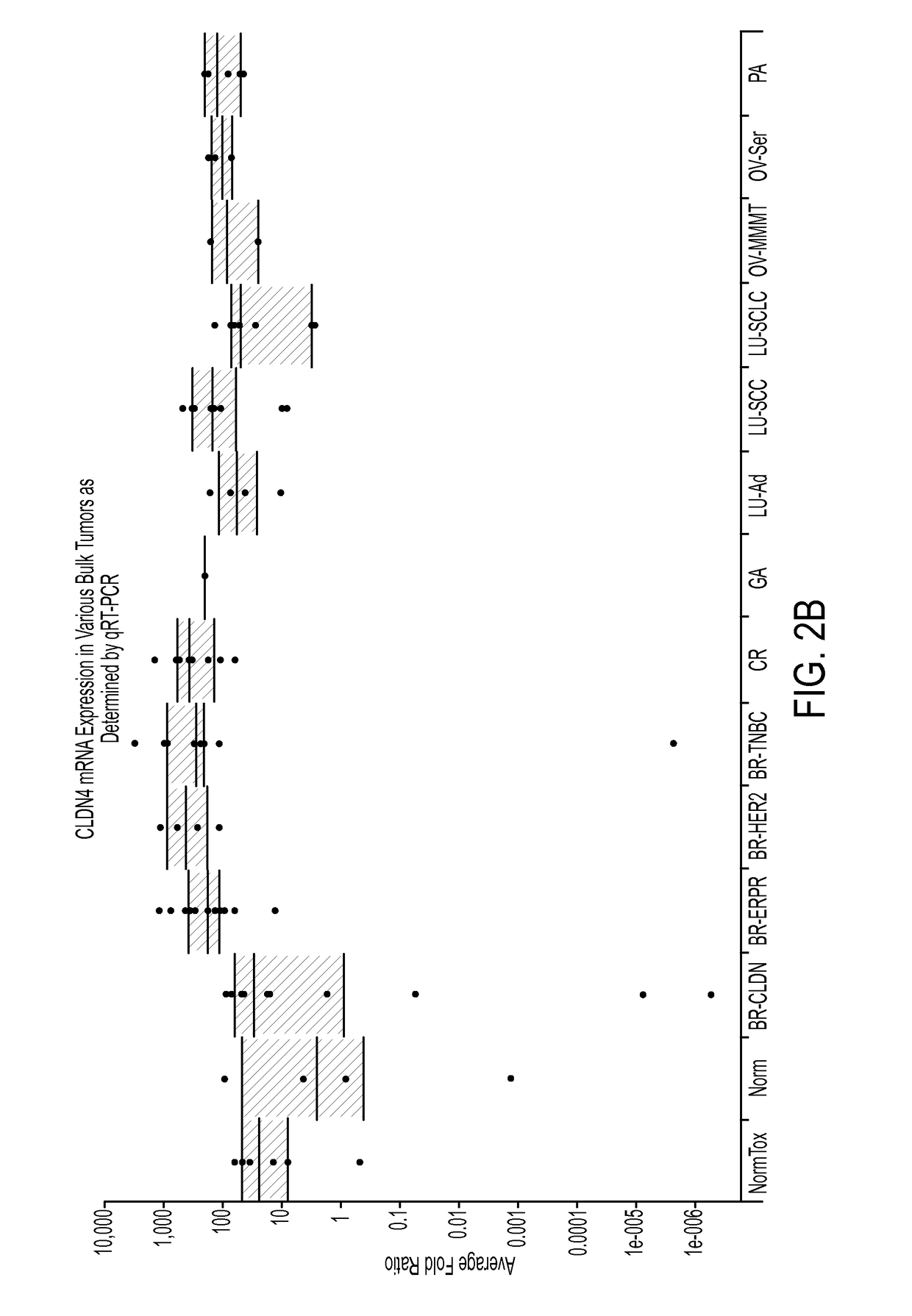
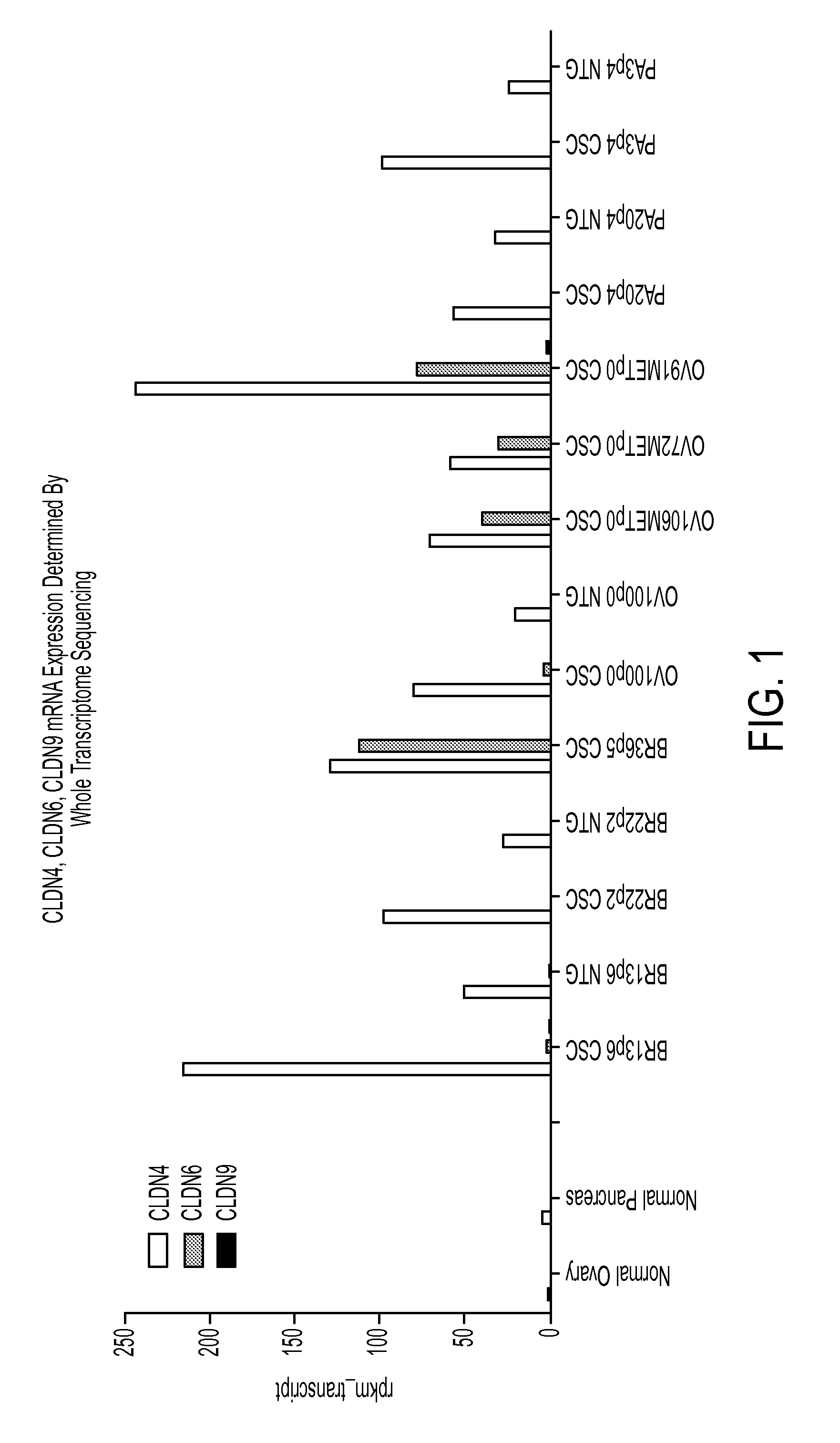
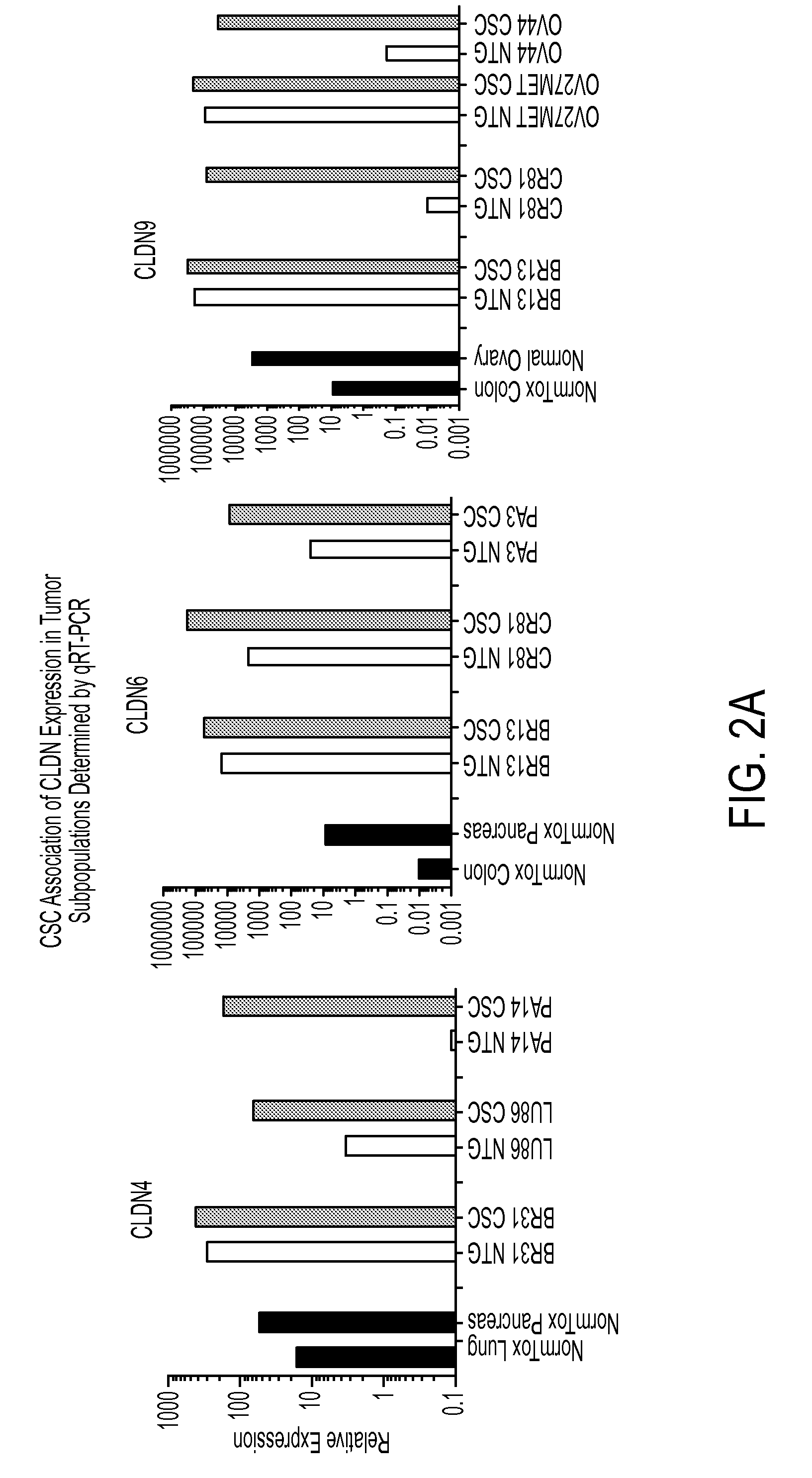
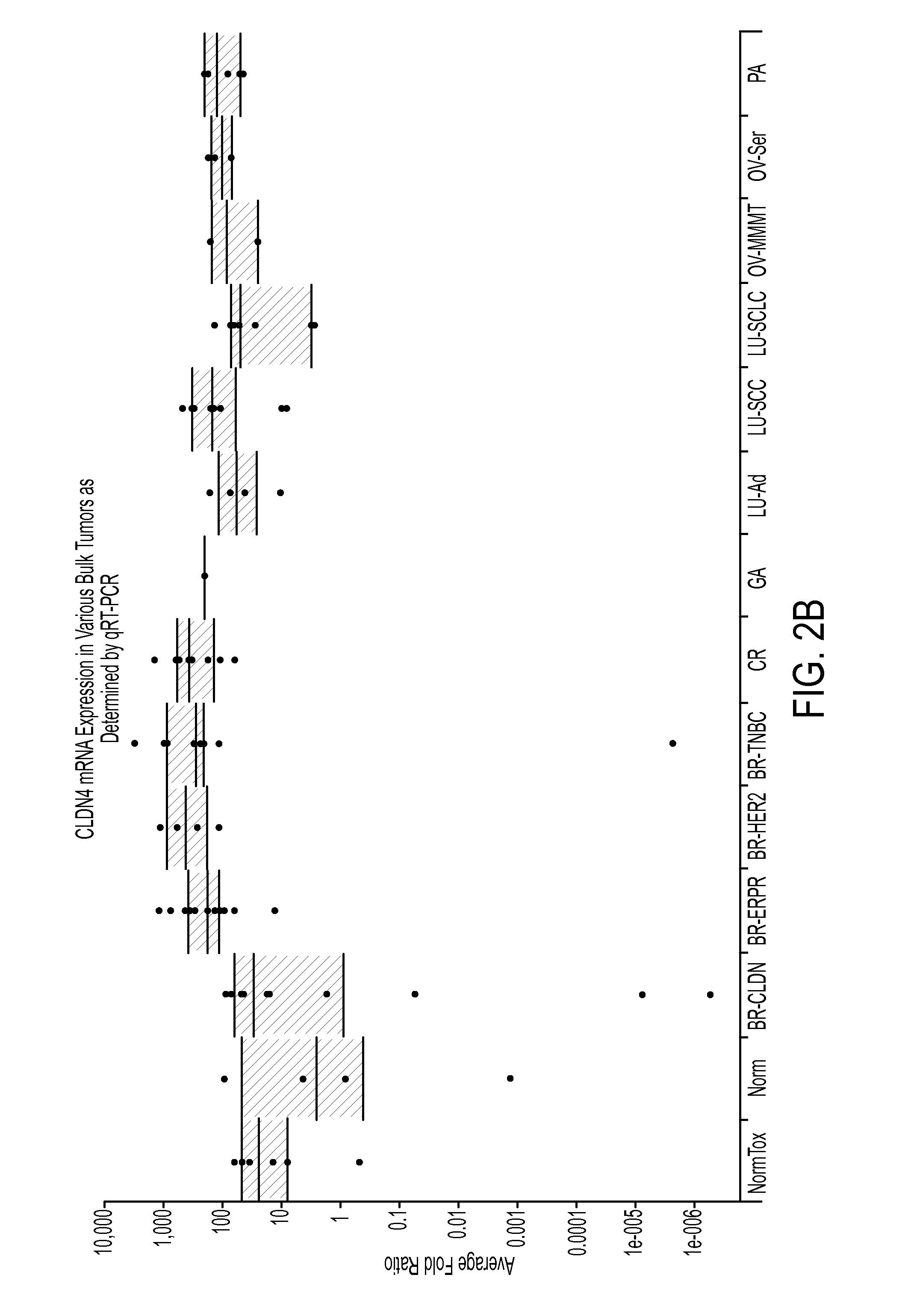
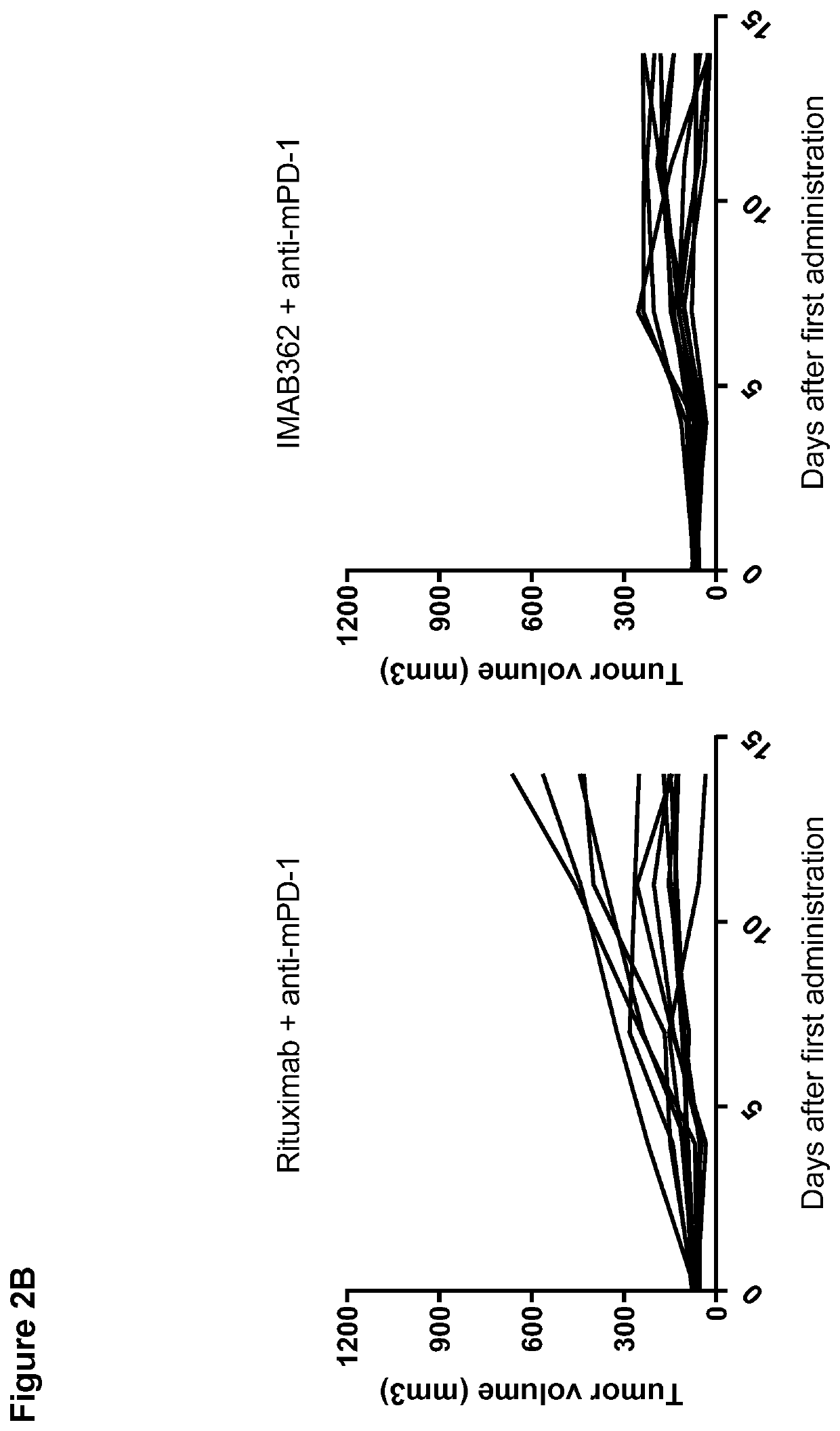
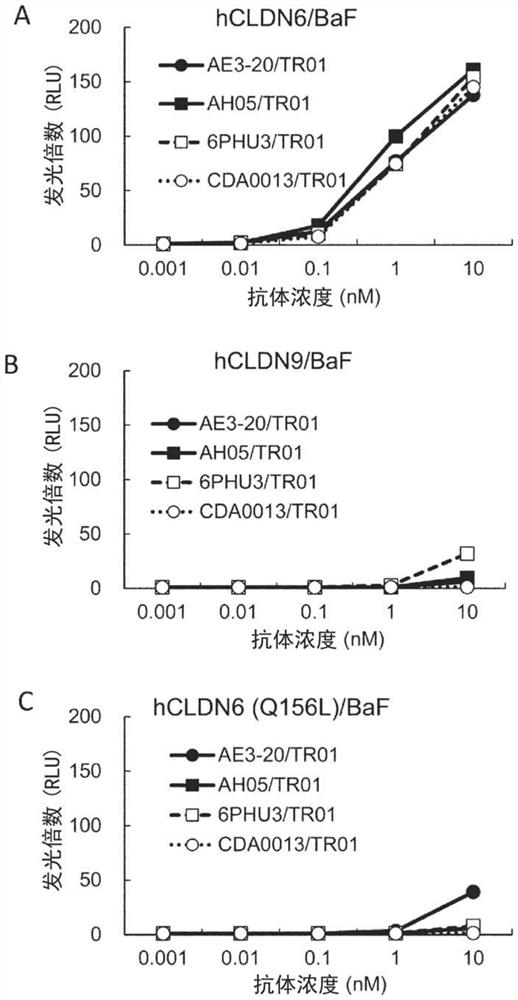
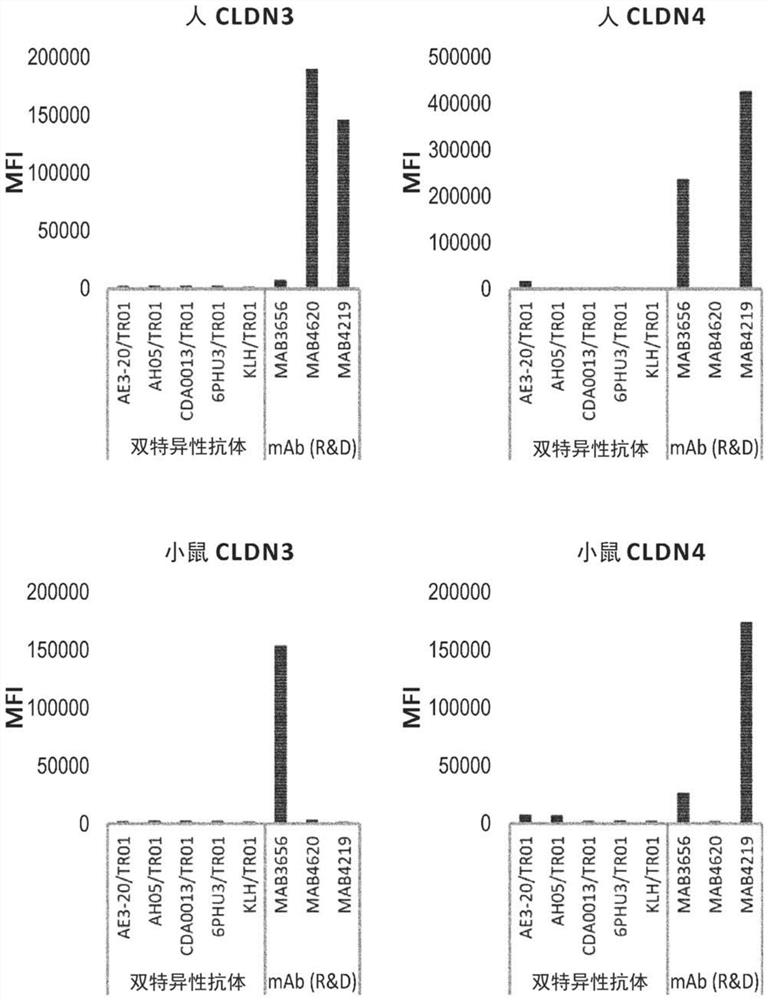
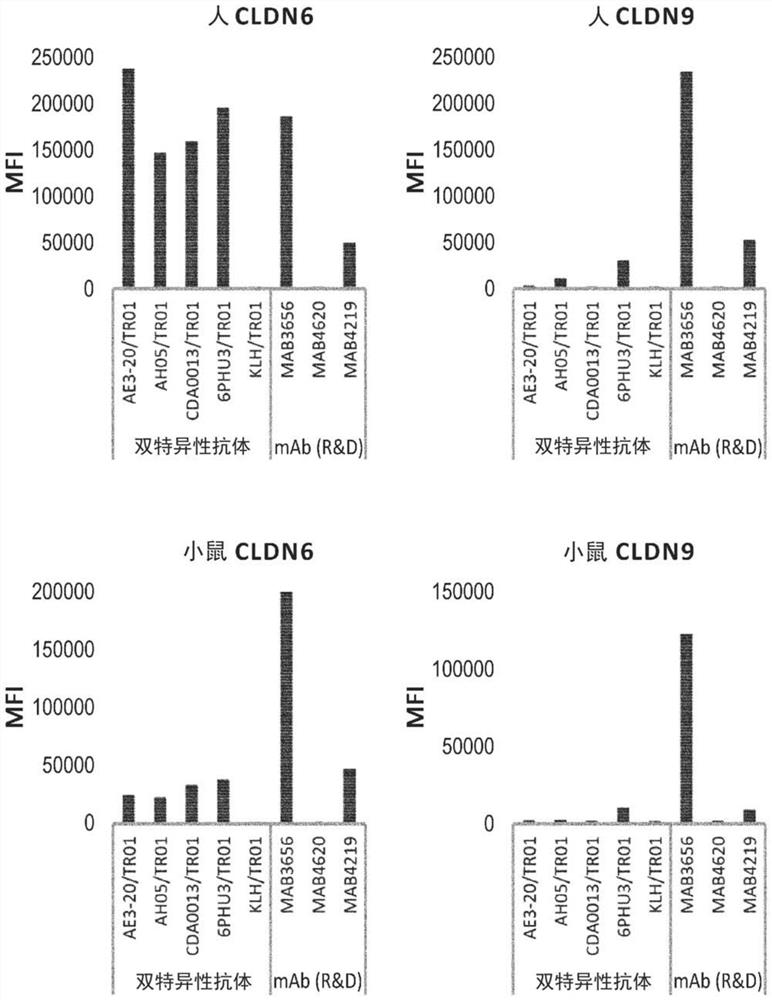
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Claudin Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
