A kind of antibody-conjugated drug against claudin 6 and its application
An antibody-conjugated drug, claudin technology, applied in the field of biomedicine, can solve problems such as high recurrence rate and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Antigen sequence and its preparation
[0047] Through the analysis and comparison of bioinformatics, a polypeptide sequence with the highest score and the least crossness with other tight junction structure-related proteins was screened out:
[0048] SEQ ID NO.1
[0049] LPMWKVTAFIGN
[0050] The polypeptide sequence corresponds to the 27th to 38th amino acids of the claudin 6 protein sequence.
[0051] In order to facilitate the access to the corresponding carrier KIH, a connection structure C was added at the end of the above sequence, and the following peptide synthesis of the polypeptide fragment with a purity of 90% was carried out. The sequence is:
[0052] LPMWKVTAFIGNC.
Embodiment 2
[0053] Example 2 Antibody Preparation
[0054] The synthesized polypeptide sequence was coupled with a carrier protein (such as KLH), and 3 BALB / c mice were boosted by standard procedures. The mouse with the best immune response was selected, and the splenocytes were fused with myeloma cells to prepare hybridoma cells.
[0055] The obtained hybridoma cells were screened by proliferation and ELISA to obtain high-titer clones; and 2 to 8 positive mother cells were subcloned by the limited dilution method, and finally a positive clone was determined for subtype identification. It was deposited