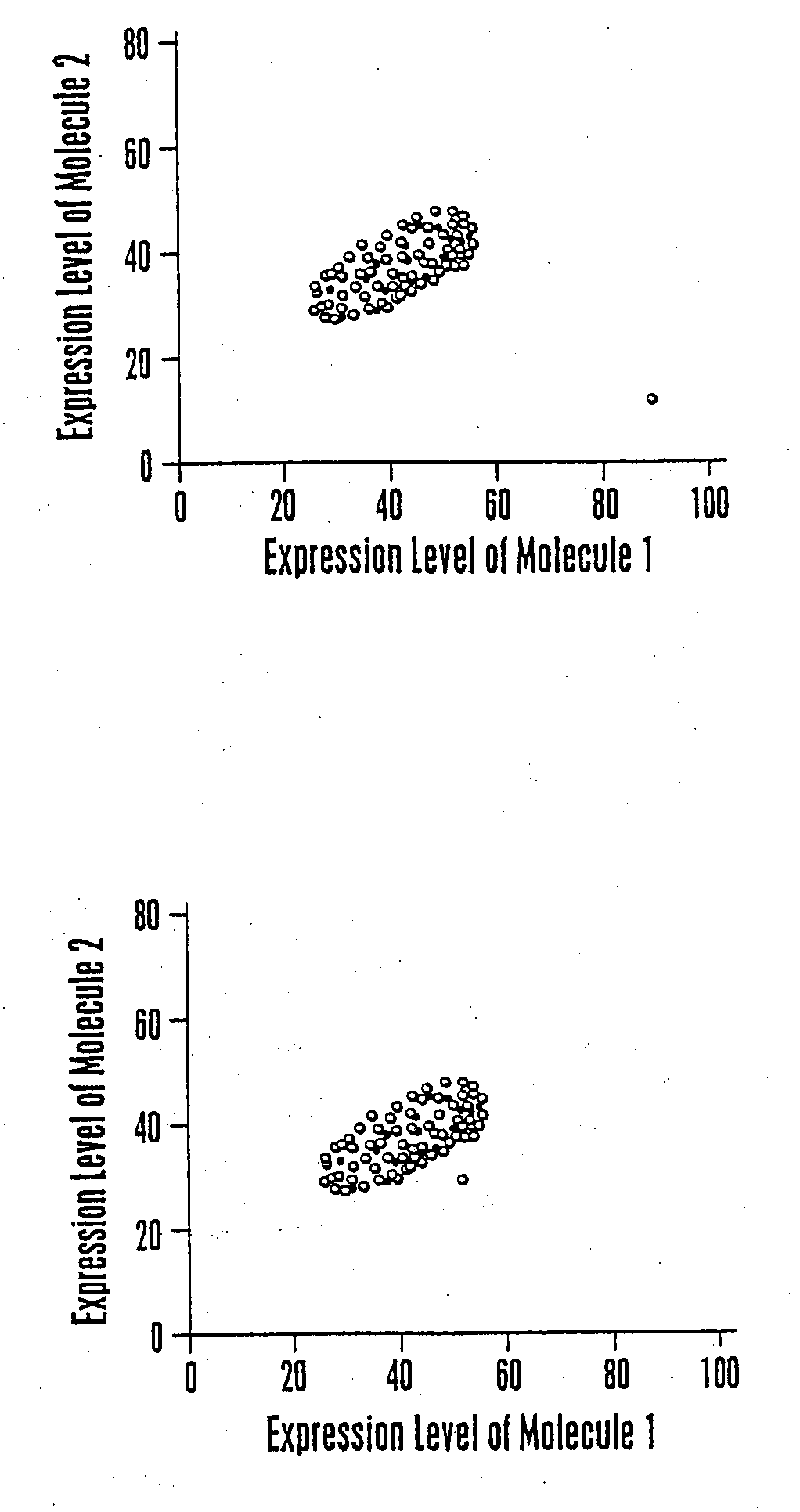
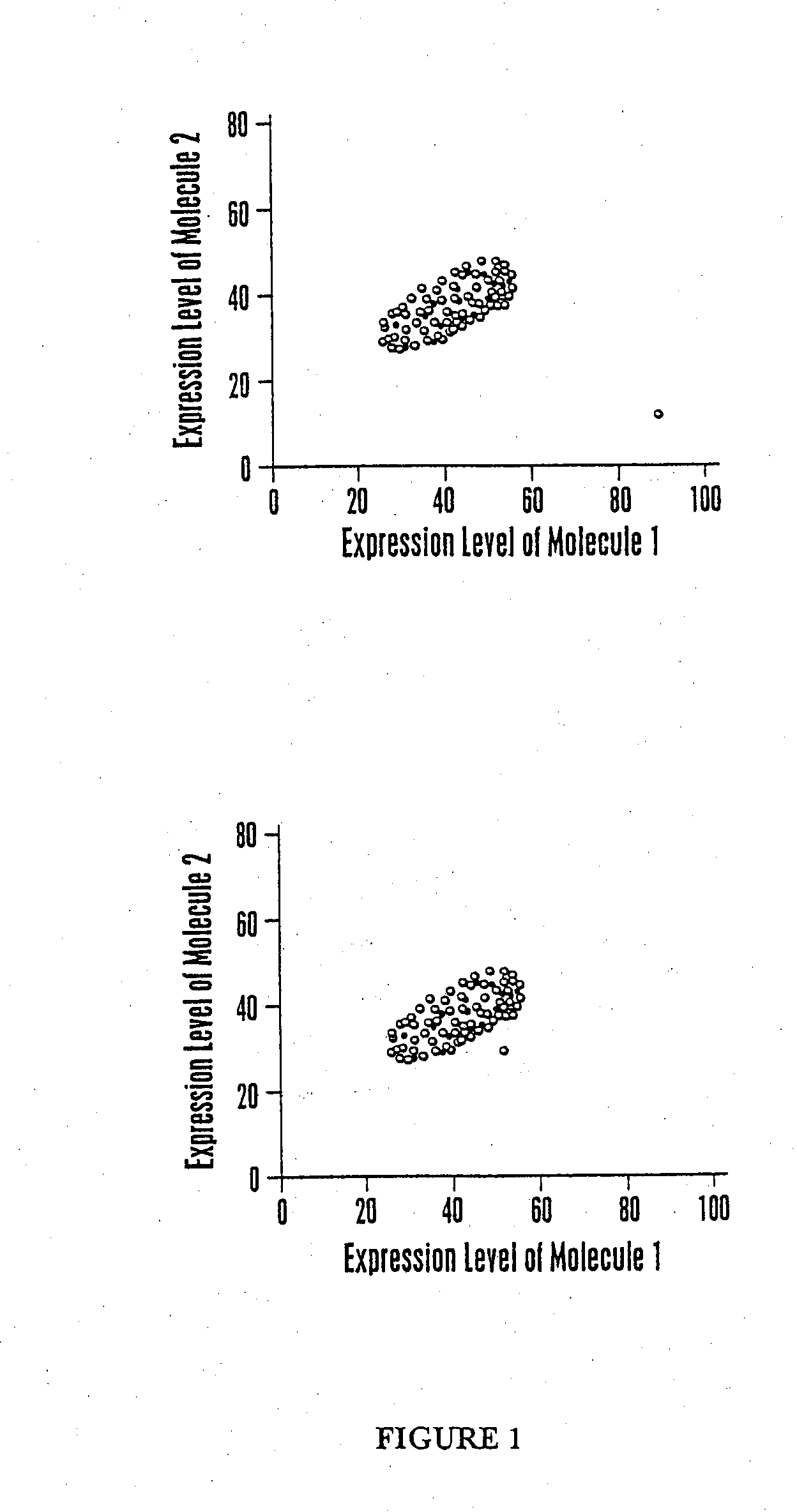
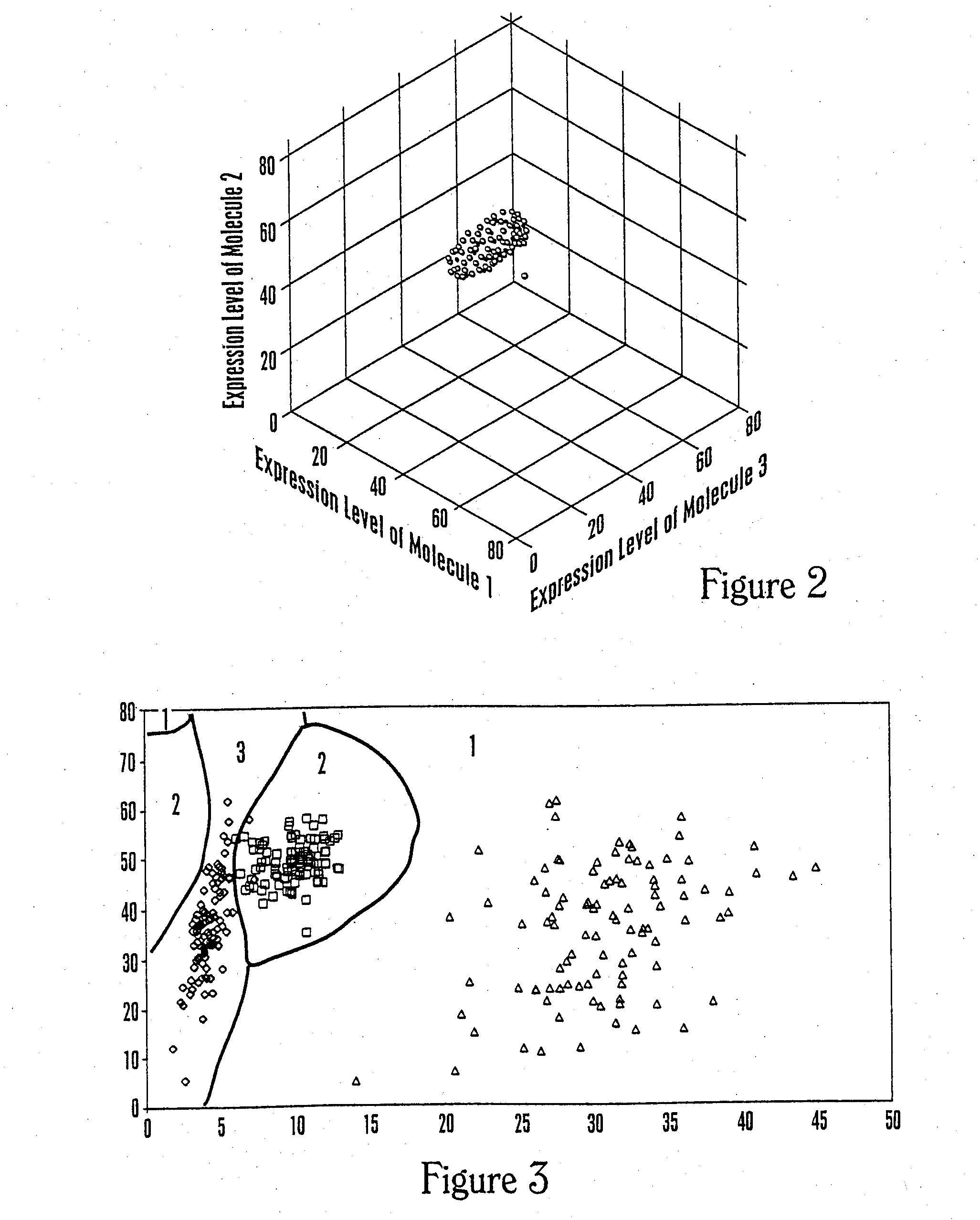
Multiparameter analysis for drug response and related methods
a multi-parameter analysis and drug technology, applied in the field of predictive medicine, can solve the problems of a large number of genes in the analysis of such a large number of genes, and a new layer of complexity arises
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Calculation Methodology Using Multivariate Classification Theory
[0254]This example describes a calculation methodology using multivariate classification theory to classify health-associated regions of multidimensional space.
[0255]Data are available on expression levels corresponding to a set of molecules for individuals with known health states, for example, healthy, ovarian cancer, prostate cancer, diabetes, and the like. The number m corresponds to the number of different health states. The calculation steps involved are: (1) estimate the probability distribution of the observed data vector for each health state; (2) estimate the costs of misclassification for each combination of health states; (3) estimate the a priori