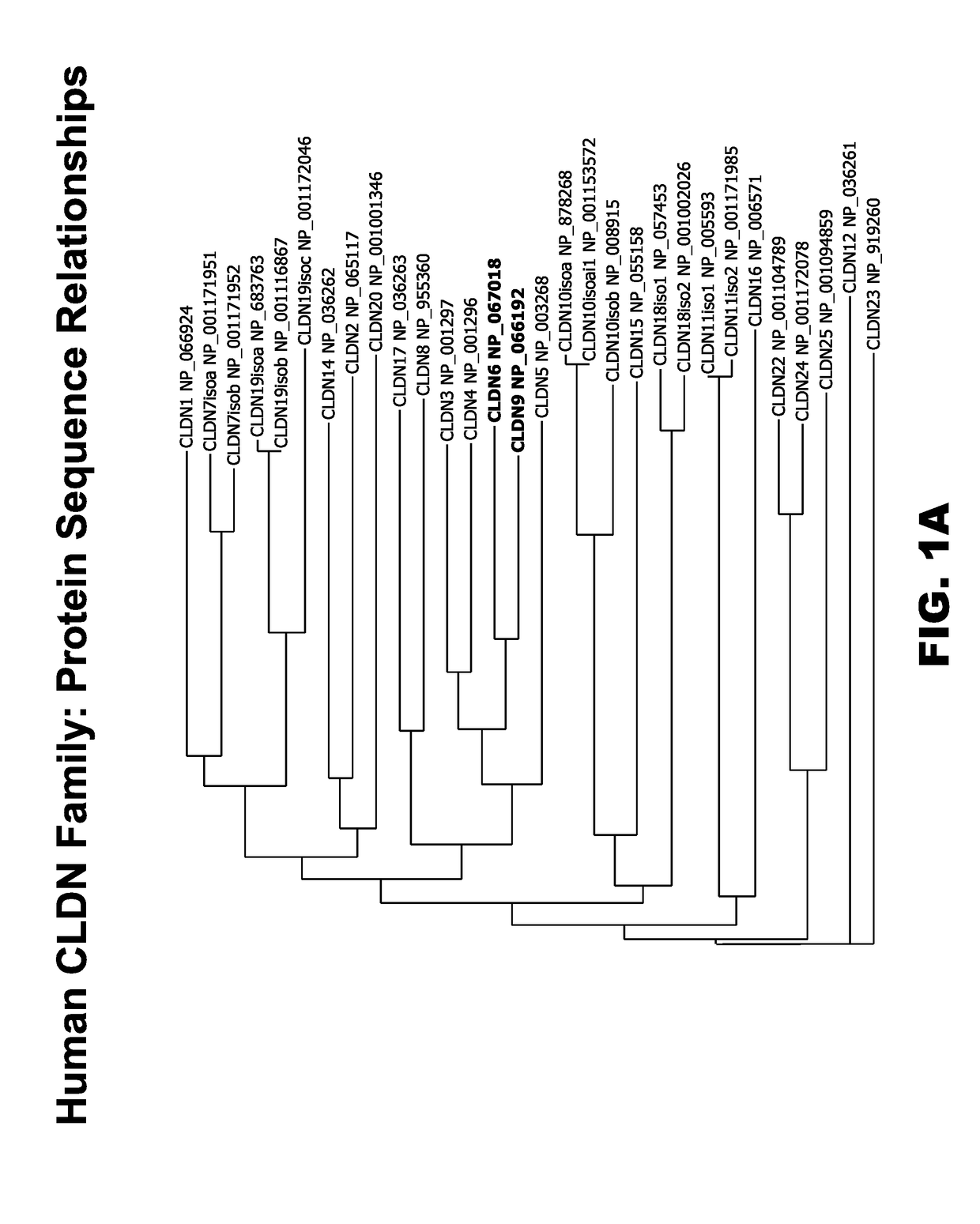
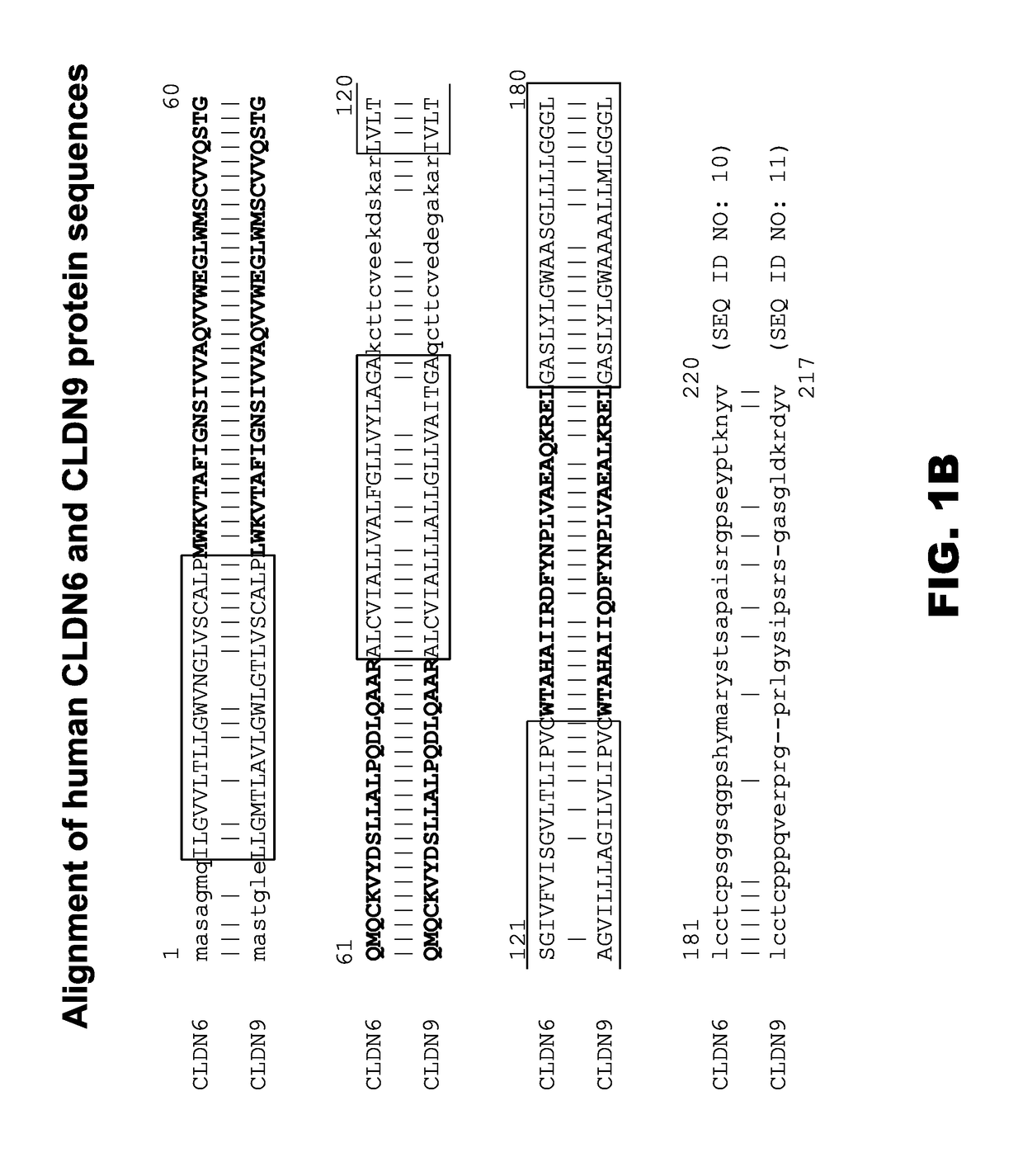
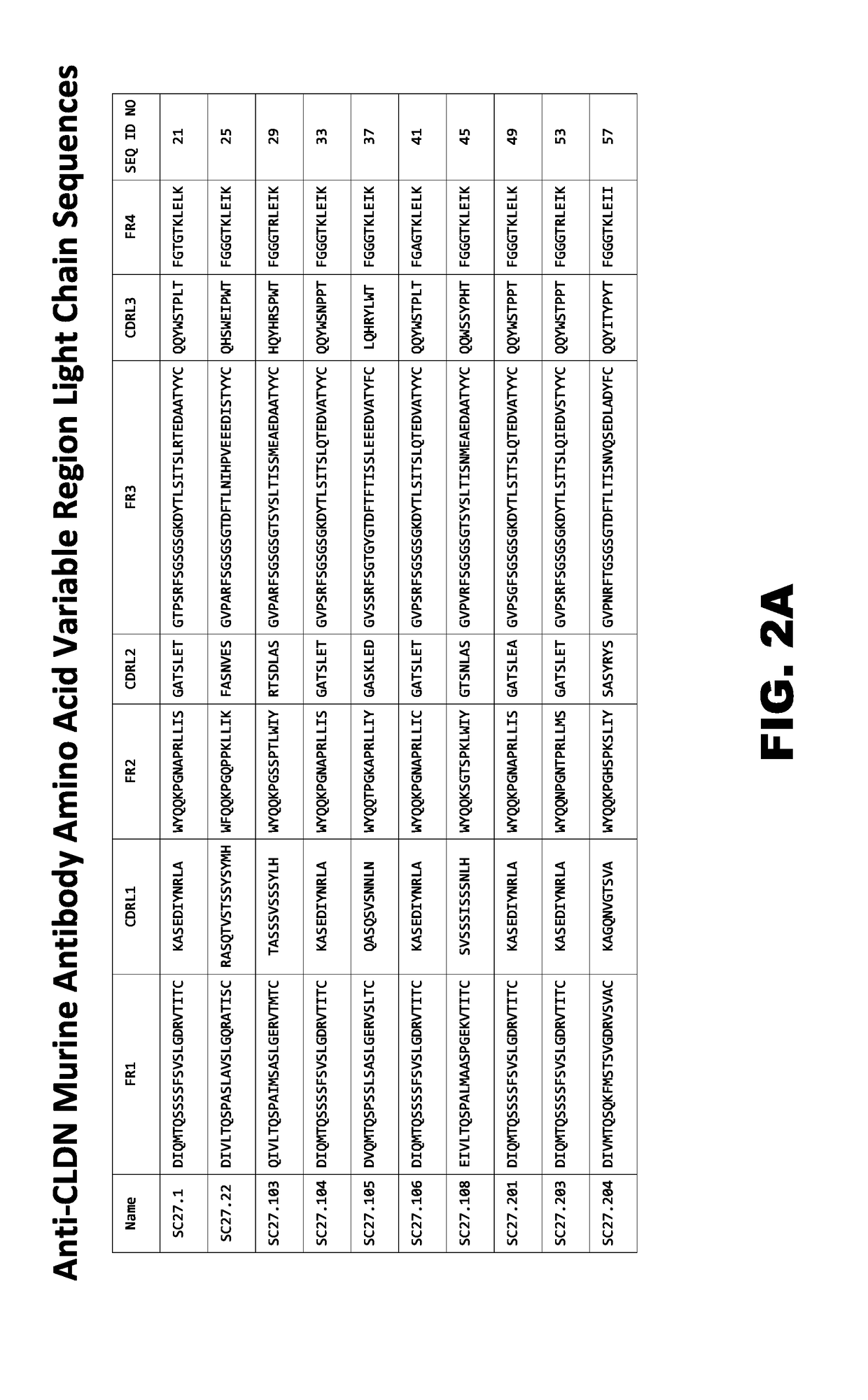
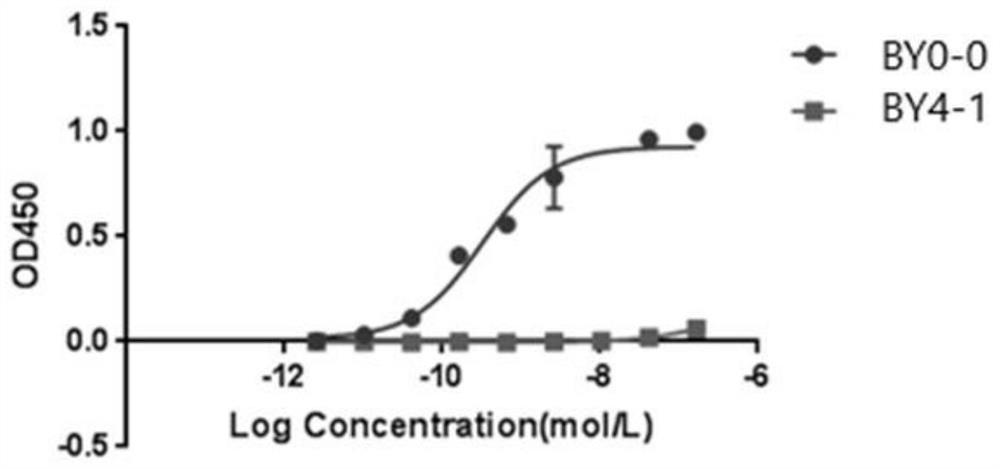
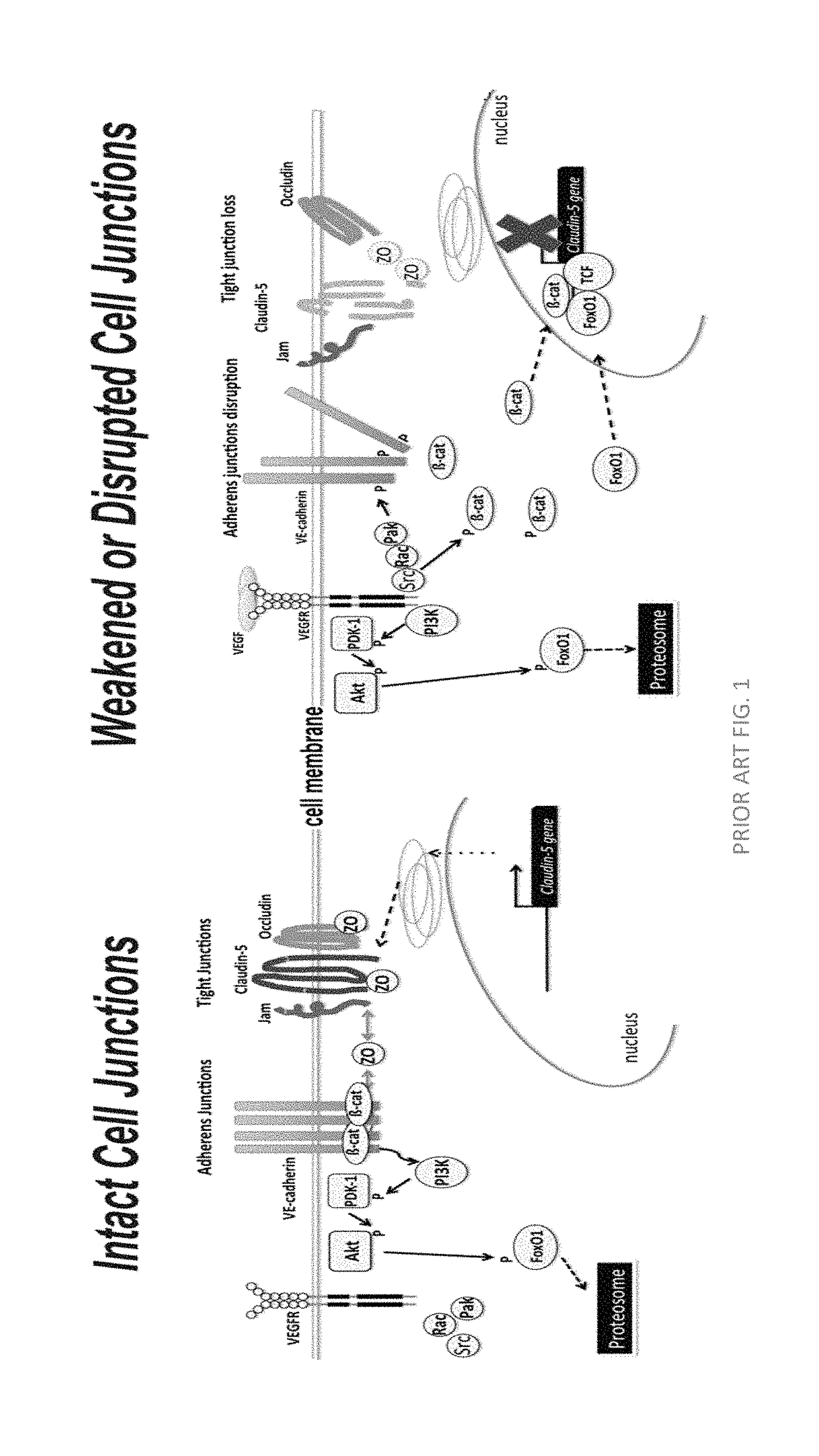
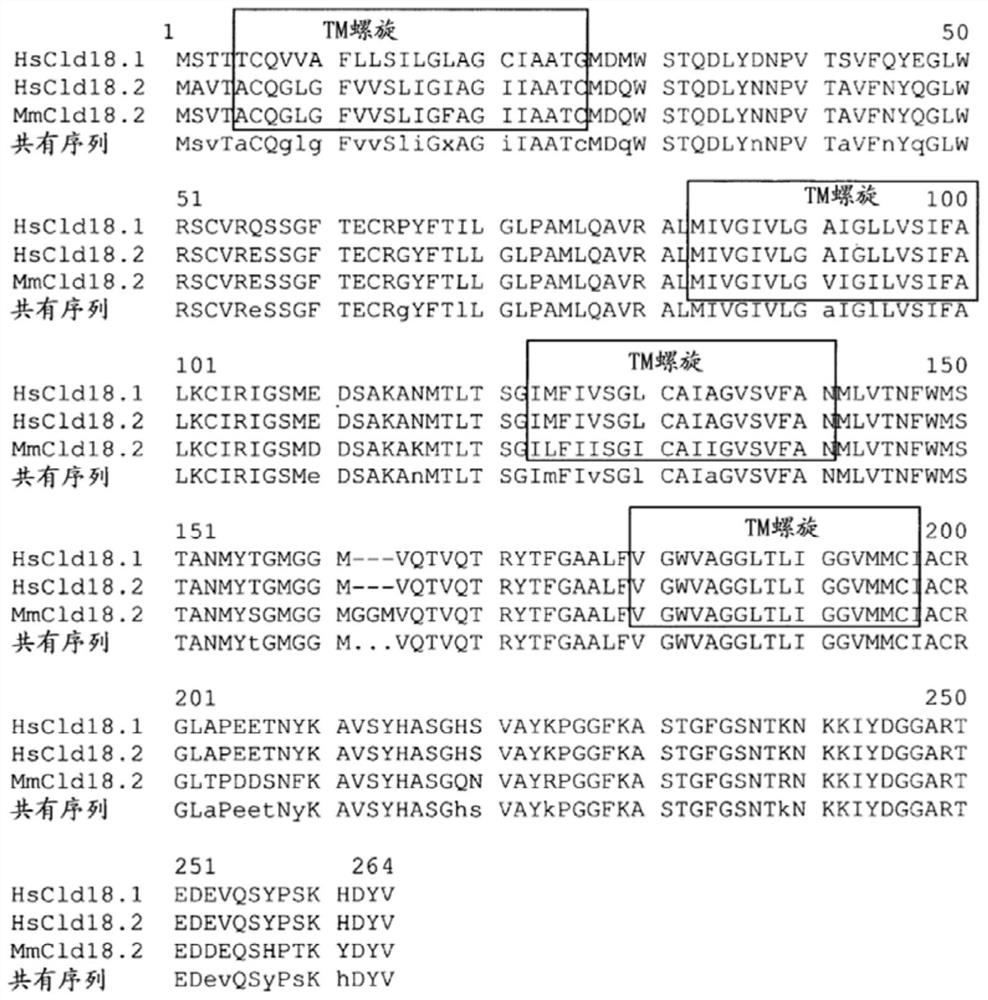
Claudins are a family of proteins which, along with occludin, are the most important components of the tight junctions) (zonulae occludentes). Tight junctions establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of an epithelium.
