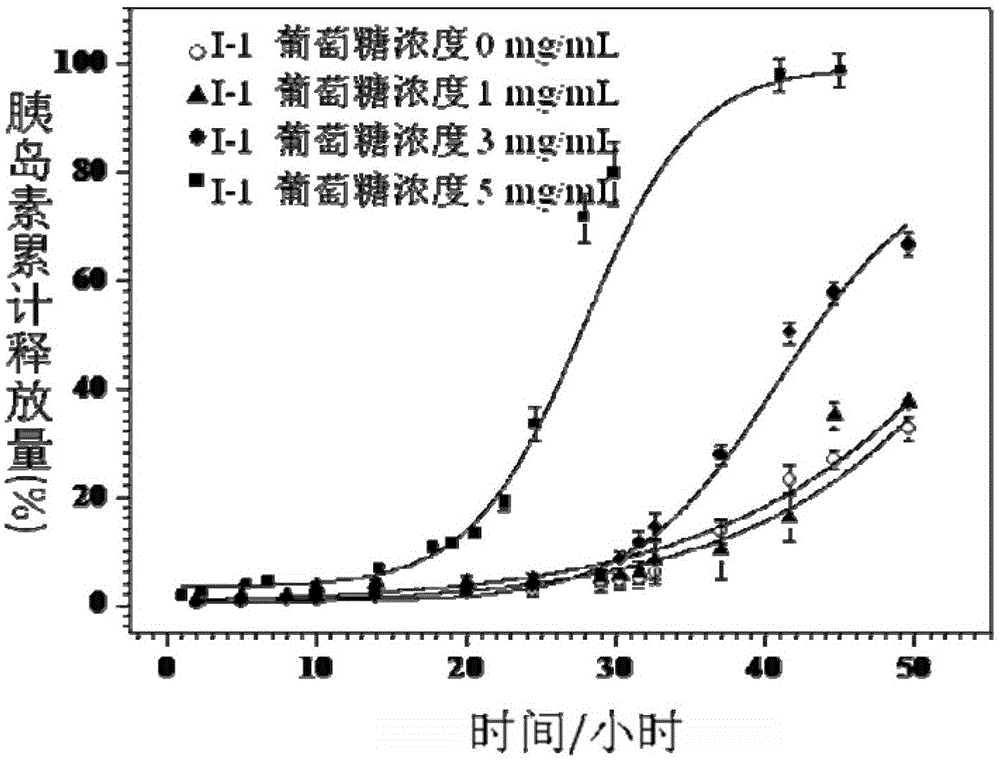
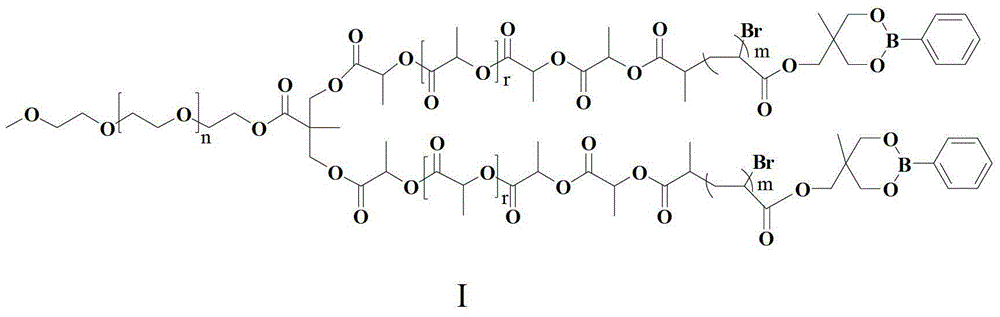
ActiveCN103408710BTo promote metabolismGood biocompatibilityMetabolism disorderPharmaceutical non-active ingredientsConcentrations glucoseD-Glucose
The invention provides a biodegradable and sugar responsive Y type polymer drug delivery material and preparation, which belongs to the field of biological materials. The structural formula of the compound is obtained by the following steps of firstly preparing a compound III, and then, preparing a compound V, and reacting the compound III with the compound V under conditions of cuprous bromide, copper bromide and divinyl pentamethylene triamine. The material provided by the invention has glucose responsive self-discipline insulin release ability and has selectivity on glucose concentration.
Owner:BEIJING UNIV OF CHEM TECH
Methods for predicting drug responsiveness in cancer patients
ActiveUS11421284B2Organic active ingredientsMicrobiological testing/measurementPharmaceutical drugOncology
The present invention features methods, devices, and kits for detecting expression in a patient having cancer or determining responsive of a patient having cancer to a treatment, such as irofulven. The invention further includes methods of treating a patient having cancer by administering, e.g., irofulven.
Owner:ALLARITY THERAPEUTICS EURO APS
Early Diagnosis of Autoimmune and Inflammatory Disorders
The disclosure relates to methods and assay that assists in the diagnosis of autoimmune and chronic inflammatory disorders such as systemic lupus erythematosus and rheumatoid arthritis by analyzing drug-responsiveness of an interferon signal in a hematological sample (e.g., blood) obtained from a human subject. The assay involves comparing the interferon signal in a control aliquot of the sample with the same interferon sample in an aliquot that has been exposed to a therapeutic modality (e.g., combined with a drug) that is known to be efficacious to treat the disorder. A significant difference between the interferon signals of the control and treated aliquots that corresponds to a characteristic interferon signature for the disorder indicates that the subject is afflicted with, or is likely to develop, the disorder.
Owner:PAPPU RAMESH
Mitochondrial protease oma1 as a marker for breast cancer
PendingUS20190369100A1Early detectionHydrolasesImmunoglobulins against cell receptors/antigens/surface-determinantsProteinase activityTumor progression
The use of mitochondrial protease OMA1 as a theranostic marker for breast cancer, tumor progression, metastatis and drug responsiveness is disclosed herein.
Owner:NUTECH VENTURES
Methods for predicting drug responsiveness in cancer patients
PendingUS20220362240A1Minimize exposureOrganic active ingredientsHealth-index calculationPharmaceutical drugPharmaceutical medicine
The present invention features methods, devices, and kits for detecting gene expression in a patient with a cancer or determining responsive of a patient with a cancer to a treatment, such as treatment with dovitinib or a pharmaceutically acceptable salt thereof. The invention further includes methods of treating a patient with a cancer by administering a treatment, e.g., treatment with dovitinib or a pharmaceutically acceptable salt thereof, in particular when the patient is determined to be responsive to the treatment based on the expression of the biomarkers described herein.
Owner:ONCOLOGY VENTURE APS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com