probabilities of a random individual being a member of each health state; and (4) determine the optimal calculation to be performed when classifying a new individual. The development given here is based upon multivariate statistical methods such as those of T. W. A...
example ii
Logistic Regression Analysis
[0262]This example describes the analysis of a data set for three health states and two molecular expression levels using logistic regression analysis.
[0263]The data set was created starting with pseudorandom computer-generated numbers and then applying a different mathematical transformation for each health related reference group. For the data set shown in FIG. 4A for three health states and two molecular expression levels, the resulting classification regions are shown using logistic regression analysis under the assumptions that the costs of misclassification are all equal, and the prior probabilities are 0.2, 0.5, and 0.3 for the three groups. Because health state 2 is the most common in the population, the classification tends to favor this group at the upper right where data are sparse.
[0264]The classification regions are based on three separate logistic regression analyses, one to predict each health state, where each analysis used the molecular e...
example iii
Machine Learning by Boosting of Individual Molecules
[0270]This example describes classification analysis using a machine learning algorithm called “boosting” to combine a chosen group of simple one-molecule-at-a-time decision rules to obtain an effective health classification.
[0271]The data set was created starting with pseudorandom computer-generated numbers and then applying a different mathematical transformation for each health related reference group. For the data set shown in FIG. 5 for three health states and two molecular expression levels, the resulting classification regions are shown for a machine-learning technique that uses boosting to combine several one-molecule-at-a-time analyses to form a classification region under the assumption that the prior probabilities are 0.6, 0.3, and 0.1 for the three groups. In this case, 8 boosting steps have been taken. The method used here is based on the AdaBoost.M1 algorithm described by Freund and Schapire (J. Computer and System Sc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| genetic defects | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com