Nucleic acid-based methods and compositions for the detection of ovarian cancer
InactiveUS20090087849A1Easy diagnosisIncrease chanceMicrobiological testing/measurementSLPIFolate receptor 1
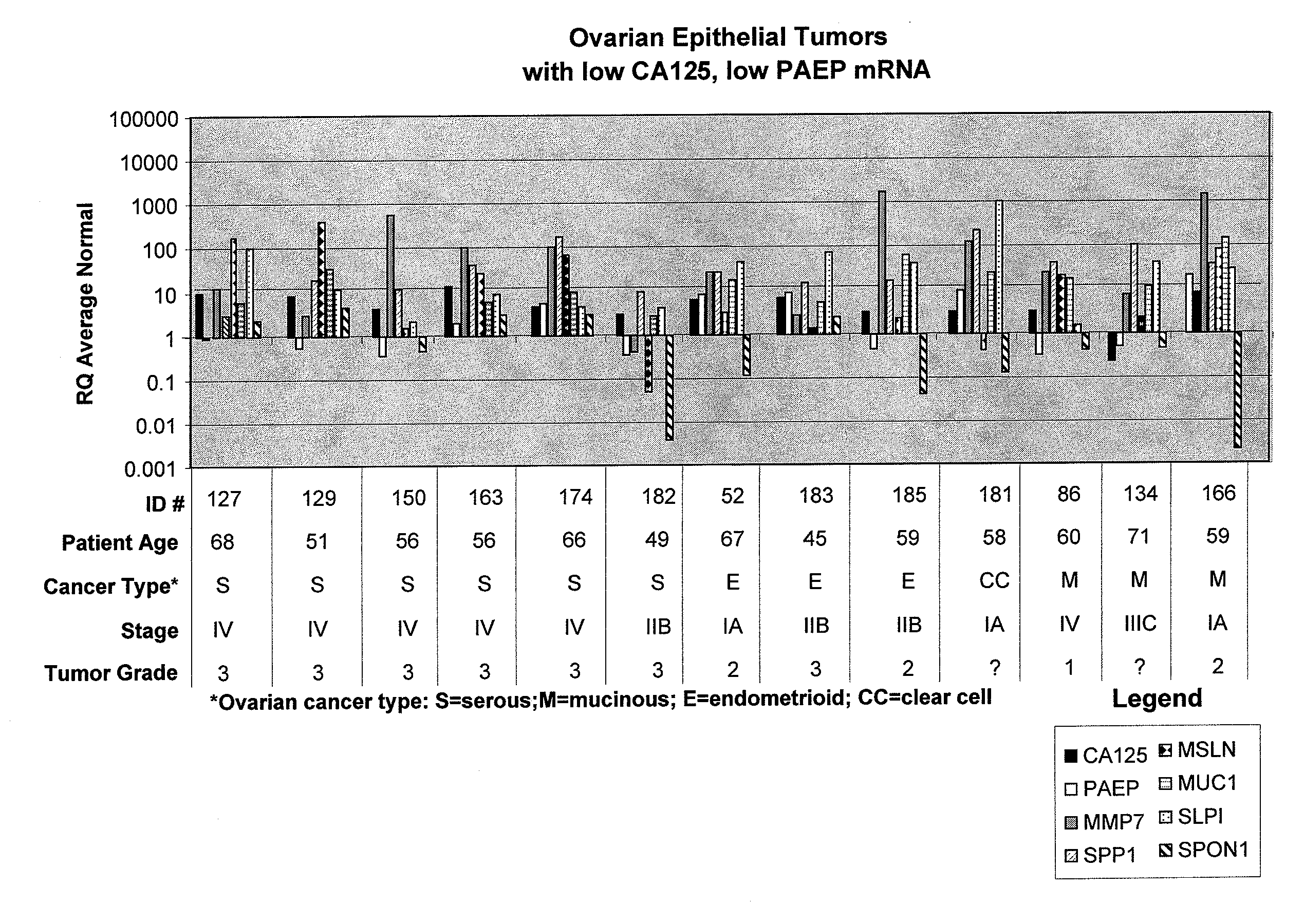
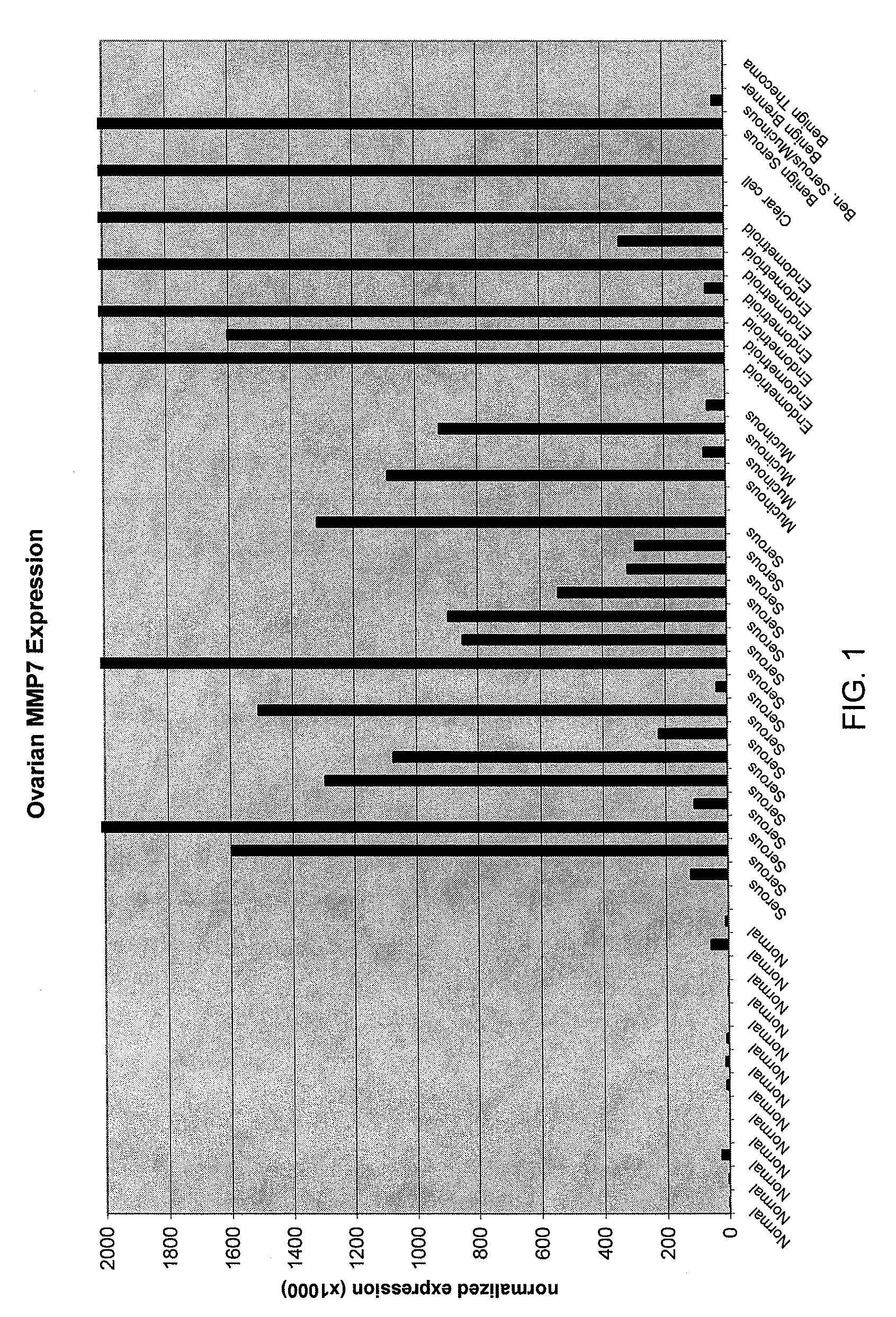
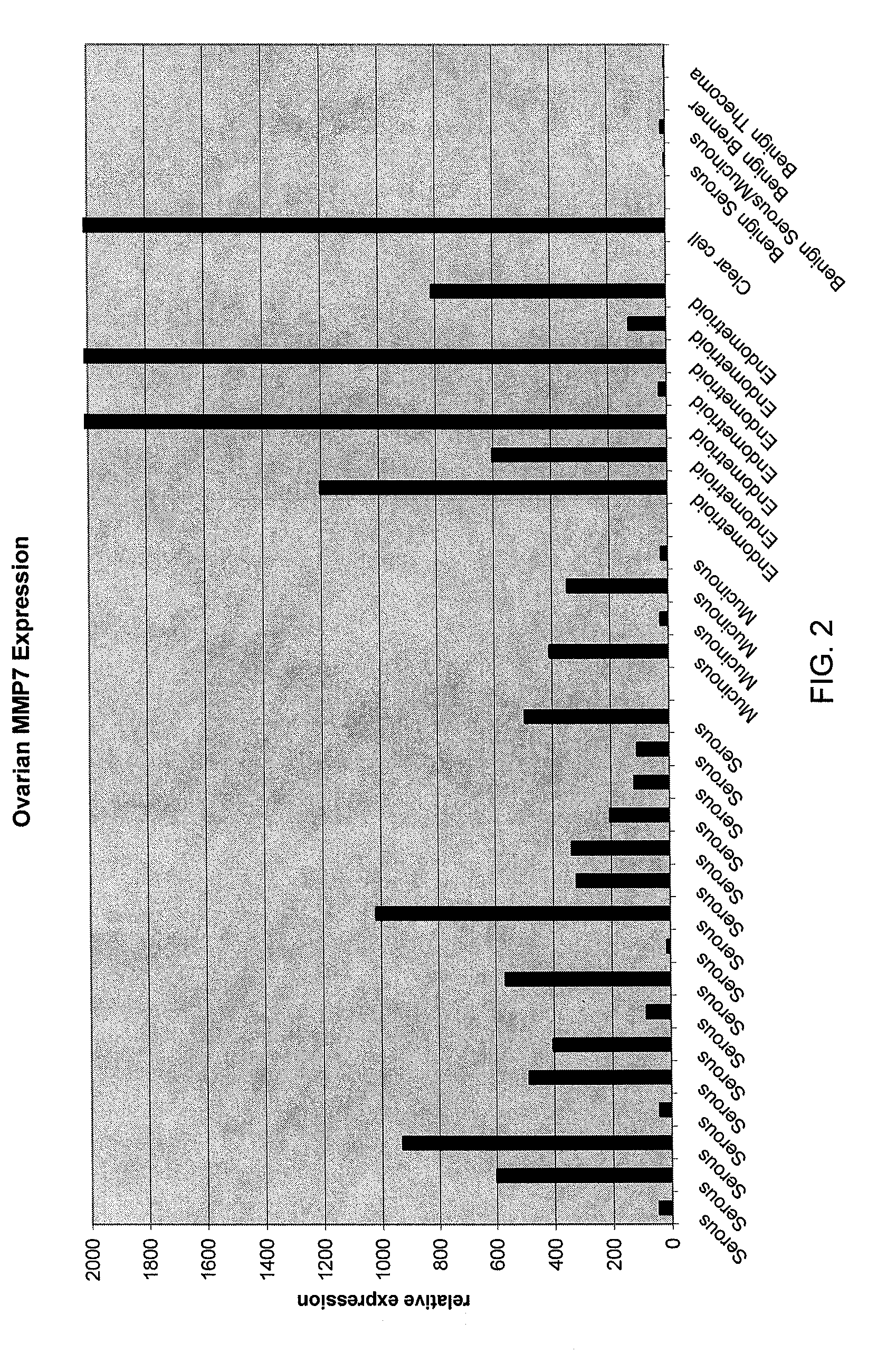
Methods and compositions for identifying ovarian cancer in a patient sample are provided. The methods of the invention comprise detecting overexpression or underexpression of at least one nucleic acid biomarker in a body sample, wherein the biomarker is selectively overexpressed or underexpressed in ovarian cancer. The body sample may be, for example, an ovarian tissue sample. The biomarkers of the invention include any nucleic acid molecule that is selectively overexpressed in ovarian cancer, including, for example, MMP-7, PAEP, CA125, HE4, PLAUR, MUC-1, SLPI, SSP1, MSLN, SPON1, interleukin-7, folate receptor 1, claudin 3, inhibin A, inhibin BB, inhibin BA, and PAI-1. Overexpression or underexpression of a biomarker of interest is detected at the nucleic acid level using such methods as real-time PCR and various nucleic acid hybridization techniques. Kits for practicing the methods of the invention are further provided.
Owner:TRIPATH IMAGING INC
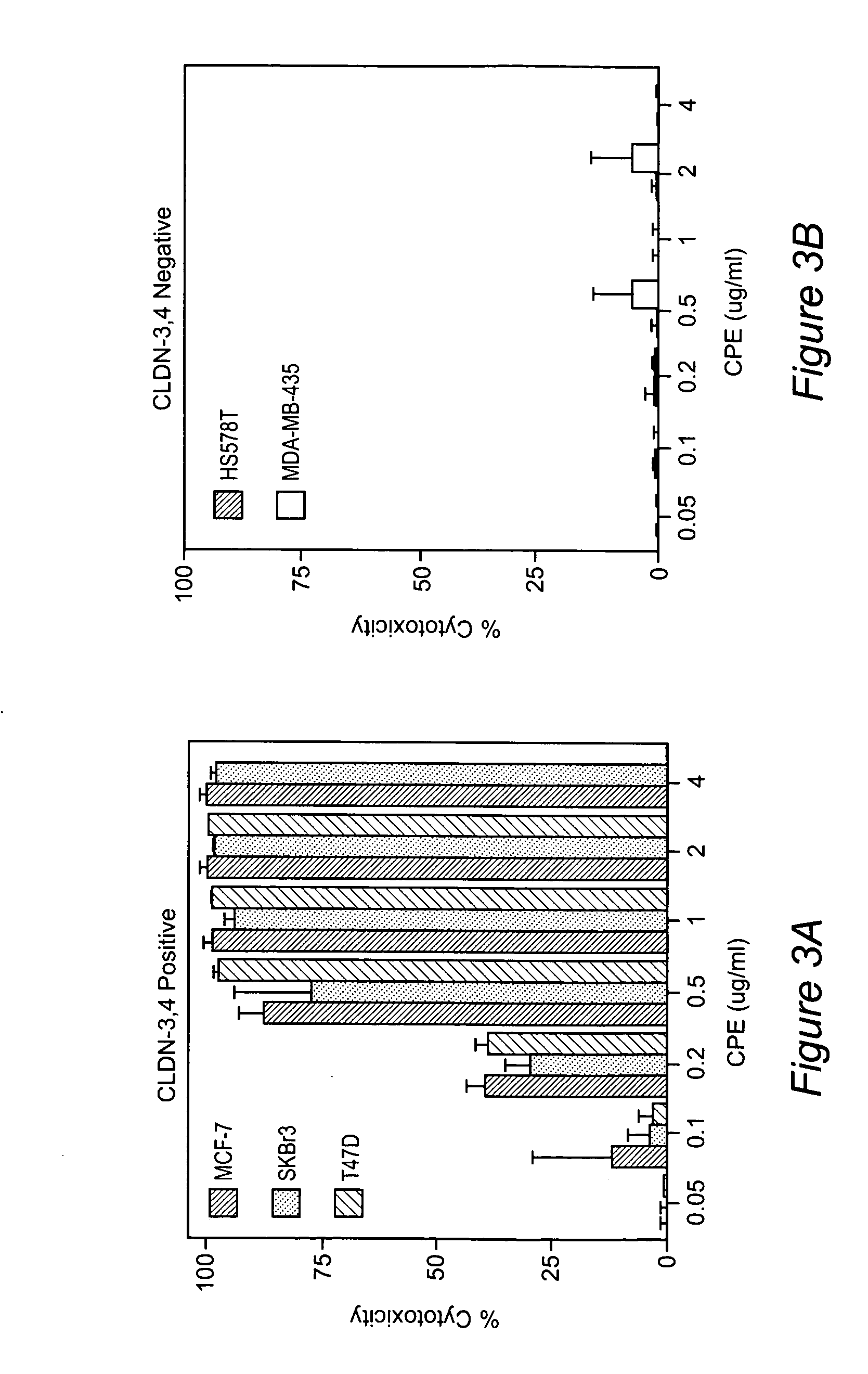
Therapy with clostridium perfringens enterotoxin to treat ovarian and uterine cancer
ActiveUS20060084594A1Low toxicityGood curative effectBacterial antigen ingredientsIn-vivo radioactive preparationsIntraperitoneal routeCancer cell
The invention discloses high levels of receptors for Clostridium perfringens enterotoxin (CPE) have been found in ovarian cancer and uterine cancer tissue samples. In addition, successful in vivo treatment of a mouse model of ovarian cancer with intraperitoneal injection of CPE is disclosed. High levels of Ep-CAM protein is also disclosed in ovarian cancer tissue samples. Thus, the invention provides a method of treating ovarian cancer and uterine cancer by administering CPE. The invention also provides a method of treating cancer in a mammal involving intraperitoneal administration of CPE, where at least some cancerous cells are located in or adjacent to the peritoneal cavity of the mammal. The invention also provides a method of treating ovarian cancer involving administering an anti-Ep-CAM antibody. The invention also provides a method of treating cancers expressing claudin-3 or claudin-4 by administering an antibody against claudin-3 and / or an antibody against claudin-4. The invention also provides a method of protecting a mammal from CPE toxicity involving administering a protective agent that binds to claudin-3 and / or claudin-4 and inhibits CPE binding to claudin-3 and / or claudin-4.
