Drug reactive metabolite mass spectrum detection probe and application thereof
A technology for metabolites and drug reactions, applied in the field of detection, can solve the problems of inability to capture hard reactive metabolites, inability to obtain comprehensive information on drug reactive metabolites, narrow capture range of drug reactive metabolites, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
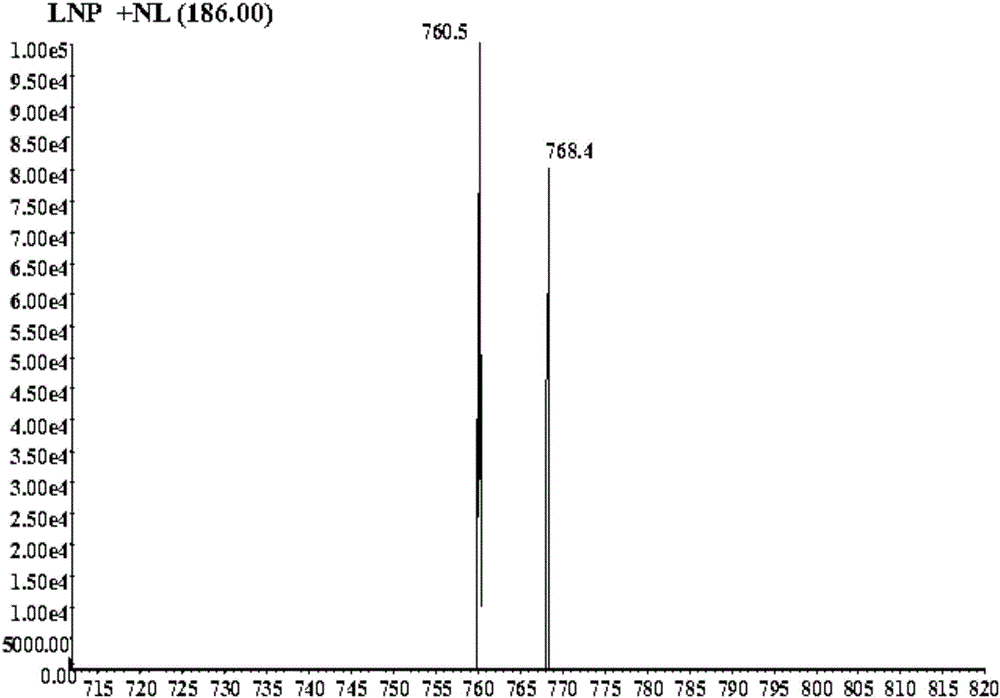
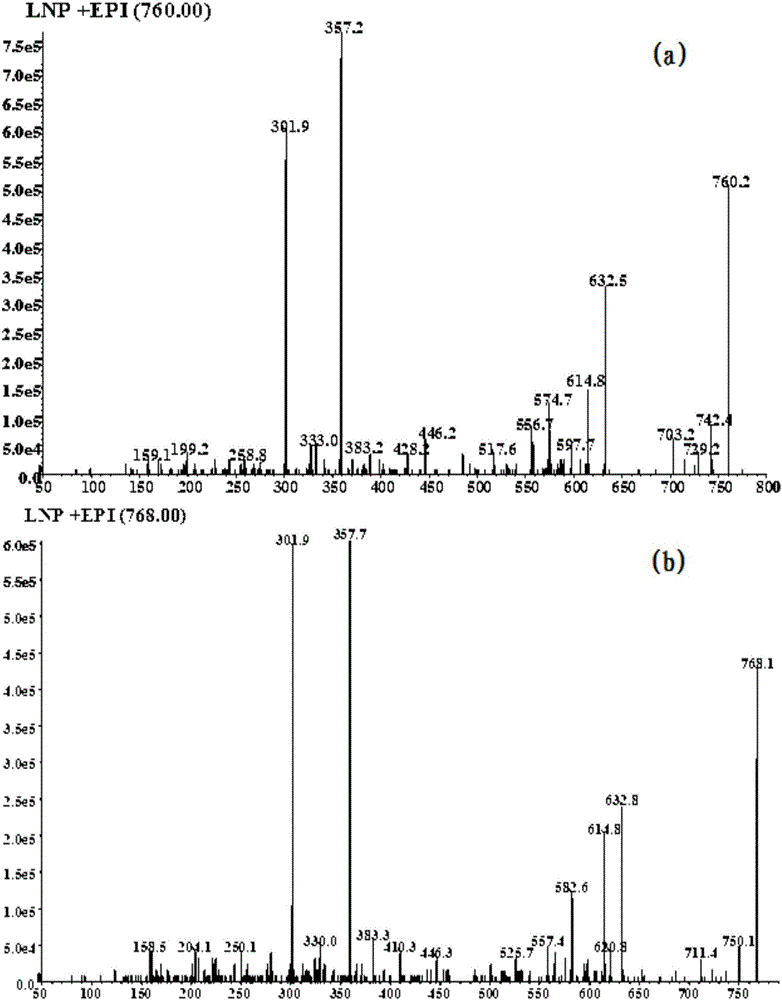
[0056] WCK (d8-WCK) is mixed with clozapine, HLM (human liver microsomes), catalysts, etc., and used as an in vitro drug metabolism reaction system of human liver microsomes. After LC-MSMS (liquid chromatography-mass spectrometry) detection, Neutral loss map and product ion scan map are obtained by neutral loss scan and product ion scan, respectively as figure 1 and figure 2 shown.