in the China Center for Type Culture Collection on November 12, 2019, with the preservation number CCTCC NO.C2019282, address: China. Wuhan. Wuhan University, Luojia Mountain, Bayi Road, Wuchang District, Wuhan City, Hubei Province.
[0056] After expanding the culture of the positive cloned cells, five mice were subjected to ascites production, and the corresponding monoclonal antibodies ...
Embodiment 3
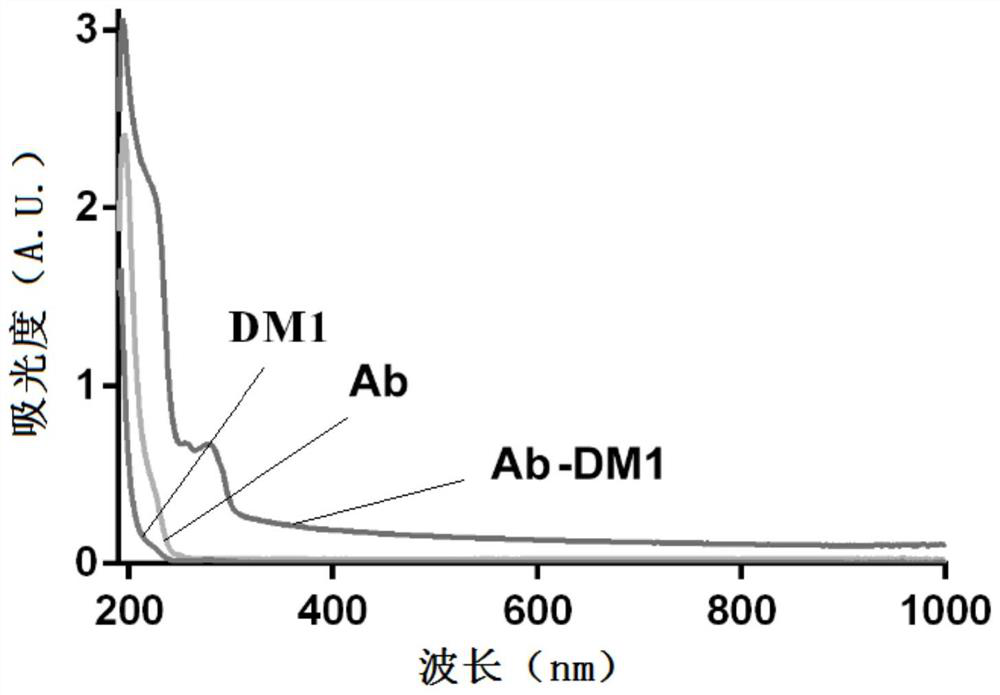
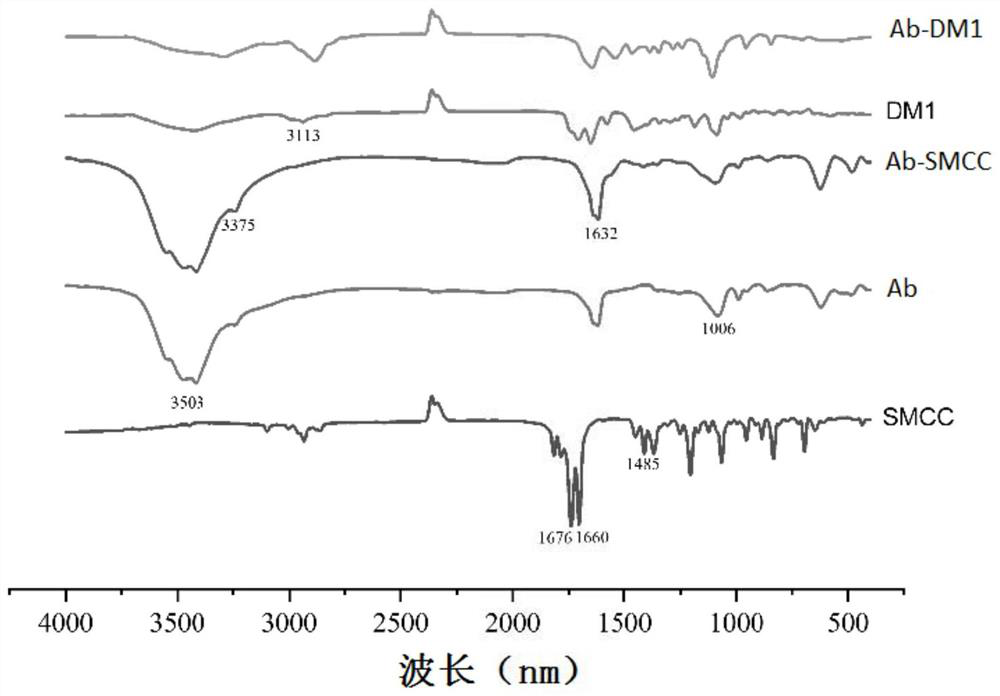
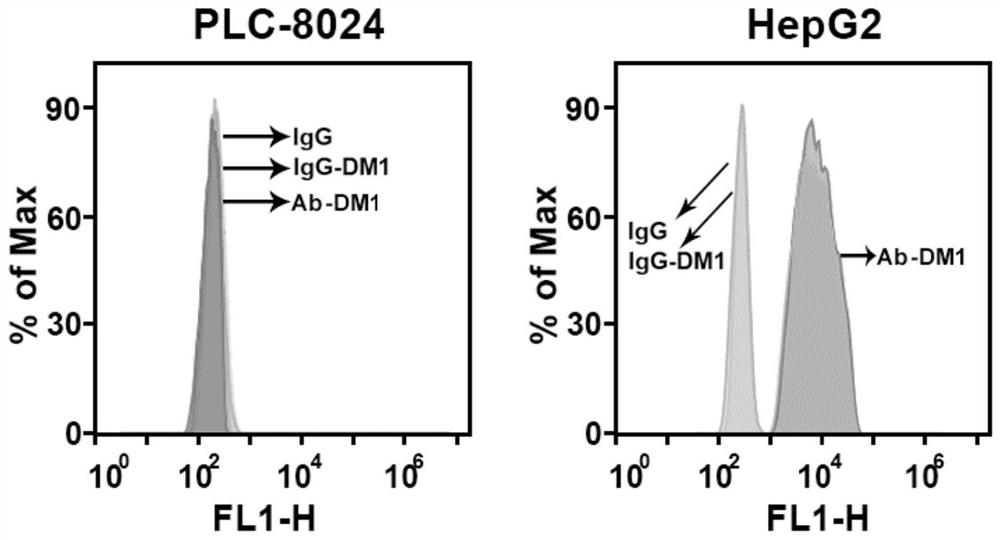
[0057] Example 3 Preparation and Identification of Antibody-Drug Conjugates
[0058] 1. Main material
[0059] Antibody: the monoclonal antibody prepared in Example 2 (hereinafter referred to as Ab);
[0060] Connection structure: SMCC, purchased from AAT Bioquest (USA);
[0061] Drug: maytansine (hereinafter referred to as DM1).
[0062] 2. Preparation method
[0063] 1) Dissolve SMCC in DMSO and store at 4°C;
[0064] 2) Combine Ab and SMCC in conjugation buffer (50mm potassium phosphate, 50mm sodium chloride, 2mm EDTA,
[0065] pH 7.2) at a molar ratio of 1:10, stirred and reacted at 4°C for 4-6 hours;
[0066] 3) After the reaction, place the mixed solution in a dialysis bag (M=5000), and dialyze at 4°C for three days;
[0067] 4) Collect the dialysate and freeze-dry it in a vacuum freeze dryer;
[0068] 5) Quantitative analysis of protein samples with BCA;
[0069] 6) The modified antibody and DM1 molecules were mixed in PBS buffer (pH=7.0) at a molar ratio of 1:1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com