Owner:YALE UNIV
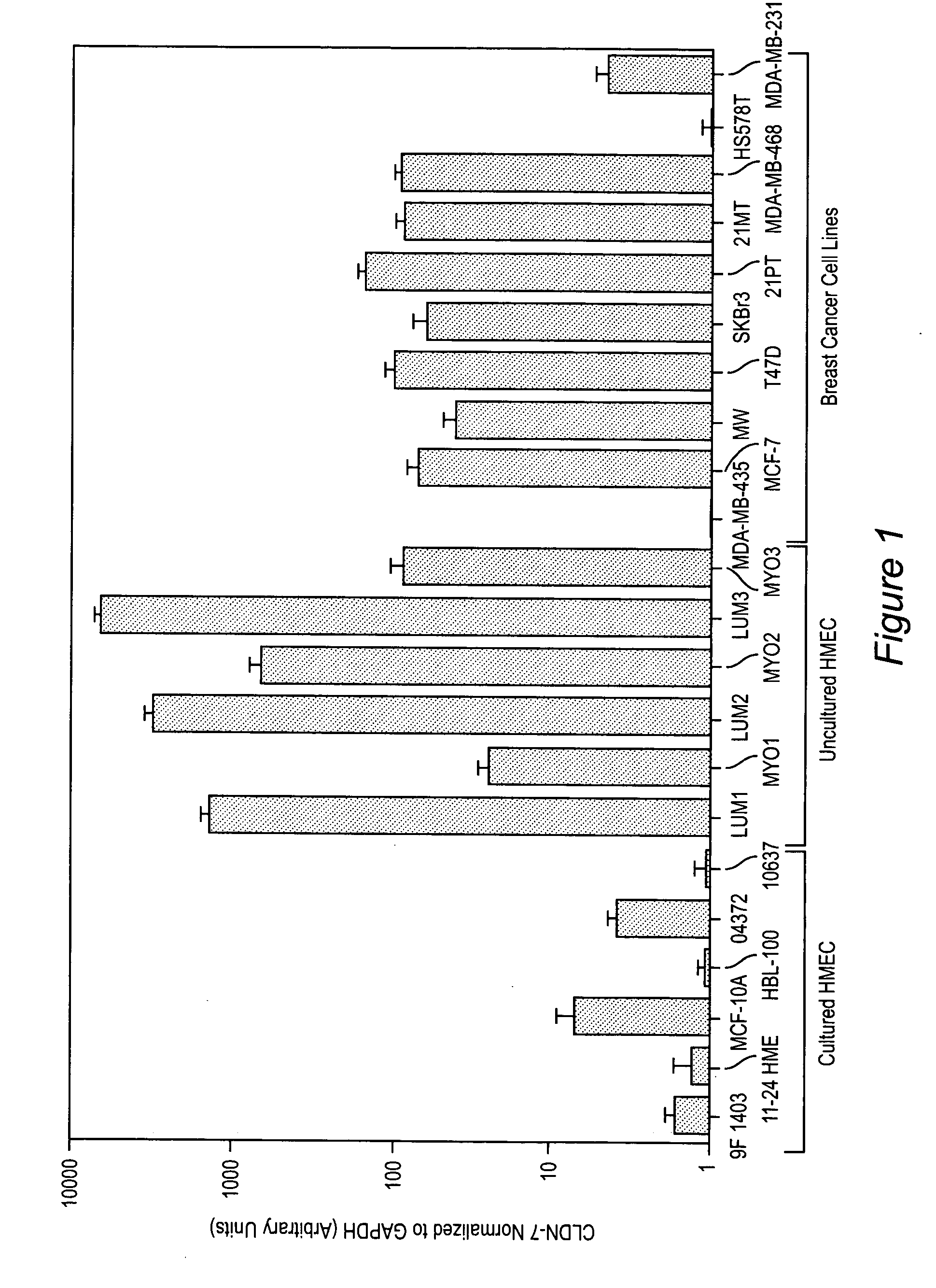
Claudins as markers for early detection, diagnosis, prognosis and as targets of therapy for breast and metastatic brain or bone cancer
InactiveUS20050074798A1Decrease cell-to-cell adhesionPromote cell migrationPeptide/protein ingredientsMicrobiological testing/measurementCancer cellBacterial enterotoxins
Methods of diagnosis, prognosis, and treatment of breast cancer, and of metastatic brain cancer, are provided The diagnostic and prognostic methods involve the immunohistochemical detection of the level of expression of the proteins claudin 1, 3, 4, and 7 in tissue or cell samples. Claudins 1 and 7 are underexpressed in the majority of breast cancers, and claudins 3 and 4 are overexpressed. The methods of treatment involve the use of Clostridium perfringens enterotoxin (or a variant thereof) to lyse metastatic cancer cells in the brain and bone that overexpress claudins 3 and 4.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
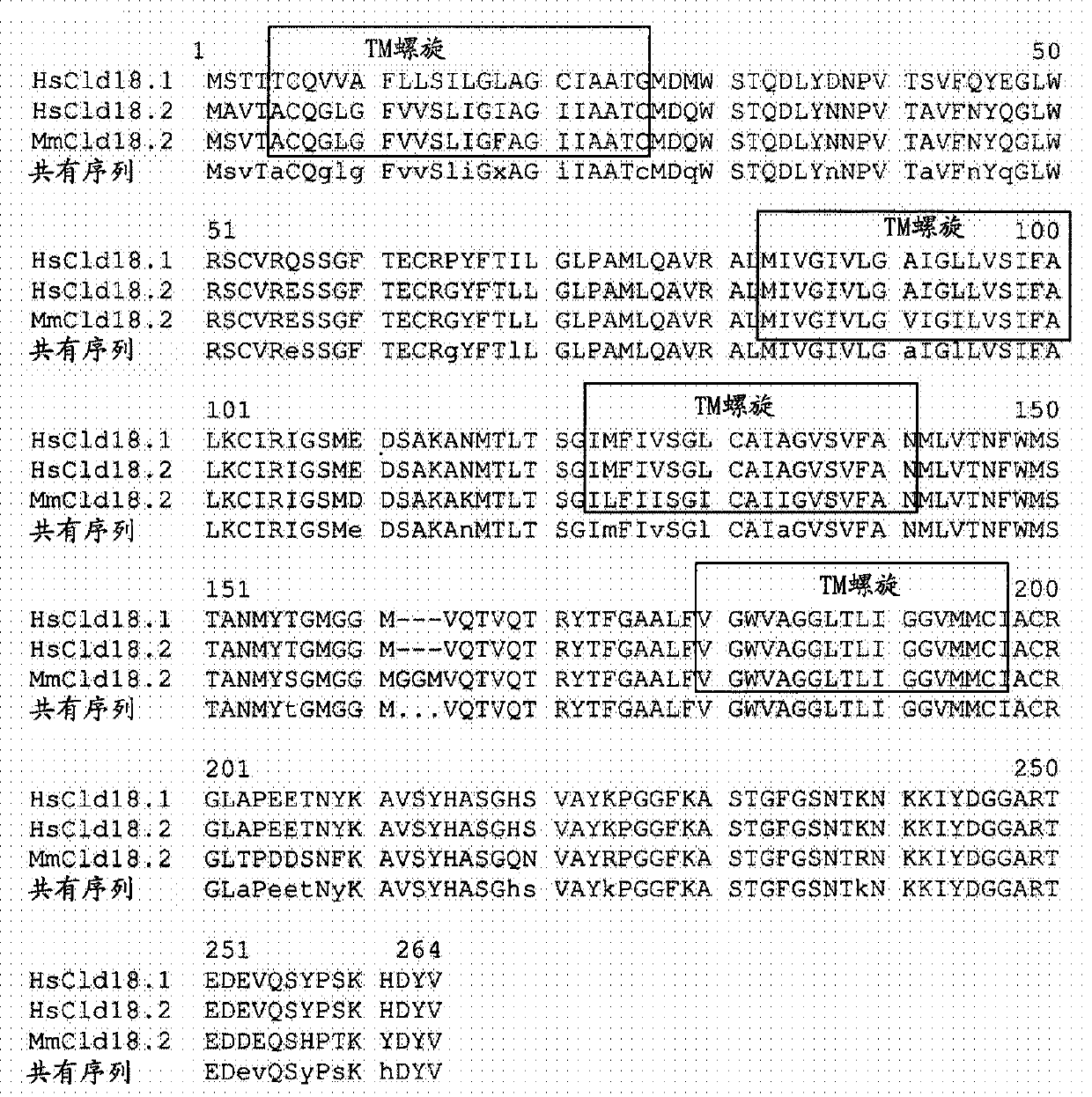
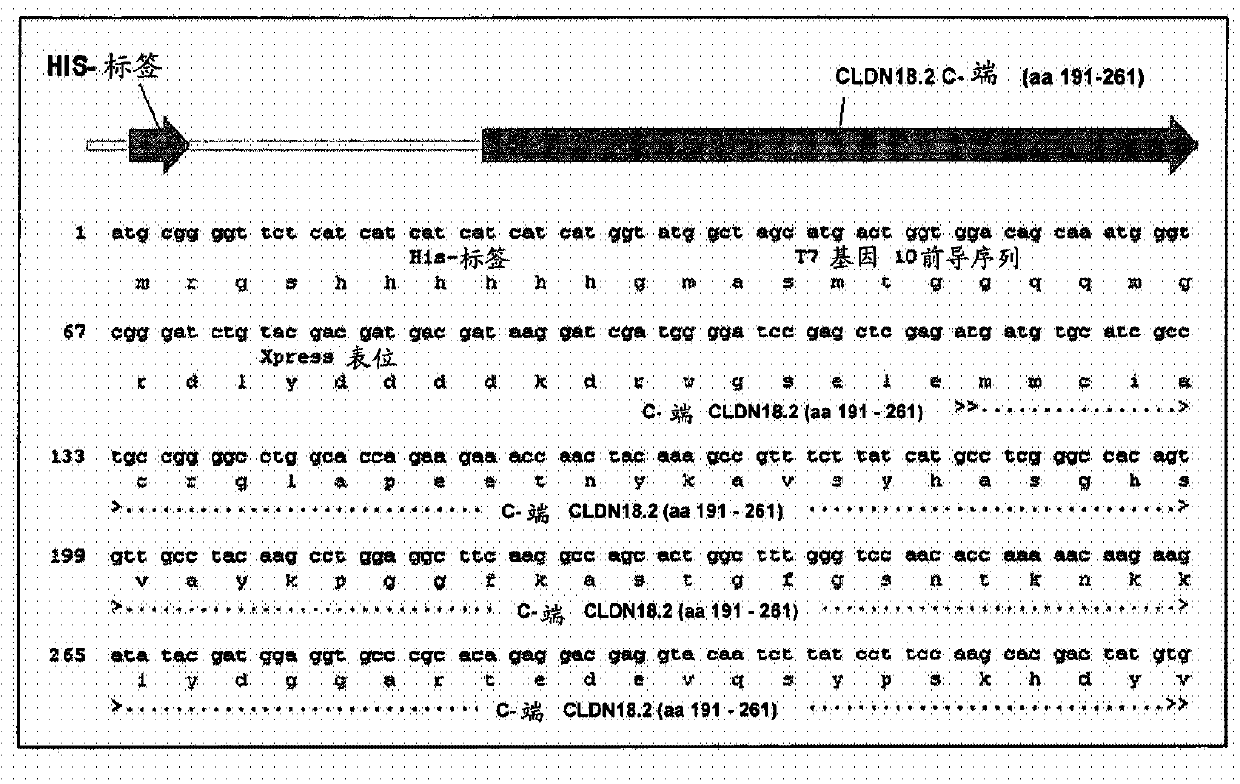
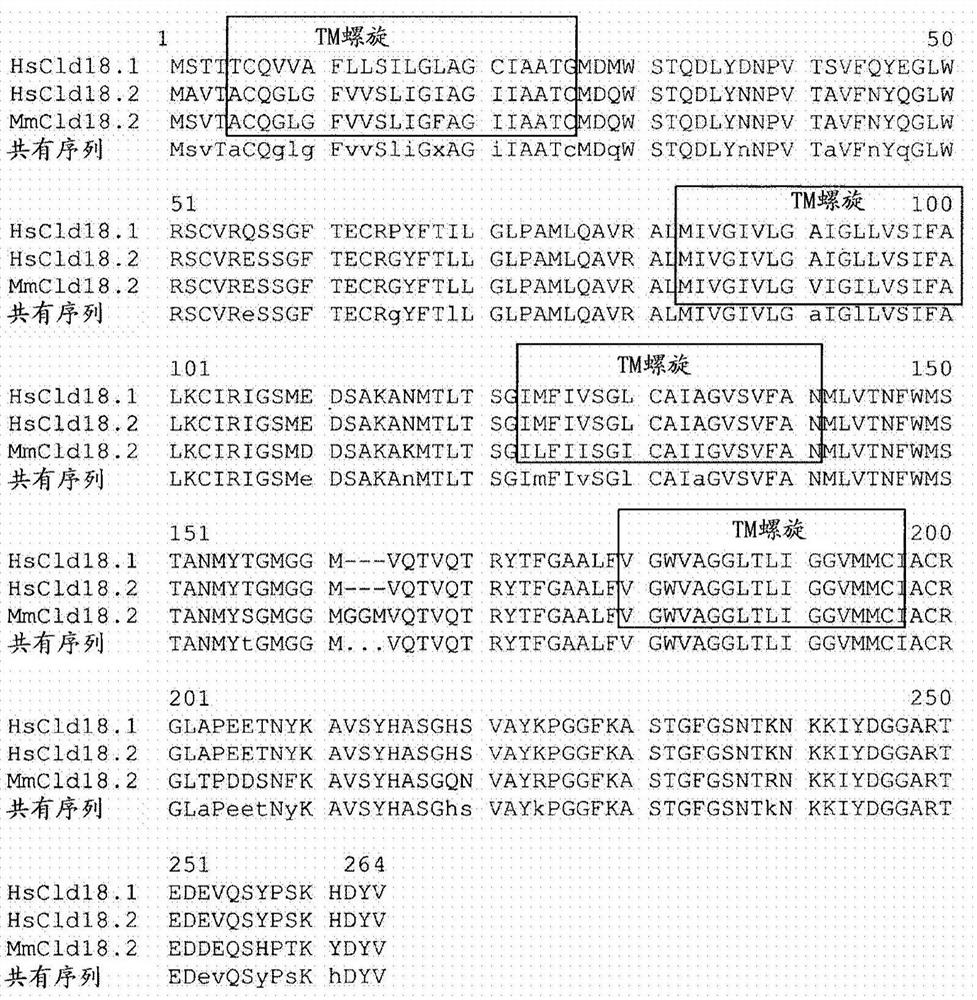
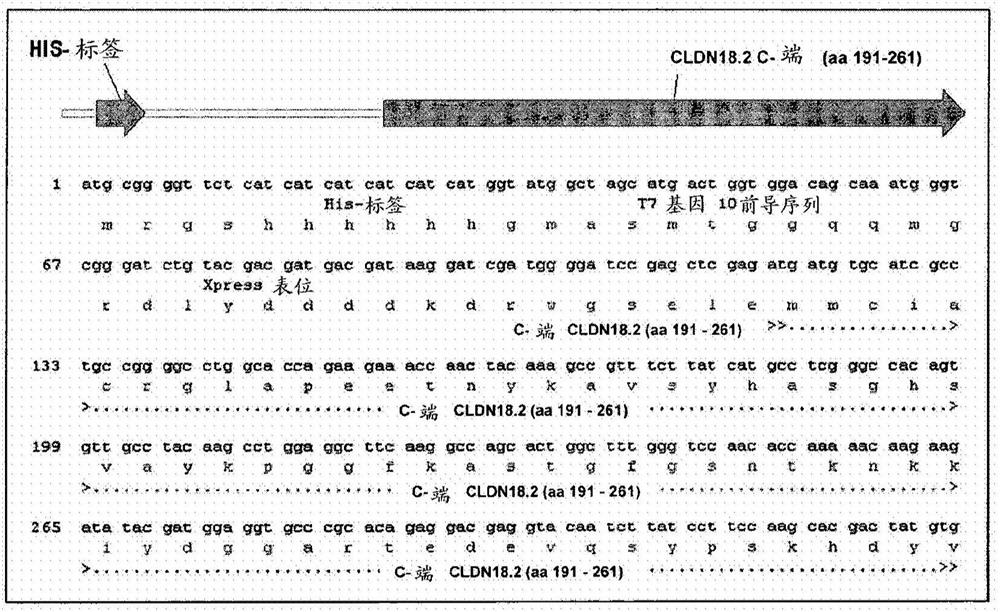
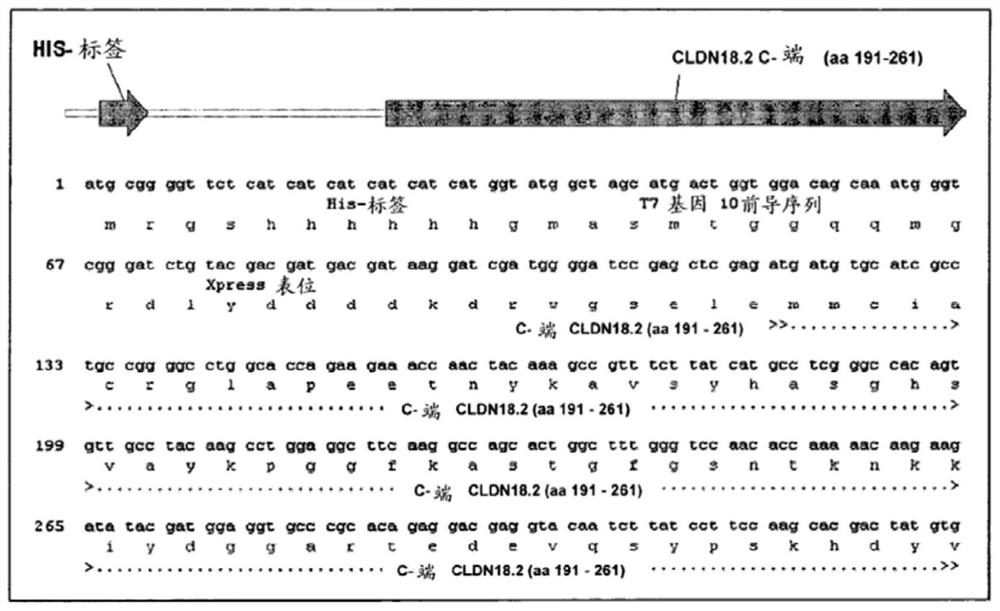
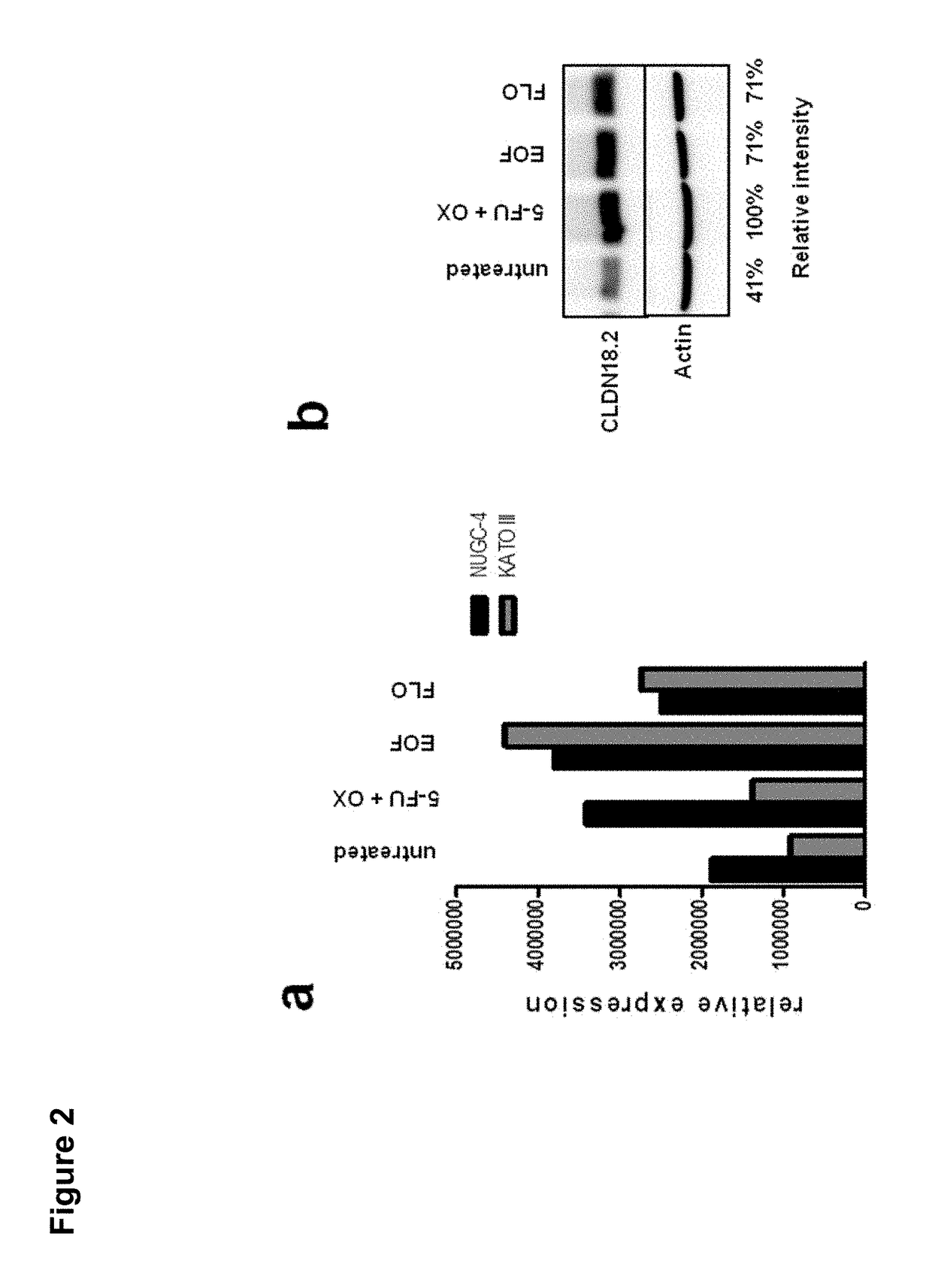
Antibodies Against Claudin 18.2 Useful in Cancer Diagnosis
ActiveCN108047331AMicroorganism based processesImmunoglobulins against cell receptors/antigens/surface-determinantsCancers diagnosisCancer research
The invention relates to antibodies against claudin 18.2 useful in cancer diagnosis. The invention relates to antibodies directed against an epitope located within the C-terminal portion of CLDN18.2 which are useful, for example, in diagnosing cancer and / or in determining whether cancer cells express CLDN18.2.
Owner:ASTELLAS PHARMA INC +1
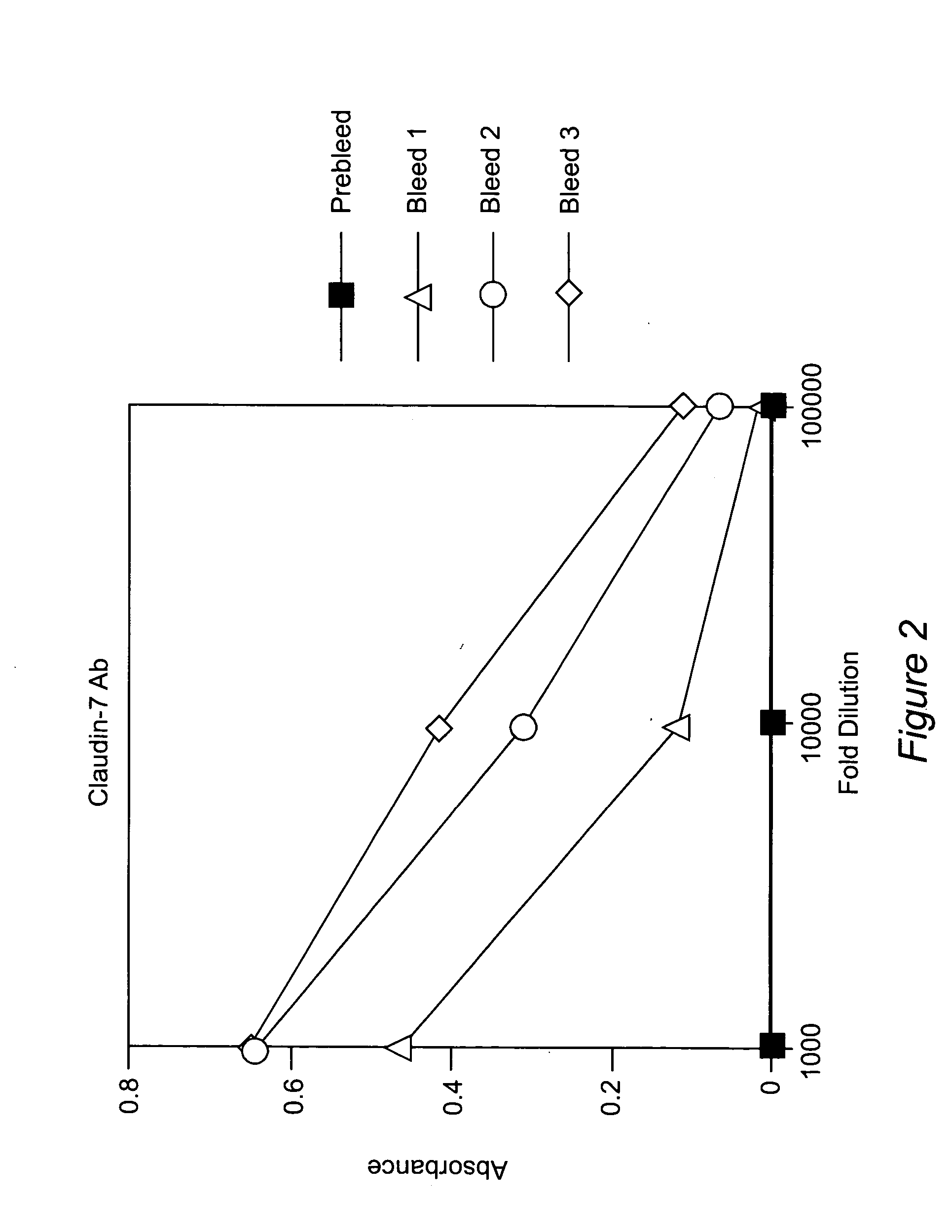
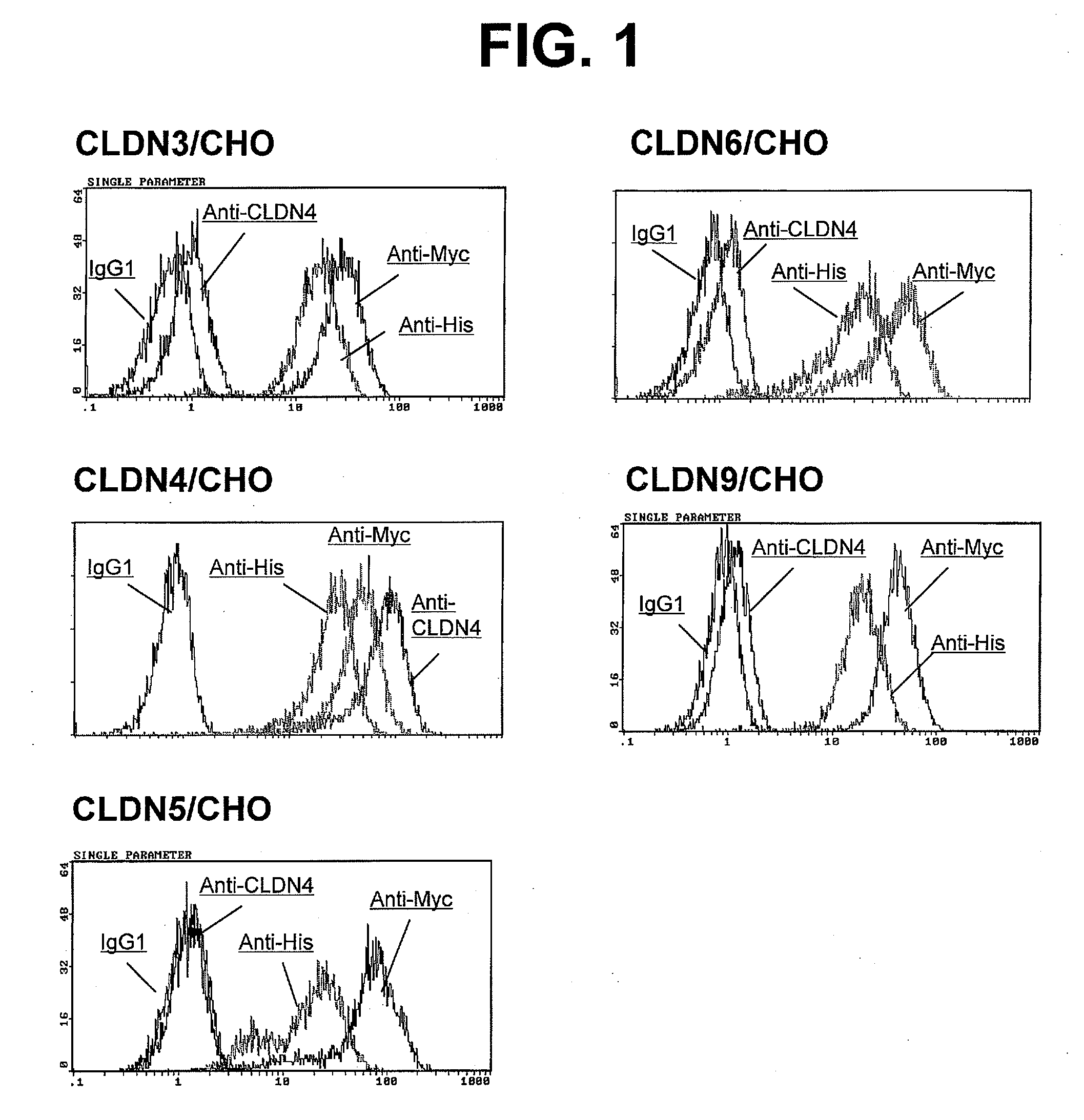
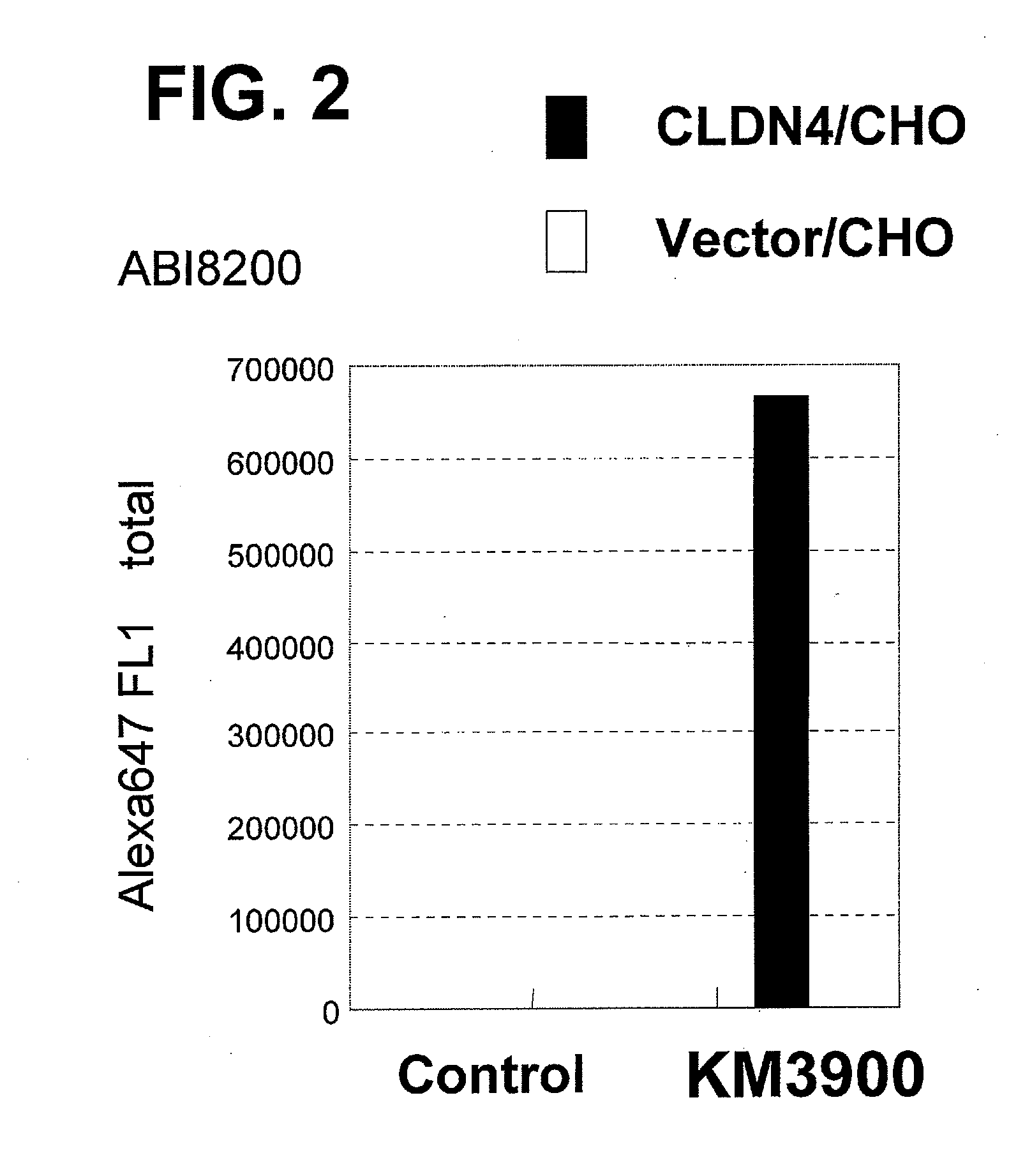
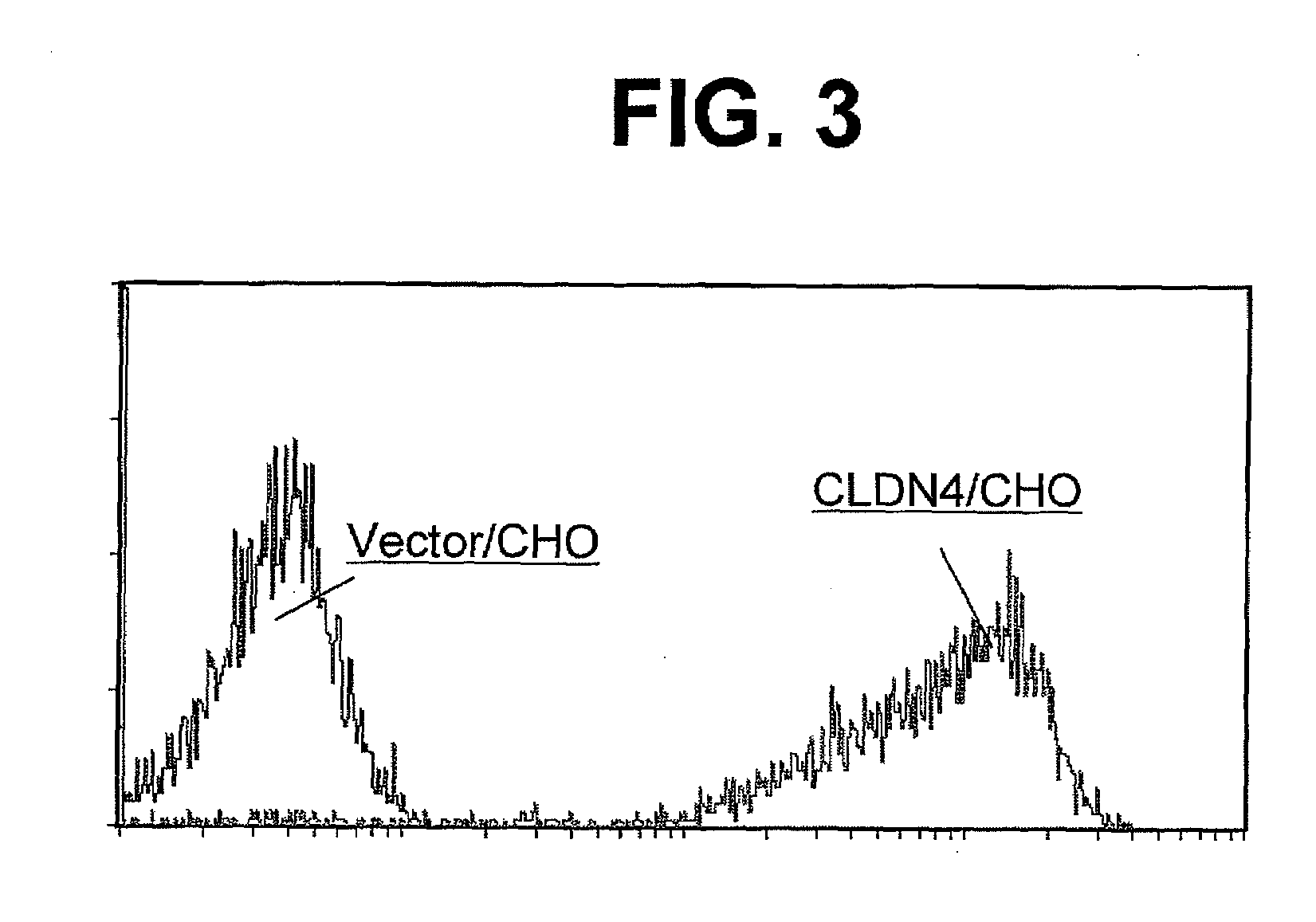
Anti-claudin-4 antibody
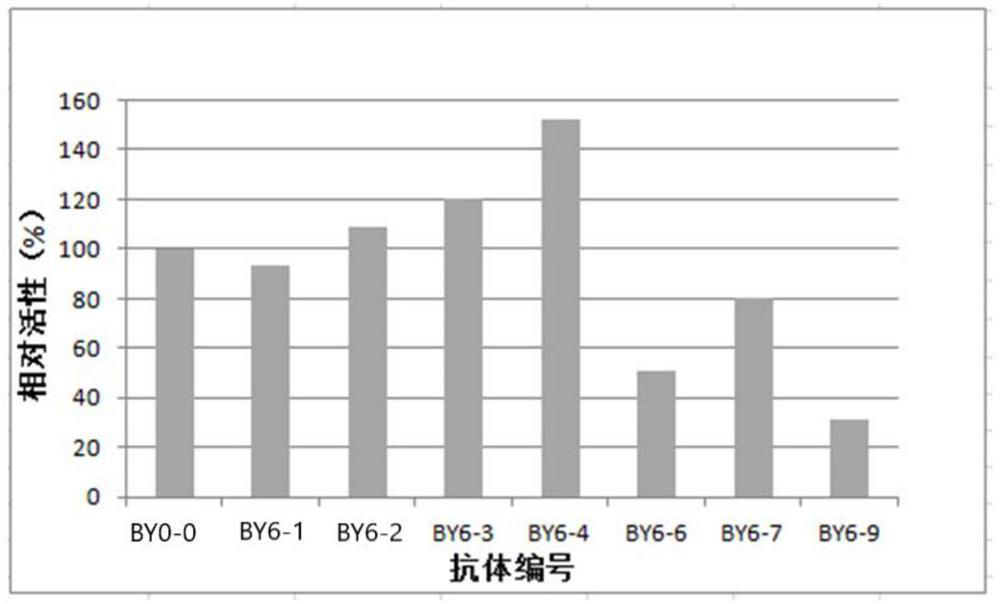
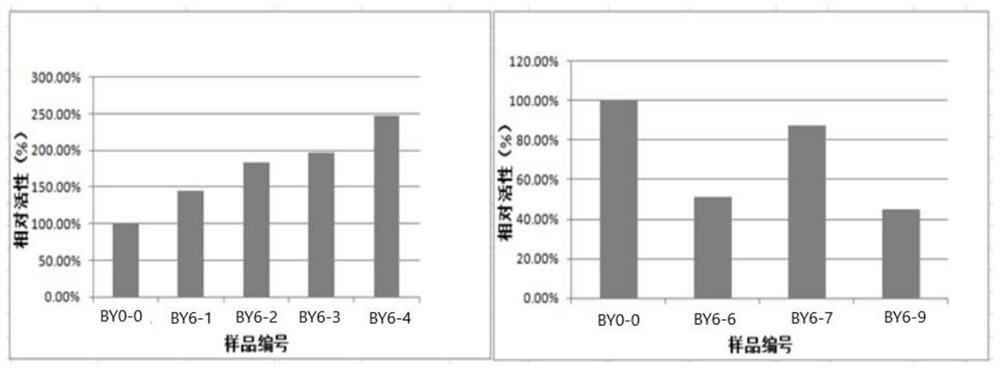