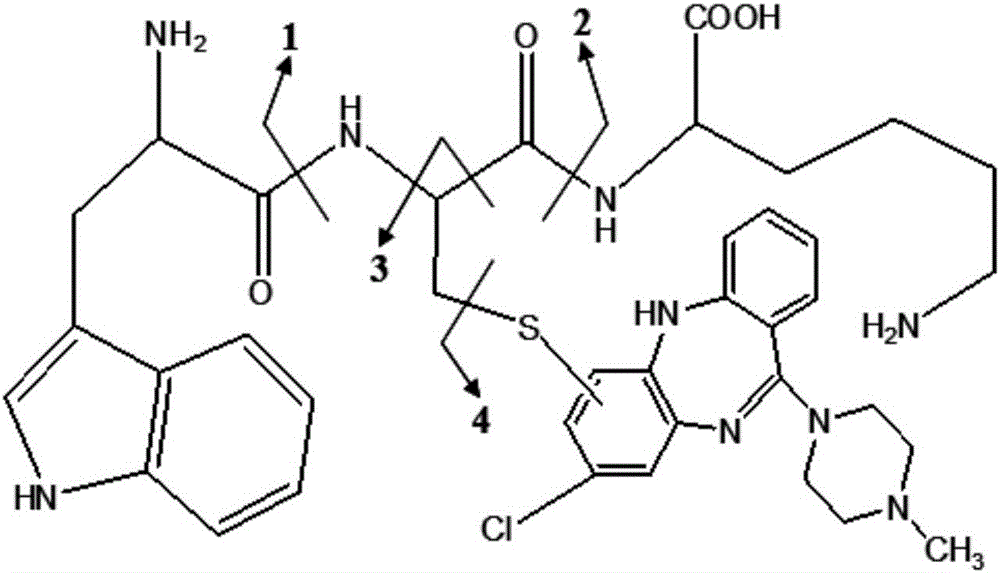
[0057] Clozapine is dehydrogenated during the enzymatic reaction to produce reactive metabolites, which rapidly combine with the sulfhydryl groups in WCK (d8-WCK) to form almost equal adducts with molecular weights of 759 and 767, respectively, such as image 3 shown. Under the neutral loss scan of the electrospray positive ion mode, the neutral group with a molecular weight of 186Da can be lost and detected, and the precursor ions with a molecular weight difference of 8Da appear at the same time, which can better eliminate the interference of impurities in complex biological matrices, such as ...
Embodiment 2
[0060] WCK (d8-WCK) was mixed with thienylfuran and enzyme reaction incubation solution. After LC-MSMS detection, thienylfuran produced two reactive metabolites during the metabolic process. One of them combines with the thiol and amino groups in the oligopeptide probe to form a stable adduct I, and the other combines with a strong nucleophilic amino group to form a stable adduct II due to its strong electrophilicity, such as Figure 4 shown. like Figure 4 The neutral loss diagram and product ion scanning diagram corresponding to adduct Ⅰ are as follows: Figure 5 and Figure 6 shown. like Figure 4 The neutral loss diagram and product ion scanning diagram corresponding to adduct II are as follows: Figure 7 and Figure 8 shown. This shows that the oligopeptide probe WCK can be successfully applied to the capture of hard drug-reactive metabolites in actual samples, and has a good mass spectrometry response.
Embodiment 3
[0062] Similarly, WCK (d8-WCK) was reacted with acetaminophen and mefenamic acid respectively, and after LC-MSMS detection, the neutral loss diagrams of each adduct were respectively obtained, as shown in Figure 9 Shown in A and B. This shows that the oligopeptide probe WCK can be successfully applied to the capture of reactive metabolites in actual samples, and has a good mass spectrometry response.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com