An object of the present invention is to provide a monoclonal antibody which is useful as a diagnostic agent or a therapeutic agent for a disease relating to a polypeptide encoded by Claudin-4 (hereinafter referred to as “CLDN4”) gene or a polypeptide encoded by a Claudin-3 (hereinafter referred to as “CLDN3”) gene, or a method for using the same. Accordingly, the present invention provides a monoclonal antibody or an antibody fragment thereof, which specifically recognizes three-dimensional structure of an extracellular region of CLDN4 and binds to the extracellular region; a monoclonal antibody or an antibody fragment thereof, which specifically recognizes three-dimensional structure of both of extracellular regions of CLDN3 and CLDN4 and binds to the extracellular regions; a hybridoma which produces the antibody; a DNA which encodes the antibody; a vector which comprises the DNA; a transformant obtained by transforming the vector; a process for producing an antibody or an antibody fragment thereof using the hybridoma or the transformant; and a diagnostic agent or a therapeutic agent for a disease relating to a polypeptide encoded by CLDN4 gene and / or CLDN3 gene using the antibody or the antibody fragment.
Owner:KYOWA HAKKO KIRIN CO LTD
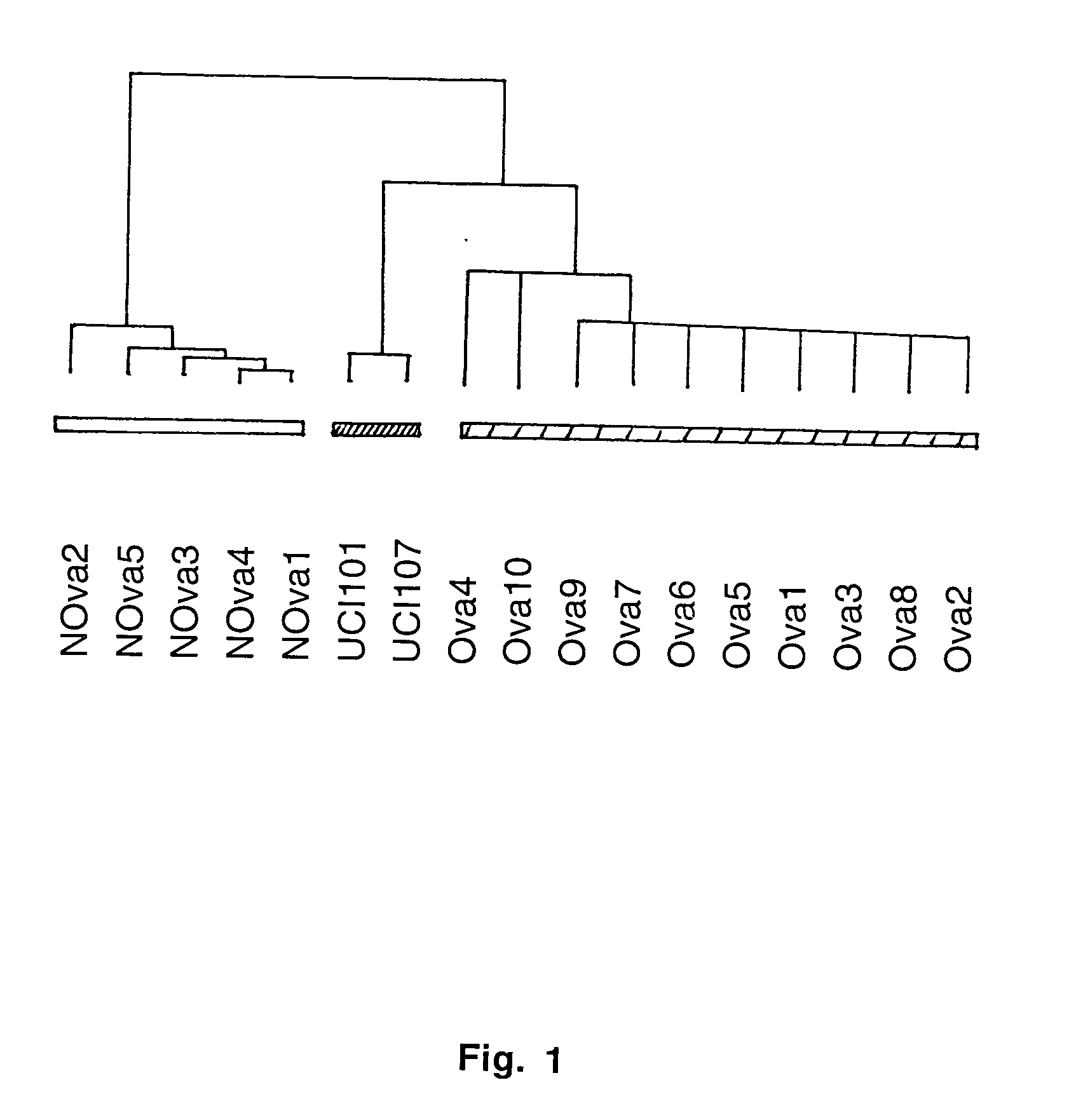
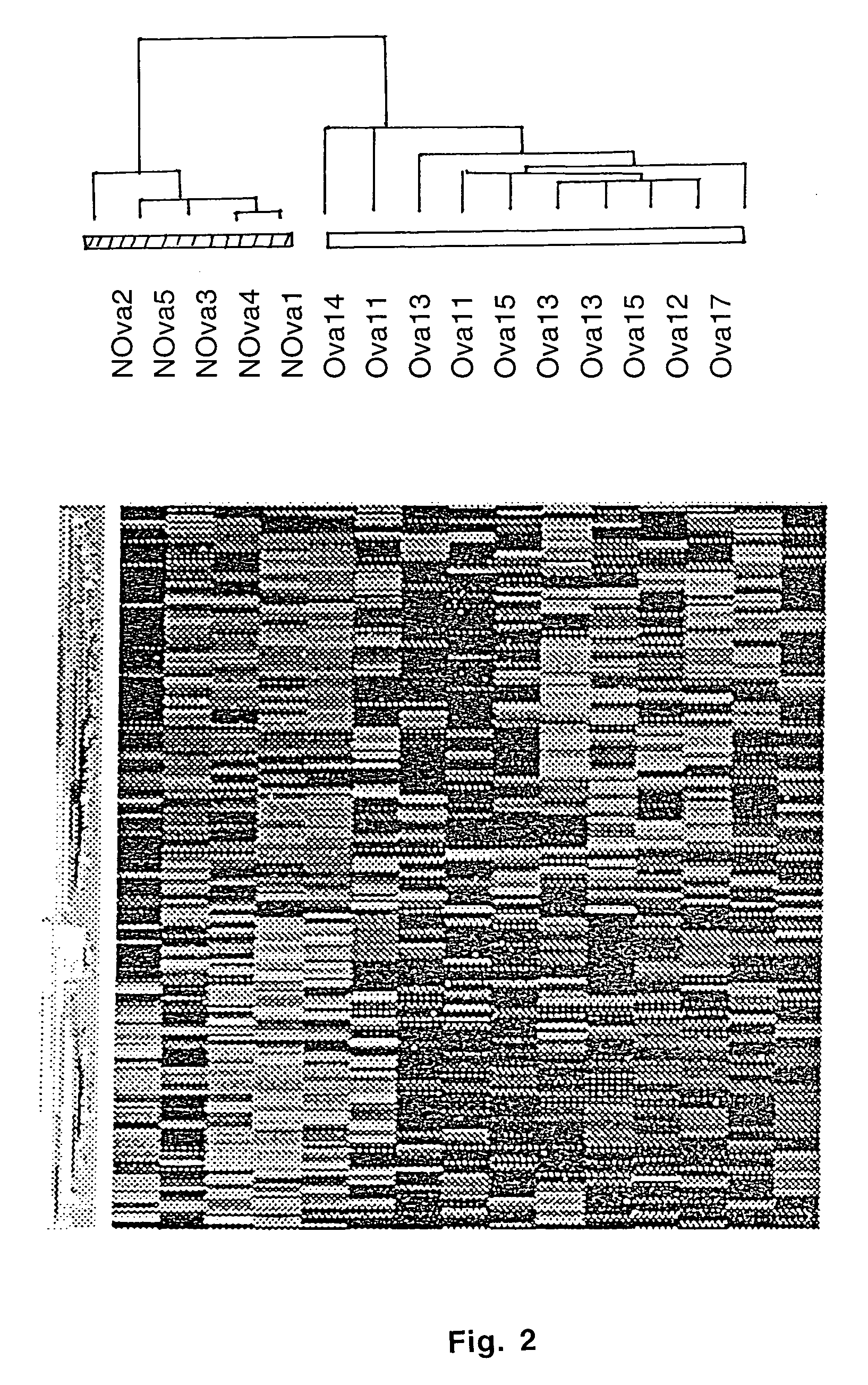
Gene expression profiling in primary ovarian serous papillary tumors and normal ovarian epithelium
InactiveUS20050048535A1Highligthing the divergence of gene expressionBioreactor/fermenter combinationsNanotechAbnormal tissue growthKinin
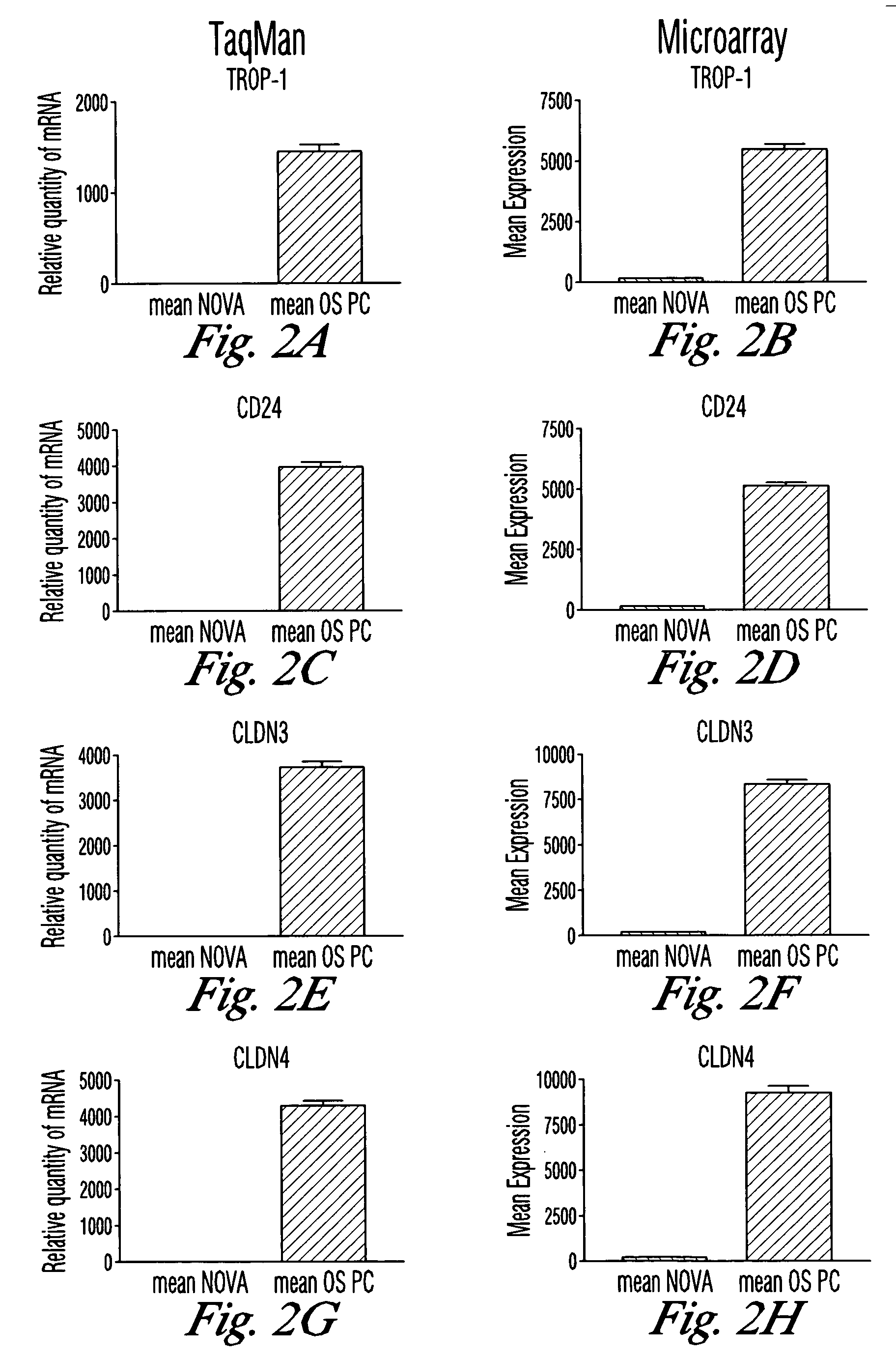
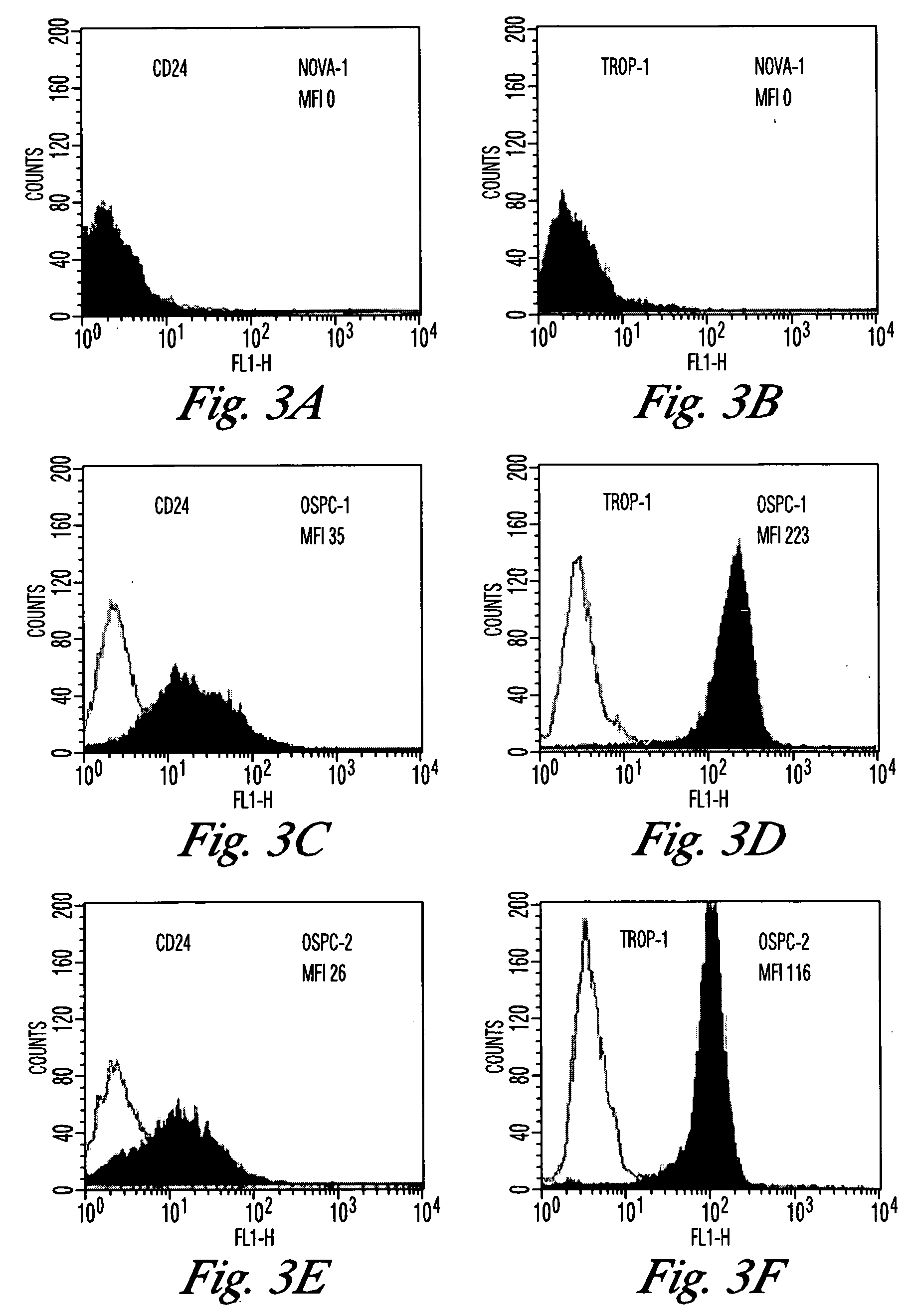
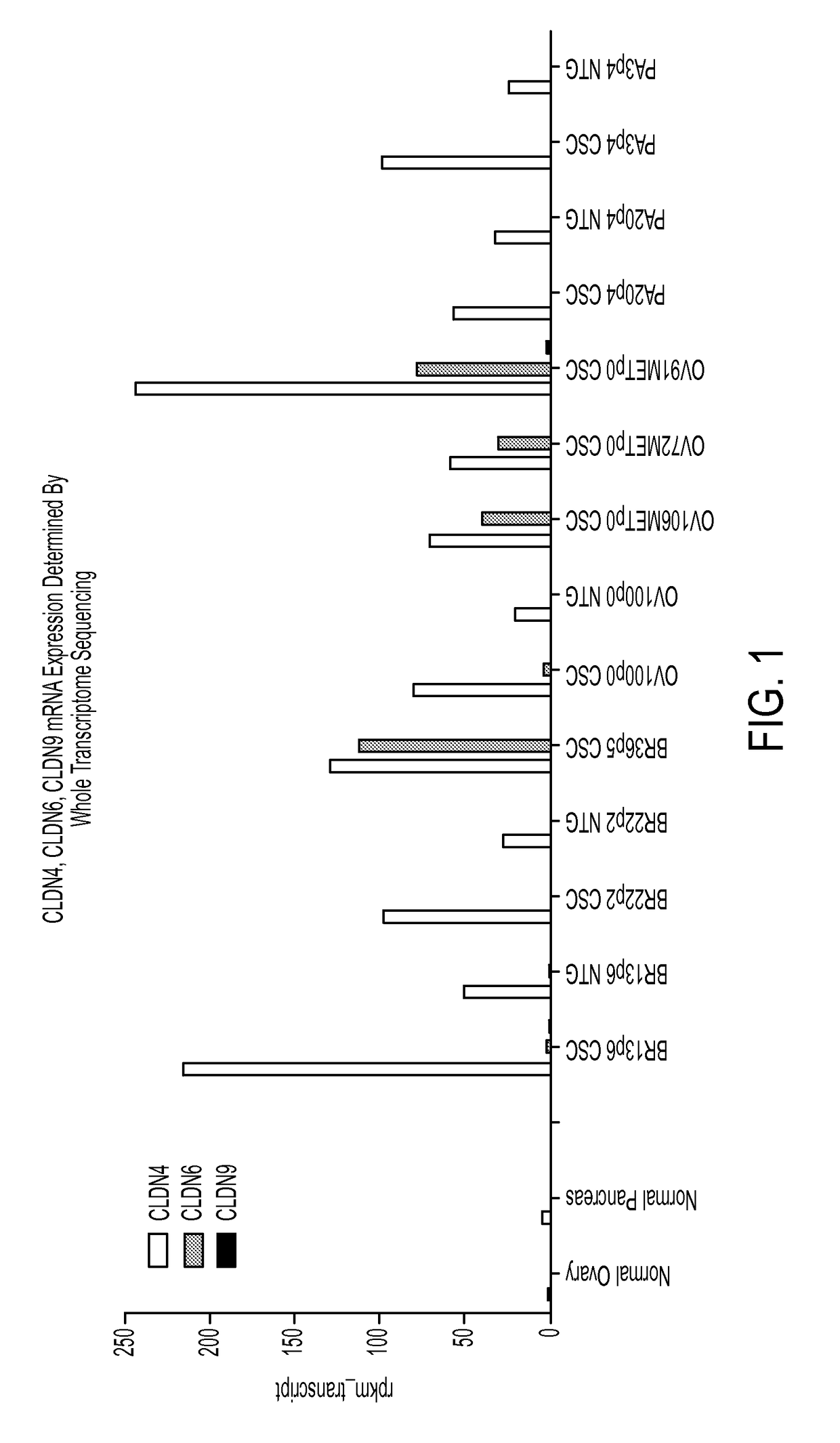
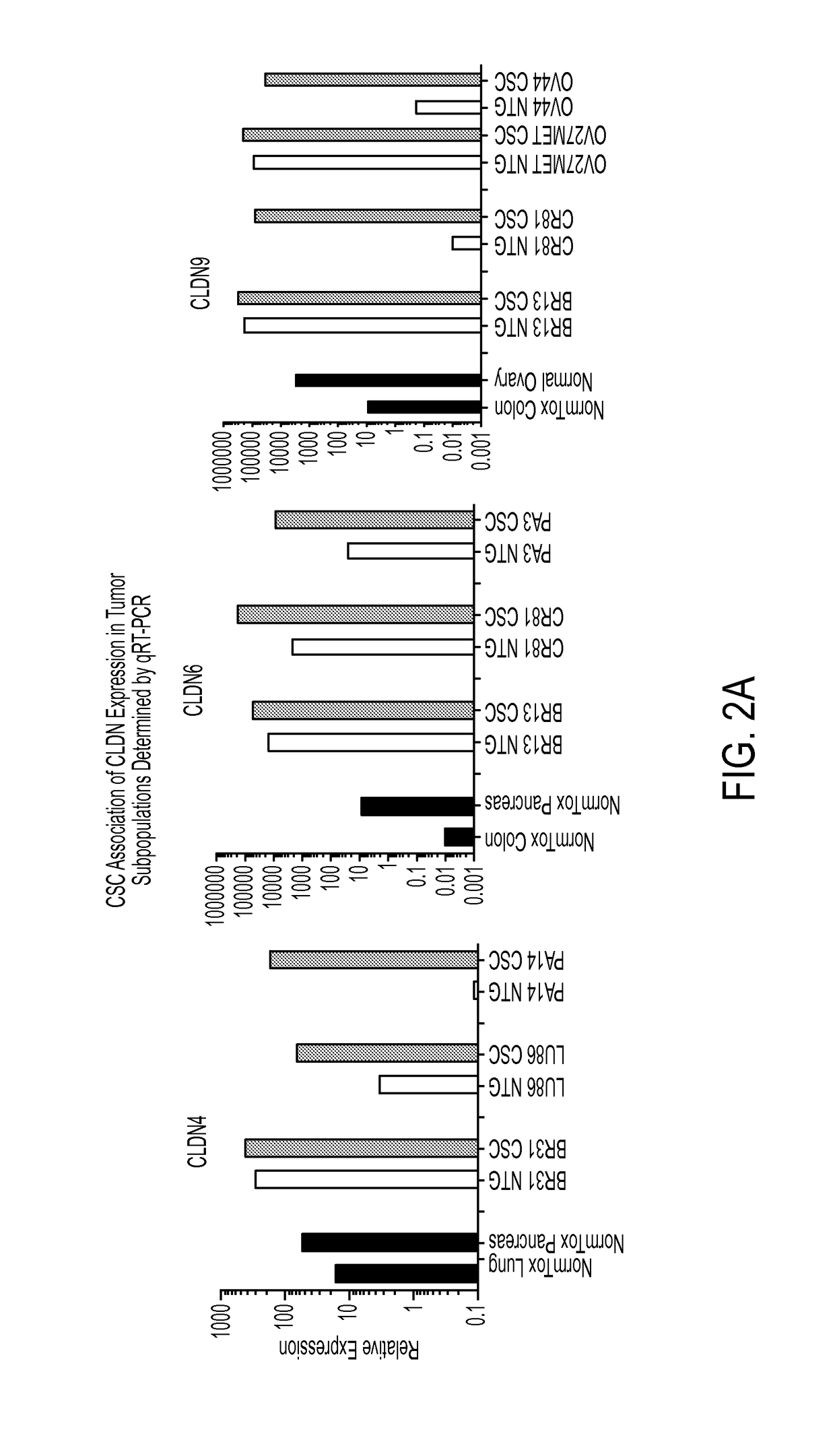
Gene expression profiling and hierarchial clustering analysis readily distinguish normal ovarian epithelial cells from primary ovarian serous papillary carcinomas. Laminin, tumor-associated calcium signal transducer 1 and 2 (TROP-1 / Ep-CAM; TROP-2), claudin 3, claudin 4, ladinin 1, S100A2, SERPIN2 (PAI-2), CD24, lipocalin 2, osteopontin, kallikrein 6 (protease M), kallikrein 10, matriptase and stratifin were found among the most highly overexpressed genes in ovarian serous papillary carcinomas, whereas transforming growth factor beta receptor III, platelet-derived growth factor receptor alpha, SEMACAP3, ras homolog gene family, member I (ARHI), thrombospondin 2 and disabled-2 / differentially expressed in ovarian carcinoma 2 (Dab2 / DOC2) were significantly down-regulated. Therapeutic strategy targeting TROP-1 / Ep-CAM by monoclonal chimeric / humanized antibodies may be beneficial in patients harboring chemotherapy-resistant ovarian serous papillary carcinomas.
Owner:BIOVENTURES LLC
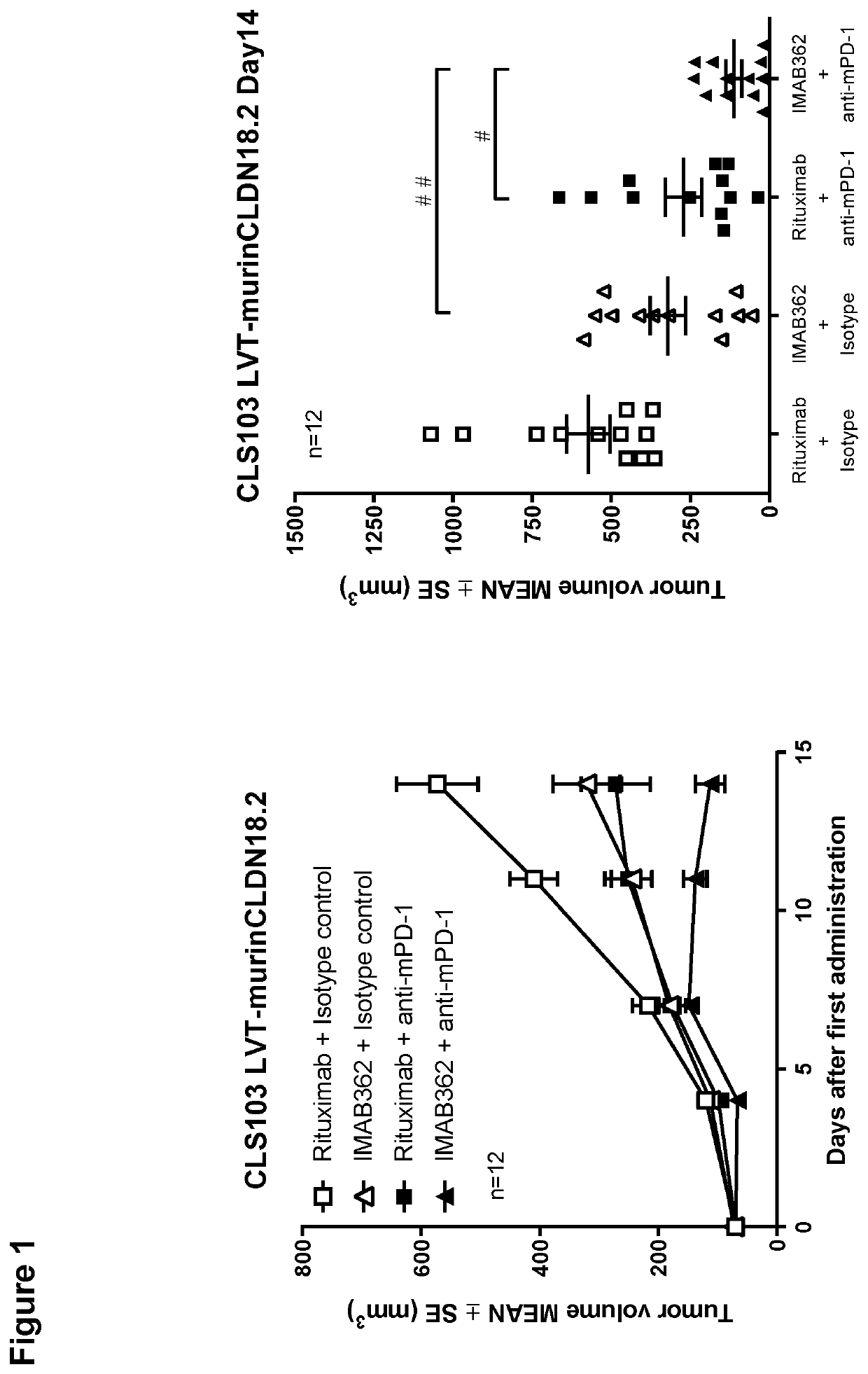
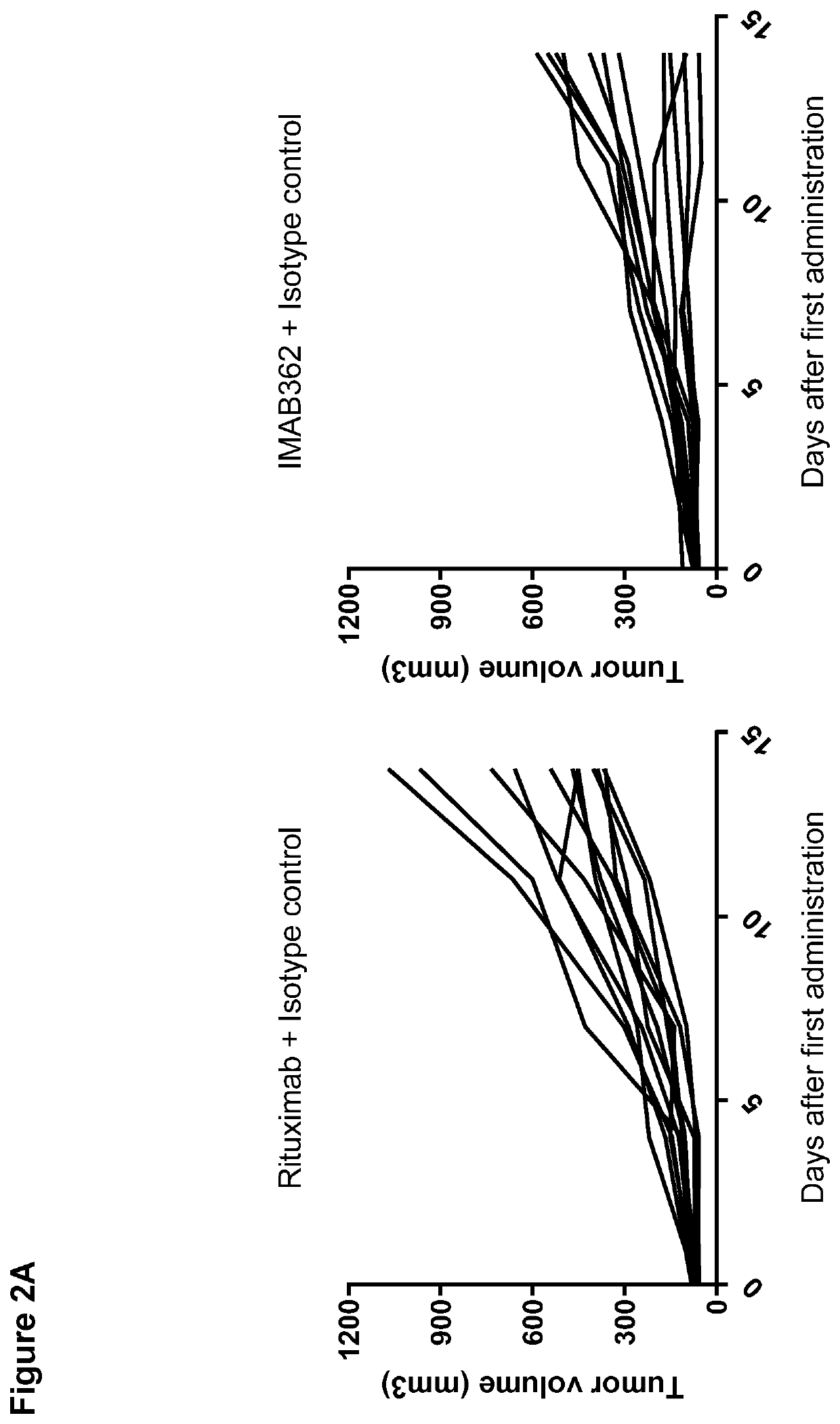
Combination therapy involving antibodies against claudin 18.2 for treatment of cancer
InactiveUS20150132253A1Effectively preventingEffectively treatingHeavy metal active ingredientsOrganic active ingredientsDiseaseCombined Modality Therapy
The present invention provides a combination therapy for effectively treating and / or preventing diseases associated with cells expressing CLDN18.2, including cancer diseases such as gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer, hepatic cancer, head-neck cancer, and cancer of the gallbladder and metastases thereof.
Owner:ASTELLAS PHARMA INC +1
Monoclonal antibodies against claudin-18 for treatment of cancer
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing CLD18, including tumor-related diseases such as gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer, hepatic cancer, head-neck cancer, and cancer of the gallbladder.
Owner:ASTELLAS PHARMA INC +1
Targeted Claudin-4 developer without influence on permeability between epithelial cells
InactiveCN101574532APermeability does not affectImprove permeabilityRadioactive preparation carriersAbnormal tissue growthApoptosis
The invention discloses a targeted Claudin-4 developer without influences on permeability between epithelial cells for positron emission computed tomography, which is a F marked polypeptide consisting of 16 amino acid residues. With shortened peptide chain, the polypeptide developer of the invention have importance biological properties of avoiding influence on the permeability between the epithelial cells, increasing the basement membrane permeability of the epithelial cells, reducing antigenicity and the like. The properties enable the developer to detect tumor lesion under the condition of constant tumor tissue microenvironments (such as blood flow, oxygenation, bioactive molecule distribution, pH value and cell apoptosis body removal) and to reduce interference from repeatedly used and inducible blocking antibodies at the same time.
Owner:SHANDONG TUMOR HOSPITAL
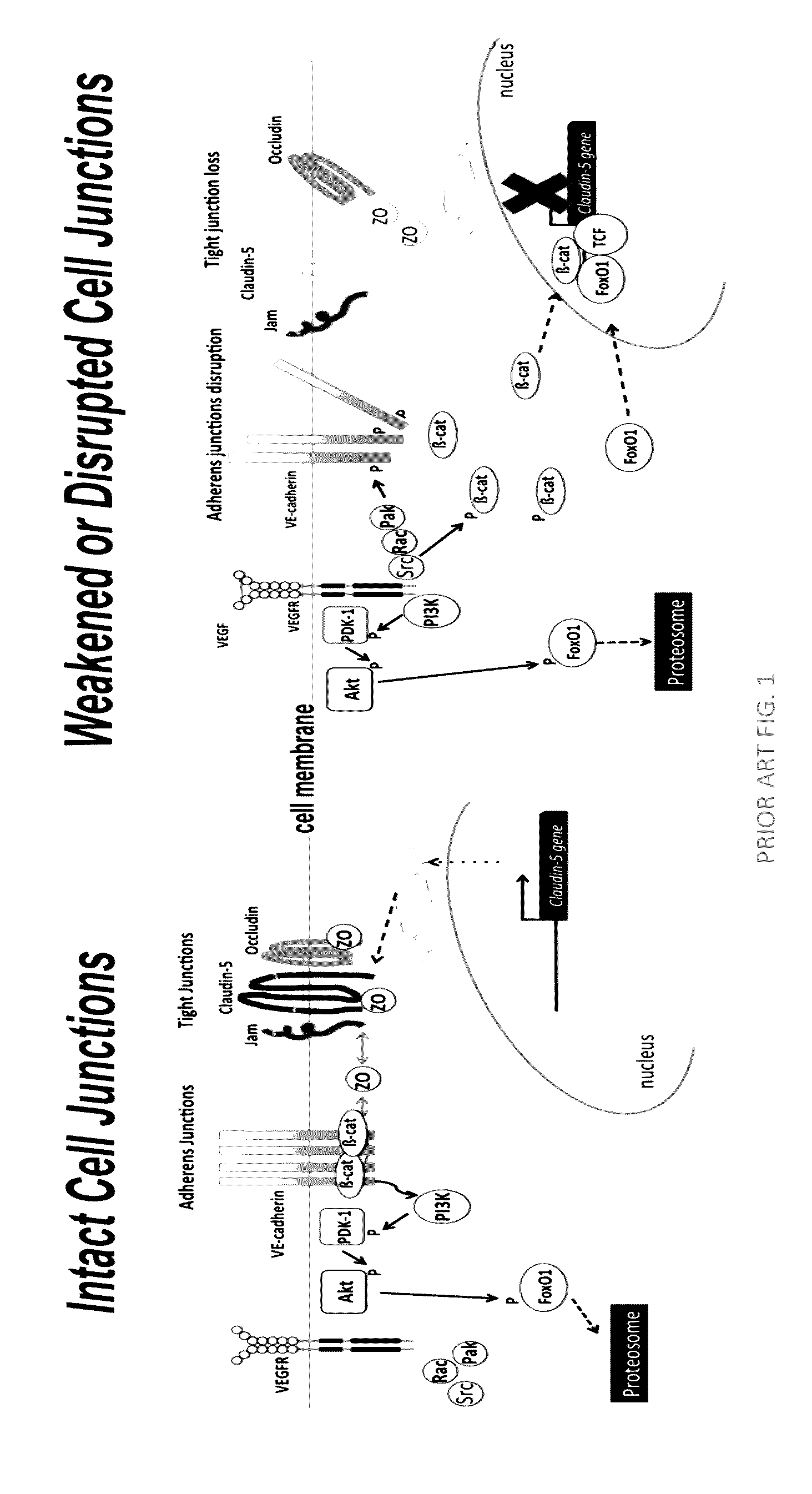
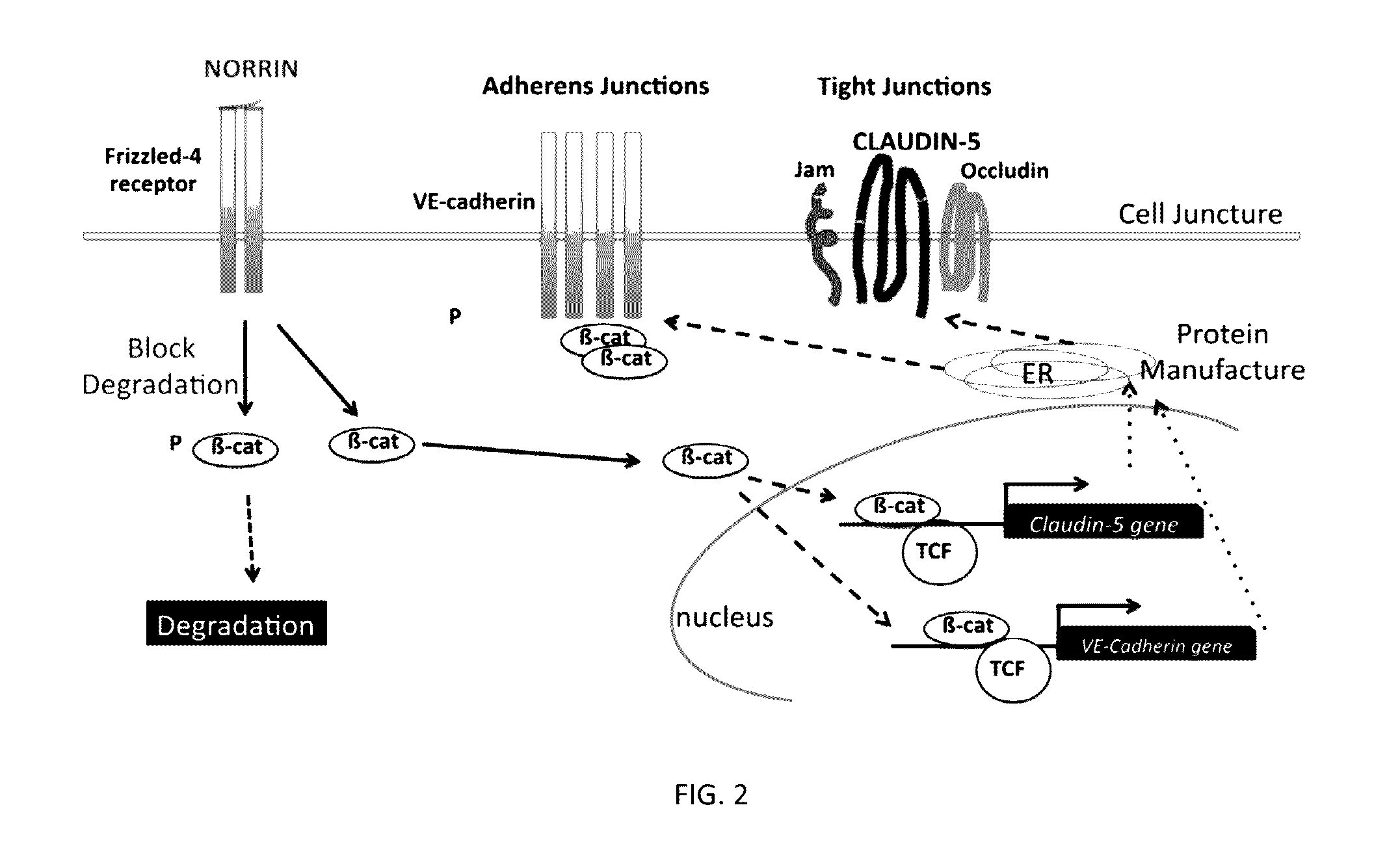
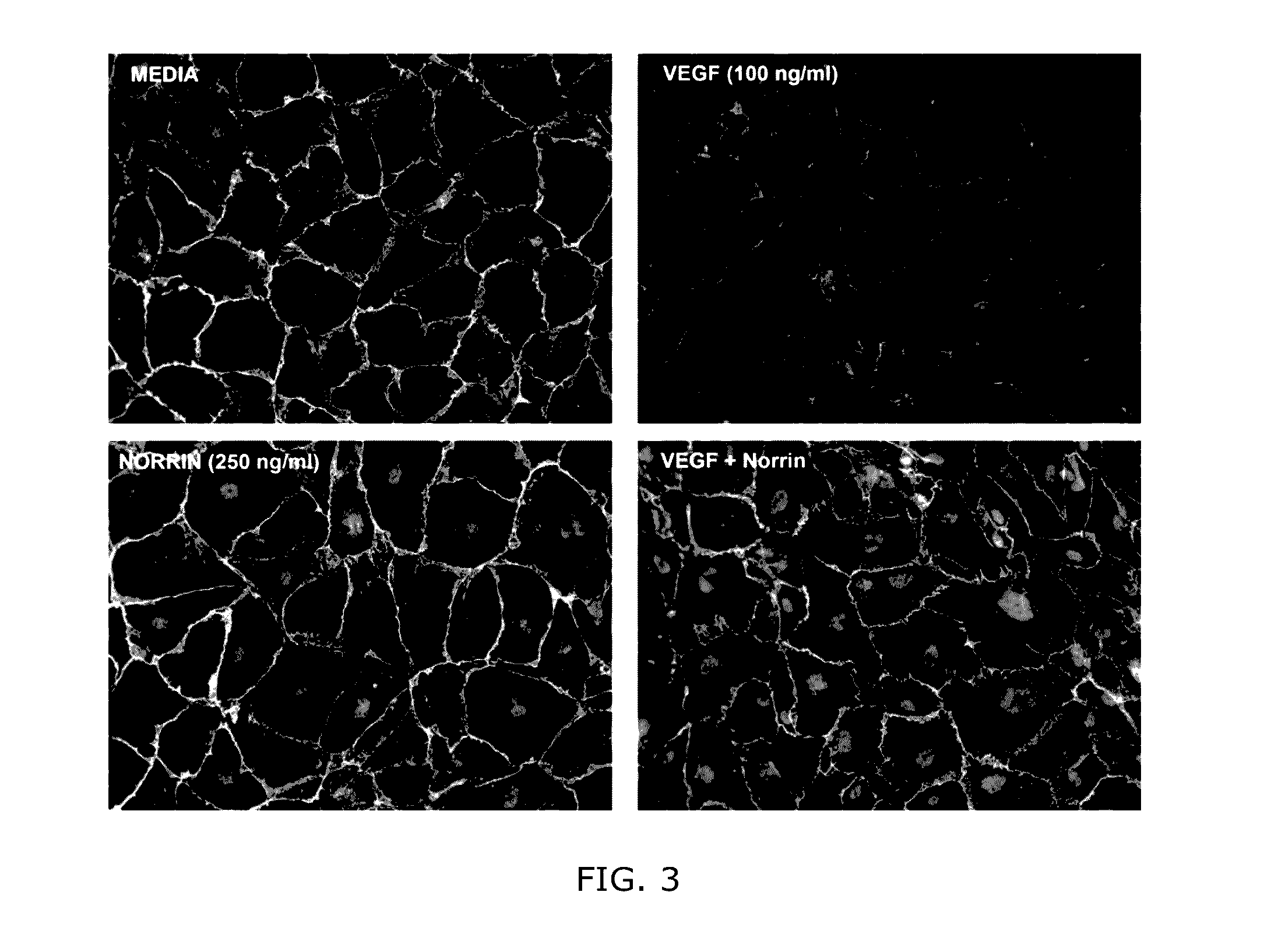
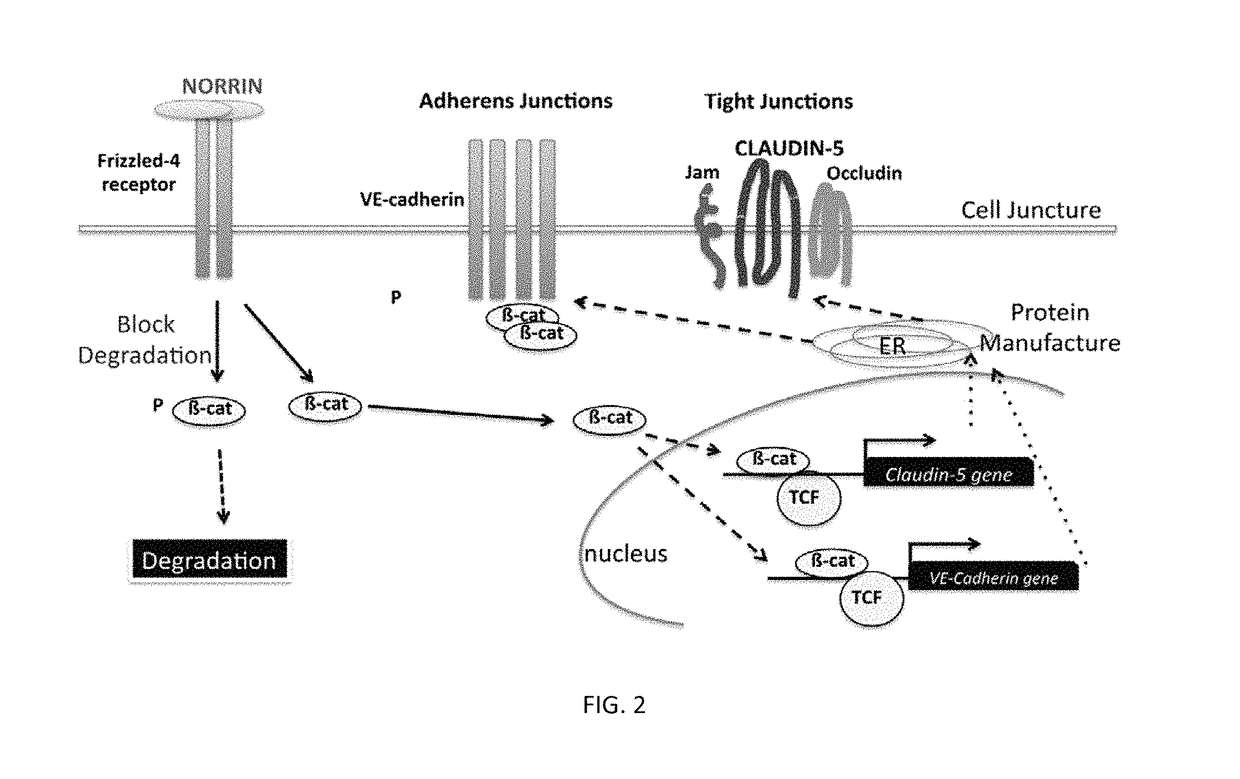
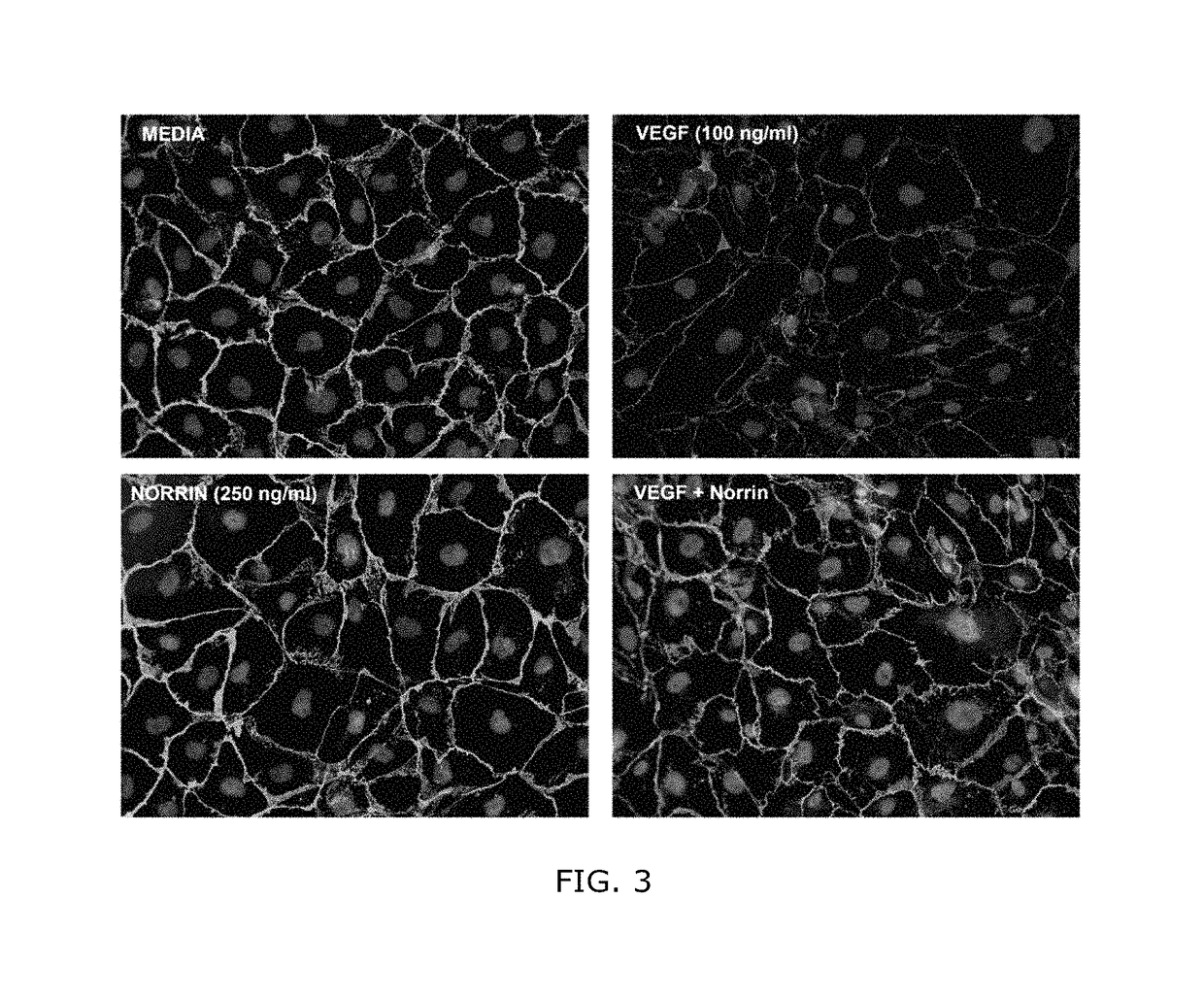
Norrin regulation of cellular production of junction proteins and use to treat retinal vasculature edema
ActiveUS20160355559A1Easy to manageSenses disorderPeptide/protein ingredientsRetinal pigment epithelial cellFhit gene
A method of tightening inter-cellular junctions in retinal or choroidal vessel cells includes exposing the retinal or choroidal vessel cells to norrin. Upon sufficient contact time, for norrin to selectively up-regulate gene expression of VE-cadherin or claudin-5 in the retinal or choroidal vessel cells, the inter-cellular junctions are tightened. The method is also suitable for treating retinal pigment epithelial cells in wet macular degeneration.
Owner:RETINAL SOLUTIONS LLC
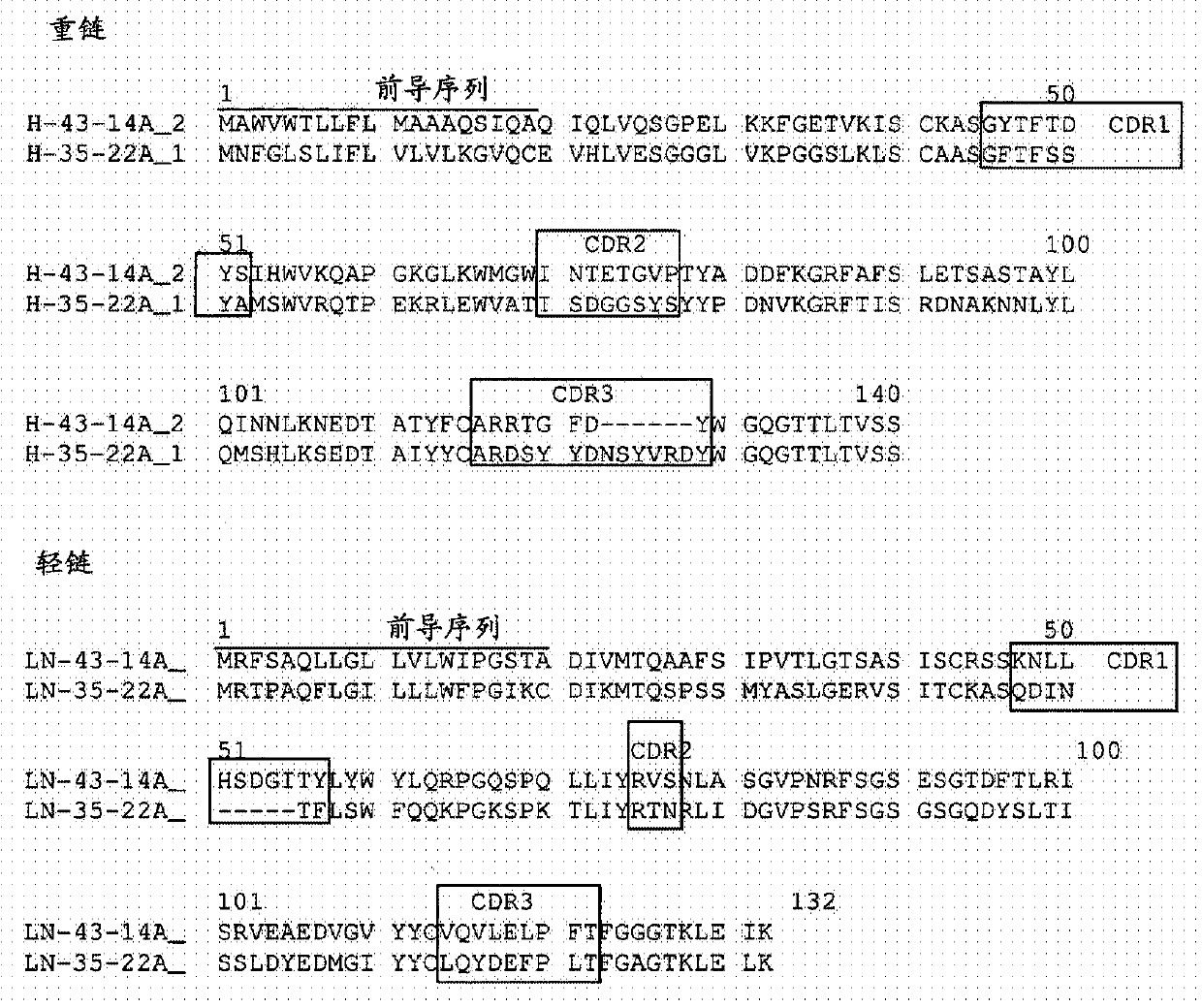
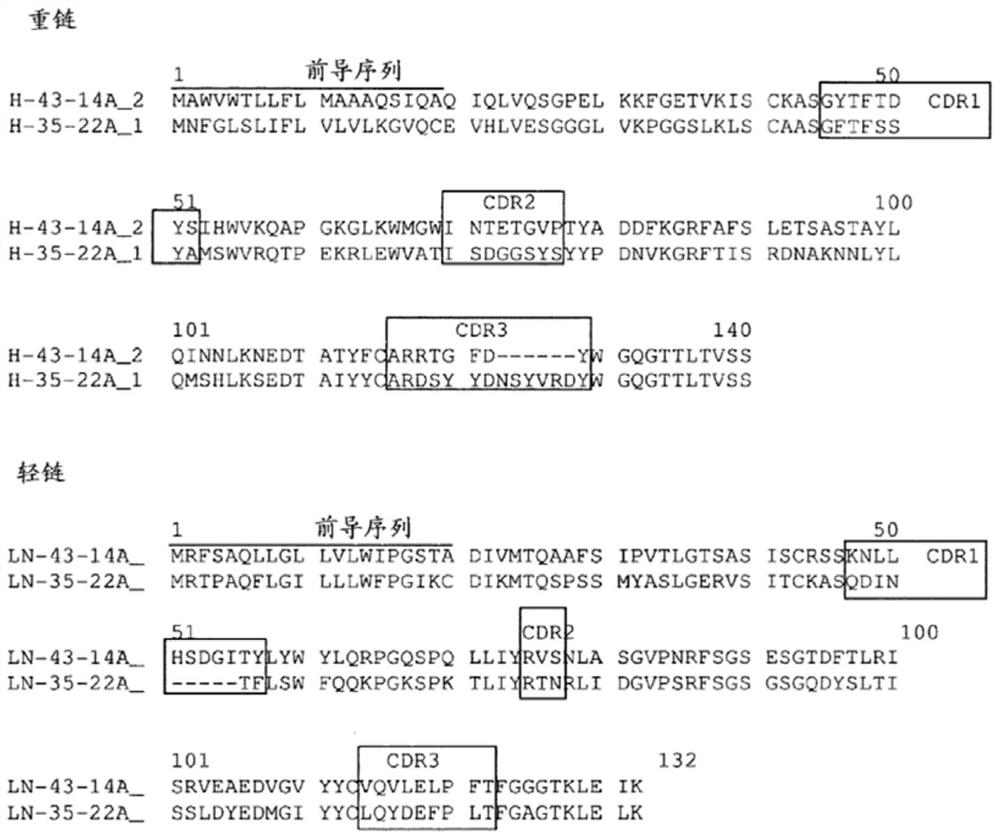
Humanized antibody binding to claudin for treating cancer
ActiveCN111808194AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesOncology
The present invention relates to a humanized antibody binding to claudin 18.2 for treating cancer. The antibody does not bind to Claudin 18.1, but selectively binds to Claudin 18.2 to destroy tight connection between cells, so that the Claudin 18.2 cannot exert normal functions, and the antibody can be used for preventing or treating tumors, especially advanced gastric cancer.
Owner:BEIJING KAWIN TECH SHARE HLDG
Anti-claudin antibodies and methods of use
ActiveUS10053511B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntibodyAntibody-drug conjugate
Owner:ABBVIE STEMCENTRX LLC
Novel Anti-claudin antibodies and methods of use
ActiveUS20160289332A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntibodyAntibody-drug conjugate
Owner:ABBVIE STEMCENTRX LLC
Novel Anti-claudin antibodies and methods of use
InactiveUS20190083645A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntiendomysial antibodies
Owner:ABBVIE STEMCENTRX LLC
Combination therapy involving antibodies against claudin 18.2 for treatment of cancer
ActiveUS9433675B2High affinityStrong specificityHeavy metal active ingredientsBiocideDiseaseEsophageal cancer
The present invention provides a combination therapy for effectively treating and / or preventing diseases associated with cells expressing CLDN18.2, including cancer diseases such as gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer, hepatic cancer, head-neck cancer, and cancer of the gallbladder and metastases thereof.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITAETSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GEMEINNUETZIGE GMBH +1
Antibody for preventing or treating cancer
ActiveCN111961135AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesPharmaceutical drug
The invention belongs to the technical field of antibodies, and provides an antibody for preventing or treating cancer. The antibody specifically binds to human claudin 18.2. The invention also provides a pharmaceutical composition comprising the antibod, application of the composition, nucleic acid molecules encoding the antibody, a vector comprising the nucleic acid molecules, host cells and a method for preparing the antibody.
Owner:北京亦庄国际蛋白药物技术有限公司
Method for diagnosing and treating cancer
A method for treating cancer in a mammal comprised of administering a therapeutically effective amount of an agent that induces the expression of one or more claudins within a cancerous cell. Preferably the claudin is claudin-3, claudin-4 aor claudin-9. In a preferred method, a nucleic acid that encodes a claudin is administered to the mammal under conditions wherein the nucleic acid is transfected into the cancerous cell and the claudin is produced in the cell. Also disclosed is a method for diagnosing cancer comprising determining the presence of a claudin 3, 4 or 9 in a cell that normally expresses the protein. If the cell does not express the claudin, then it is cancerous.
Owner:马里纳生物技术公司
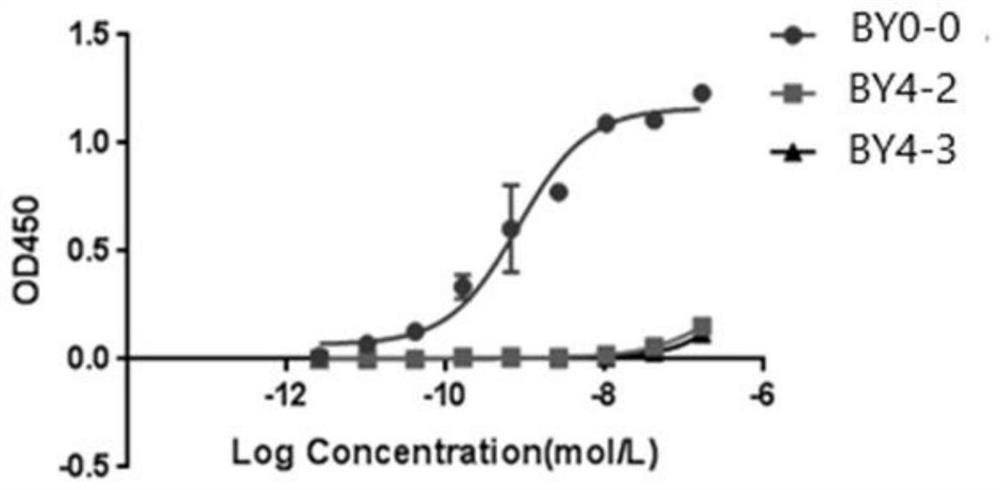
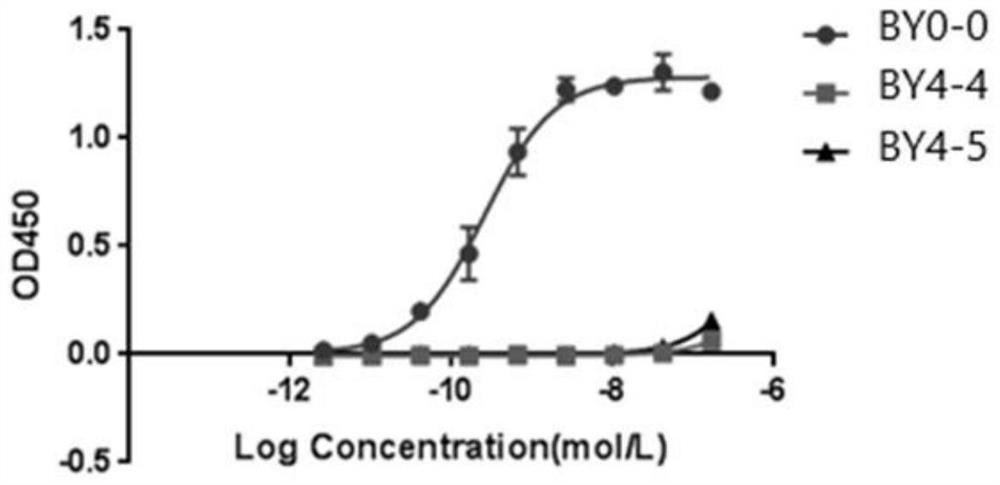
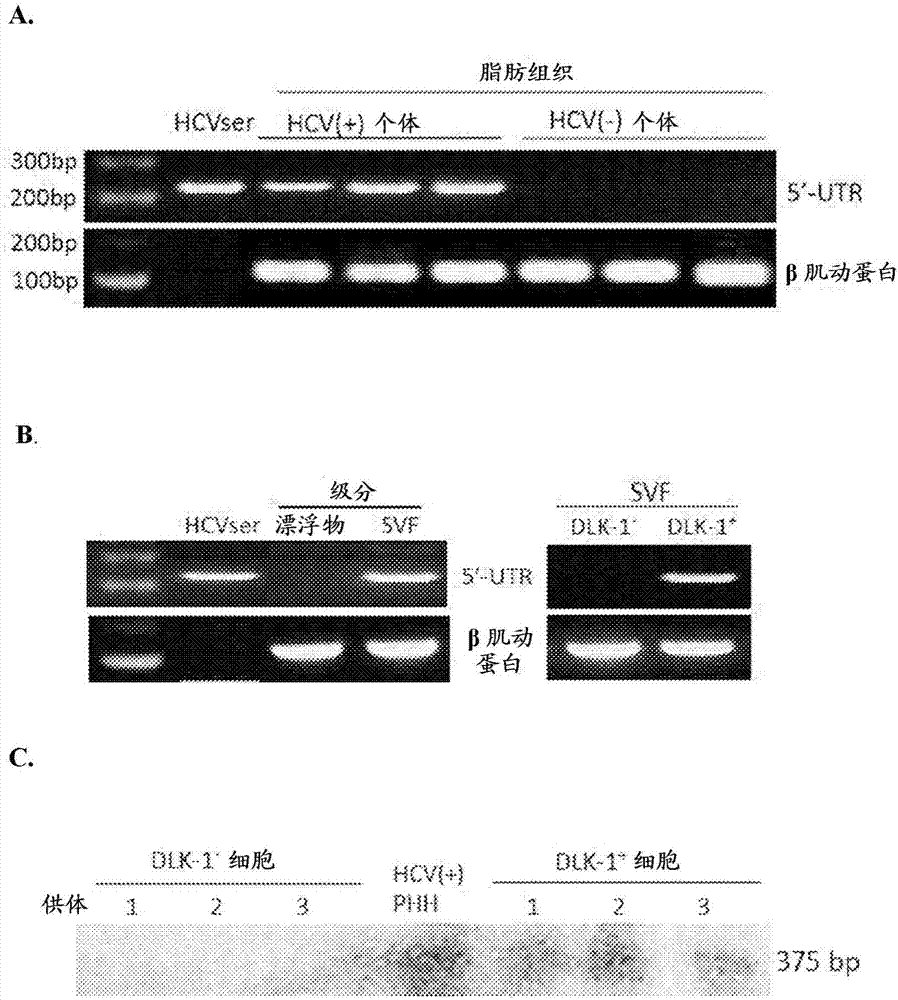
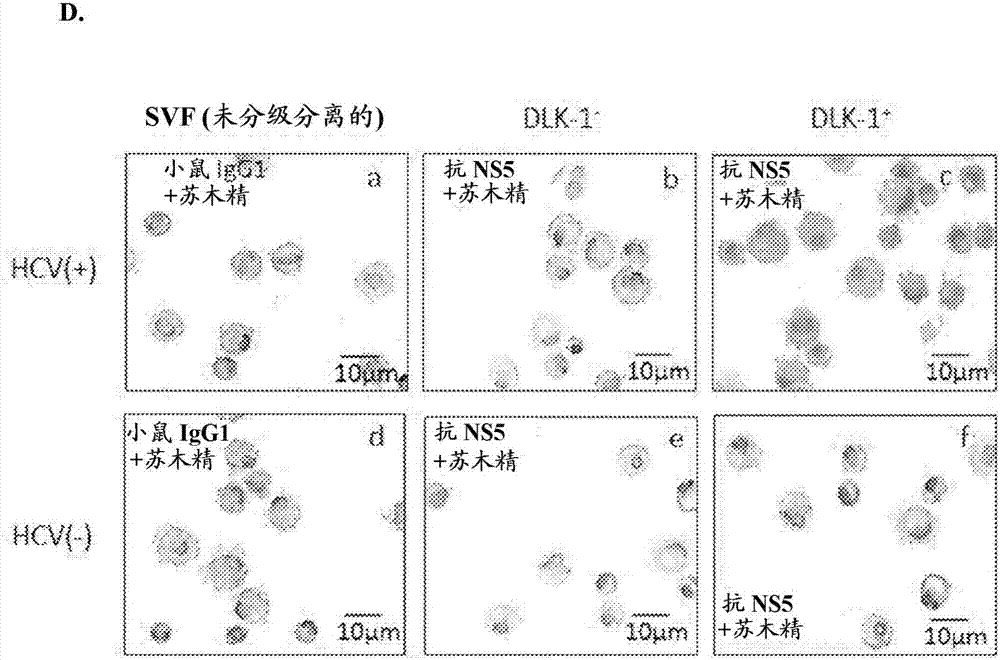
Employing human adipose-derived stem cells to propagate serum-derived hepatitis c virus and use thereof
ActiveCN107208060ASsRNA viruses positive-senseMicrobiological testing/measurementAdipogenesisHepatica
Hepatitis C virus replication at extrahepatic sites has been suggested; however, complete viral replication has only been confirmed in hepatocytes. Here we show that human adipogenic DLK-1+stem cells (hADSC) freshly isolated from HCV-infected individuals contained viral transcripts, replication intermediates and viral antigens in vivo, and viral transcripts increased in supernatants upon prolonged ex vivo culture. Furthermore, naive hADSC isolated from HCV(-)individuals support complete replication of clinical isolates in vitro, and the infection is donor-nonspecific for cells and cross-genotypic for viruses. Viral infection / replication is mediated through CD81, LDL-R, SR-B1, EGFR, Apolipoprotein E, occludin, claudin-1, NPC1L1 and diacylglycerol acetyltransferase-1, and can be inhibited by anti-viral drugs. In addition, the physical properties of hADSC-propagated viral particles resemble clinical isolates more than JFH1 / HCVcc, and viruses propagated by in vitro infected hADSC are infectious to primary human hepatocytes. Therefore, hADSC are an in vivo HCV reservoir and represent a novel venue of clinical virus-host interaction. hADSC can also be exploited as a physiologically relevant primary cell culture system to propagate clinical isolates.
Owner:VANWORLD PHARMA (RUGAO) CO LTD
Norrin regulation of cellular production of junction proteins and use to treat retinal vasculature edema
A method of tightening inter-cellular junctions in retinal or choroidal vessel cells includes exposing the retinal or choroidal vessel cells to norrin. Upon sufficient contact time, for norrin to selectively up-regulate gene expression of VE-cadherin or claudin-5 in the retinal or choroidal vessel cells, the inter-cellular junctions are tightened. The method is also suitable for treating retinal pigment epithelial cells in wet macular degeneration.
Owner:RETINAL SOLUTIONS LLC
Antibodies against claudin 18.2 for cancer diagnosis
ActiveCN108047331BMicroorganism based processesImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeCancer cell
The present invention relates to antibodies against claudin 18.2 for use in cancer diagnosis. The present invention relates to antibodies directed against epitopes located in the C-terminal part of CLDN18.2, which are useful, for example, in diagnosing cancer and / or determining whether cancer cells express CLDN18.2.
Owner:ASTELLAS PHARMA INC +1
Combination therapy involving antibodies against claudin 18.2 and immune checkpoint inhibitors for treatment of cancer
PendingUS20220324965A1Improve anti-tumor efficacyGood curative effectDigestive systemImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseOncology
The present invention provides a combination therapy for effectively treating and / or preventing diseases associated with cells expressing CLDN18.2, including cancer diseases such as gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer, hepatic cancer, head-neck cancer, and cancer of the gallbladder and metastases thereof.
Owner:ASTELLAS PHARMA INC
Claudin-6 binding molecules and uses thereof
PendingCN113950485AStrong specificityExtended half-lifeGenetically modified cellsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer preventionCancers diagnosis
The present disclosure provides antigen binding molecules that show binding activity towards Claudin-6 (CLDN6), methods for producing the antigen binding molecules, use of the antigen-binding molecules and immunoconjugates comprising the same in treating and / or preventing cancers, use of the antigen binding molecules in detecting the presence of CLDN6 in biological samples, and use of the antigen binding molecules in diagnosis of various cancers.
Owner:CHUGAI PHARMA CO LTD
Antibodies against claudin 18.2 for cancer diagnosis
PendingCN114573697AMicroorganism based processesImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeCancer cell
The present invention relates to antibodies against claudin 18.2 for use in cancer diagnosis. The present invention relates to antibodies directed against epitopes located in the C-terminal portion of CLDN18.2, which are useful, for example, in diagnosing cancer and / or determining whether cancer cells express CLDN18.2.
Owner:ASTELLAS PHARMA INC +1
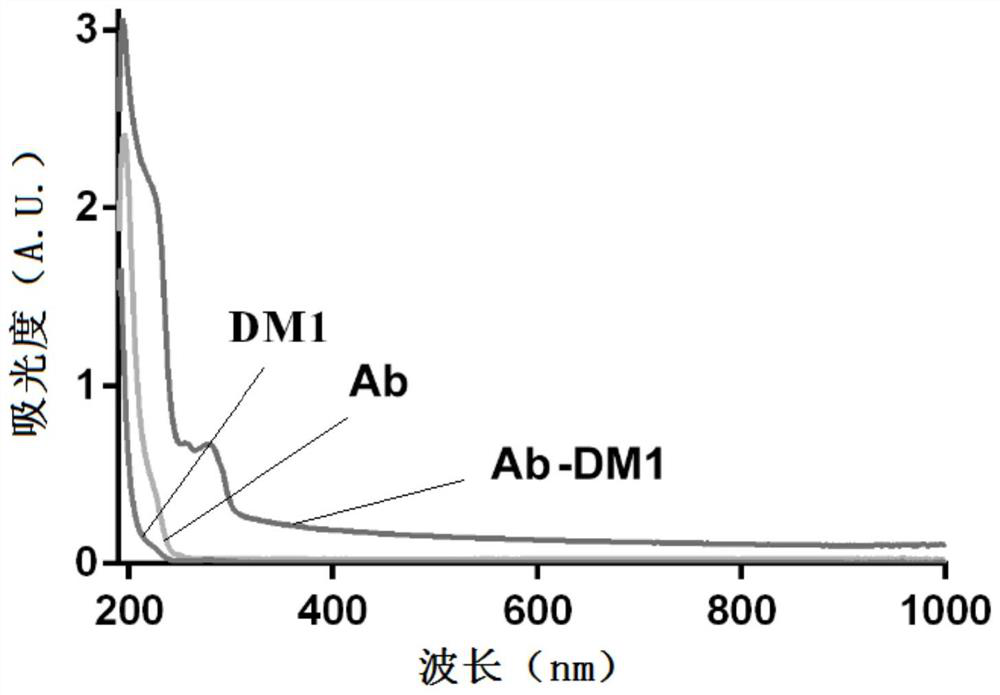
A kind of antibody-conjugated drug against claudin 6 and its application
ActiveCN111087465BLittle side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsOncogene translation productsAntiendomysial antibodiesAntibody conjugate
The invention discloses an antibody-conjugated drug against claudin 6 and its application. The invention relates to an antigen sequence for preparing claudin 6 (CLDN6) antibody, its corresponding nucleotide sequence and a method for expressing the nucleoside Genetic engineering carrier, engineering bacteria or cell line of acid sequence; animal immunization through the antigenic polypeptide can produce highly specific claudin 6 antibody; further prepare hybridoma cells by immunizing animal cells to obtain corresponding monoclonal antibodies; Combining the anti-claudin 6 antibody with a linking structure and a drug can form an antibody-conjugated drug; the antibody-coupled drug can carry the drug to the tumor expressing positive claudin 6 according to the targeting effect of claudin 6 cells, so as to achieve the effect of specifically killing tumor cells, which is of great significance to the treatment of tumors.
Owner:GUANGZHOU MEDICAL UNIV
A method for detecting multiple pancreatic cancer tumor markers by multiplex PCR and primers and probes for detection
ActiveCN106520946BStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPancreatic tissueTotal rna
The invention provides a method for detecting various pancreatic cancer tumor markers through multiple PCR and primers and probes for detection. The method comprises the following steps that (1) the primers and the probes are designed, specifically, P53 forward and reverse primer sequences and a P53 probe sequence are seen in SEQ ID No:1, 2 and 3 correspondingly, and Claudin-4 forward and reverse primer sequences and a Claudin-4 probe sequence are seen in SEQ ID No: 4, 5 and 6; (2) the pancreatic tissue total RNA is extracted; (3) the RNA concentration and purity are identified; (4) the DNA is removed; and (5) real-time PCR is conducted. By designing the specific primers and probes and optimizing the multiple PCR conditions, the method for detecting the various pancreatic cancer tumor markers through the multiple PCR is established. The method is good in specificity, high in flexibility, reliable in result data, high in repeatability and capable of saving cost and time compared with single detection.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Angiopoietin-like 4 and its use in regulating cellular leakiness
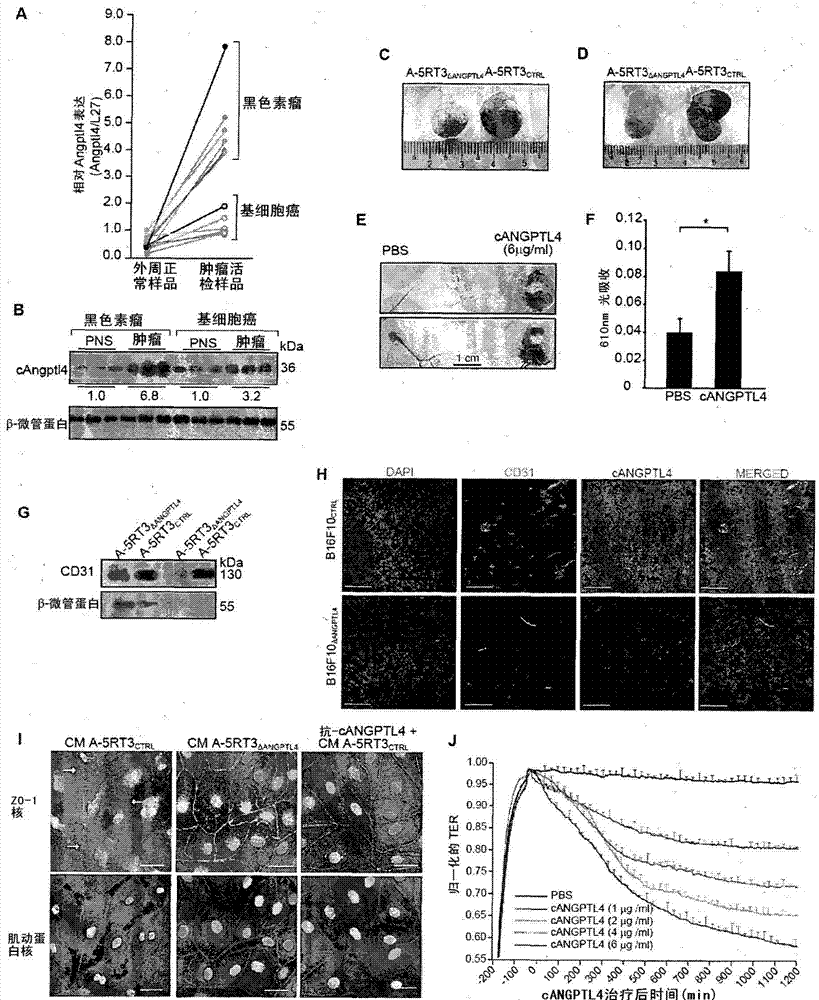
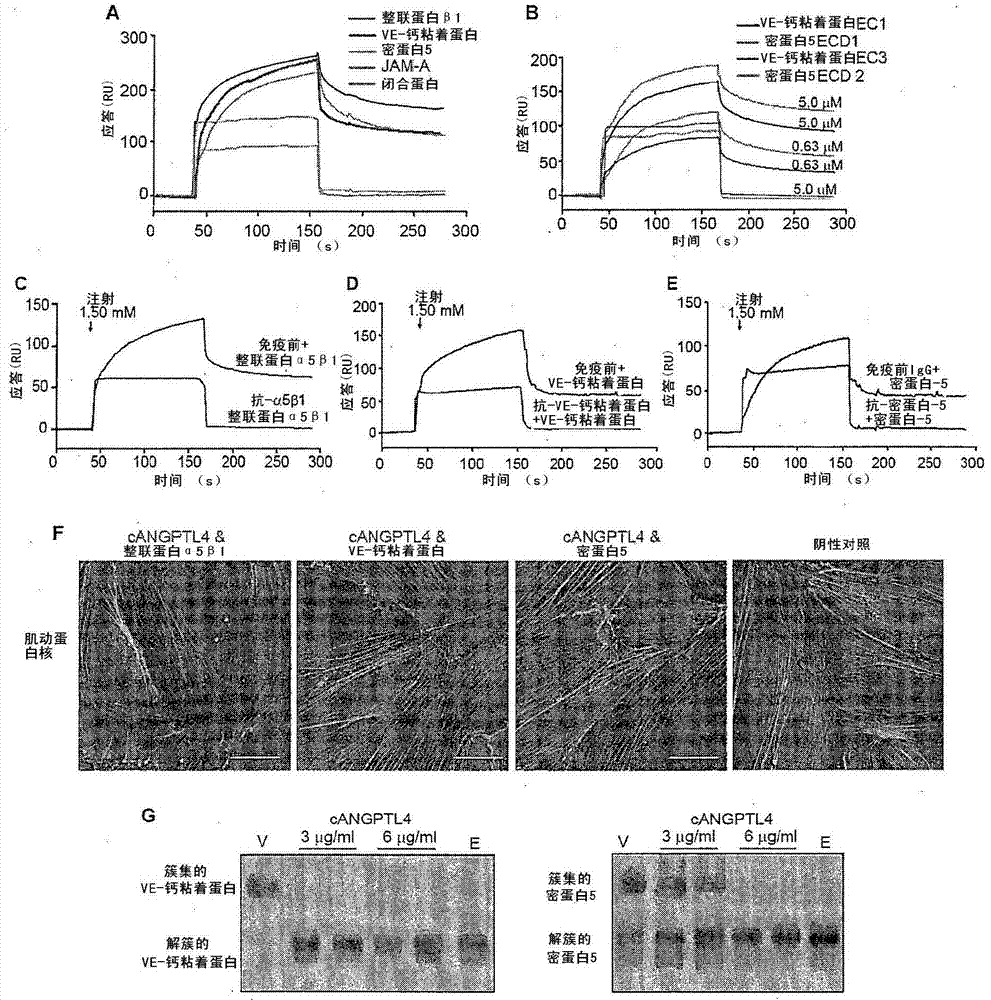
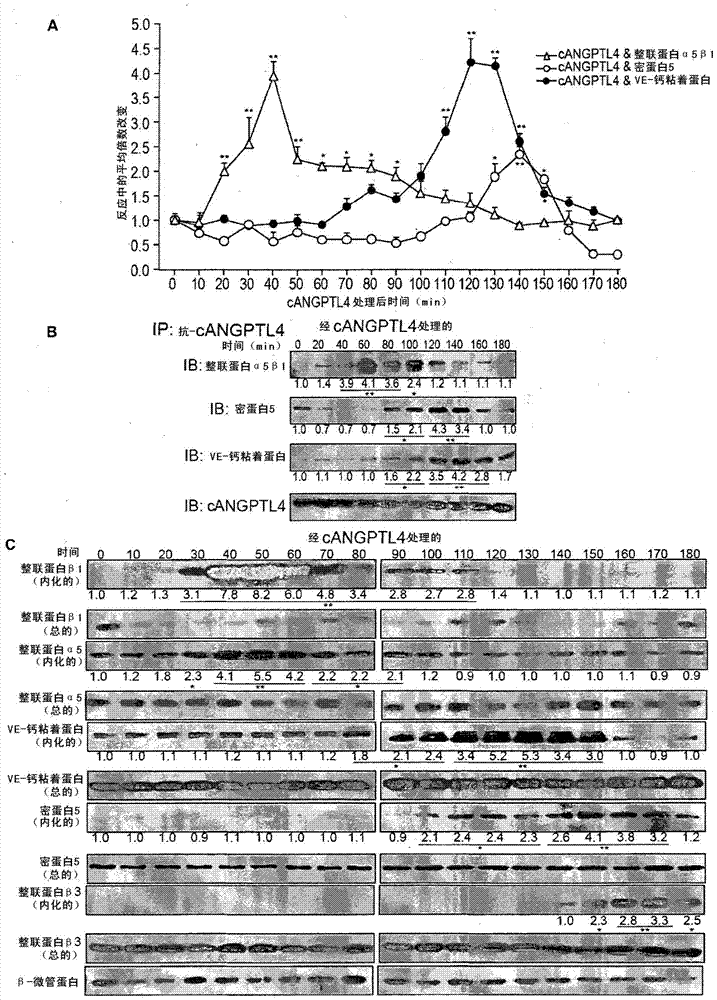
Vascular disruption induced by interactions between tumor-secreted permeability factors and adhesion proteins on endothelial cells promotes metastasis The role of tumor-secreted angiopoietin-like 4 (cANGPTL4) in vascular leakage and metastasis is controversial due to a lack of understanding of how cANGPTL4 regulates vascular integrity. Here, we show that cANGPTL4 contributes to disruption of endothelial continuity and thus metastasis by directly interacting with 3 novel binding partners integrin α5β1, VE-cadherin and claudin‑5 in a temporally sequential manner. We show that cANGPTL4 binds to and activates integrin α5β1-mediated Rac1 / PAK signaling to attenuate cell–cell contacts. cANGPTL4 subsequently associates with and dissociates VE‑cadherin and claudin‑5, leading to endothelial disruption. Interfering with the formation of these cANGPTL4 complexes delays vascular destruction. In vivo vascular permeability and metastasis assays using ANGPTL4-knockout and wild-type mice injected with control or ANGPTL4-knockdown tumors confirmed that cANGPTL4 induces vascular leakiness and promotes lung metastasis in mice. Thus, our findings explain how cANGPTL4 induces endothelial disruption. Our findings have immediate implications for targeting cANGPTL4 to treat cancer and other vascular lesions.
Owner:NANYANG TECH UNIV
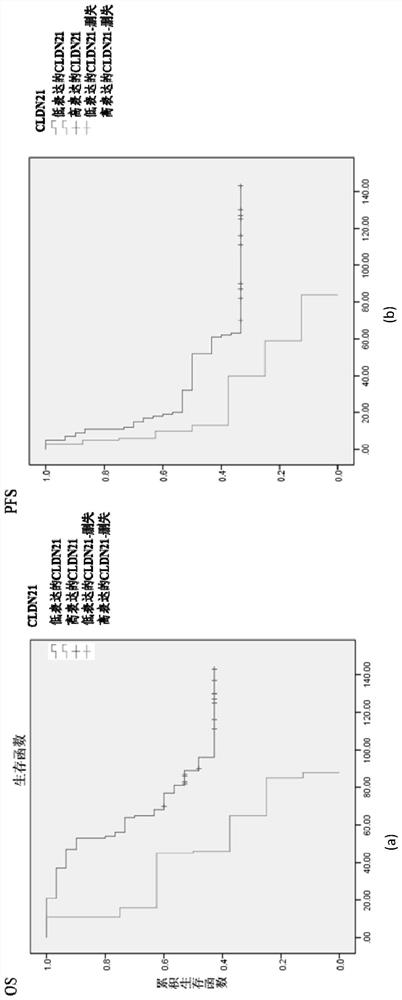
A prognostic diagnostic marker claudin21 for ovarian cancer and its application
The present invention is a prognostic diagnostic marker Claudin21 for ovarian cancer and its application. For the first time, it is found that the expression level of the ovarian cancer drug resistance marker Claudin21 is related to the prognosis of ovarian cancer, specifically the progression-free survival and overall survival when Claudin21 is highly expressed. Among the medium prognostic indicators, ovarian cancer cases show poor prognosis, that is, the discovery of the present invention provides prognosis hints for clinical treatment of ovarian cancer including chemotherapy and surgical treatment.
Owner:ZHEJIANG CANCER HOSPITAL
Combination therapy involving antibodies against claudin 18.2 for treatment of cancer
InactiveUS20180258180A1High affinityStrong specificityOrganic active ingredientsHeavy metal active ingredientsDiseaseAntiendomysial antibodies
The present invention provides a combination therapy for effectively treating and / or preventing diseases associated with cells expressing CLDN18.2, including cancer diseases such as gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer, hepatic cancer, head-neck cancer, and cancer of the gallbladder and metastases thereof.
Owner:ASTELLAS PHARMA INC +1
A humanized antibody that binds claudin for the treatment of cancer
ActiveCN111808194BImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesOncology
The present invention relates to a humanized antibody that binds to claudin 18.2 and is used for treating cancer. The antibody does not bind to Claudin18.1, but selectively binds to Claudin18.2, destroys the tight junction between cells, and makes Claudin18. 2 can not play its normal function, can be used for the prevention or treatment of tumors, especially advanced gastric cancer.
Owner:BEIJING KAWIN TECH SHARE HLDG
A Microsphere Turner
InactiveCN105289432BEasy to operatePeaceful conditionsMicroballoon preparationMicrocapsule preparationPolyvinyl alcoholMicrosphere
Owner:LASER FUSION RES CENT CHINA ACAD OF ENG PHYSICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com