Treating and preventing disease with tma and tmao lowering agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
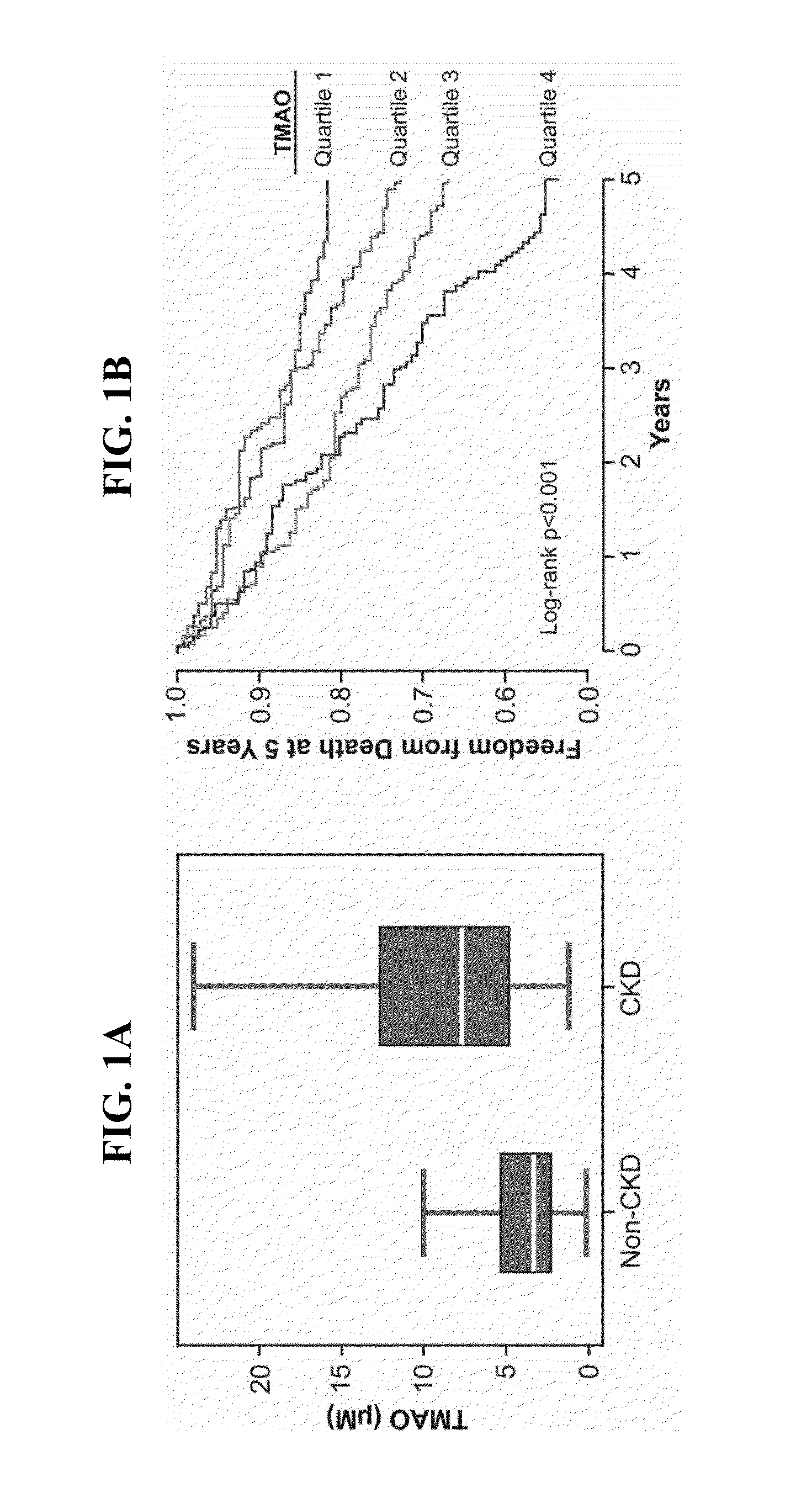
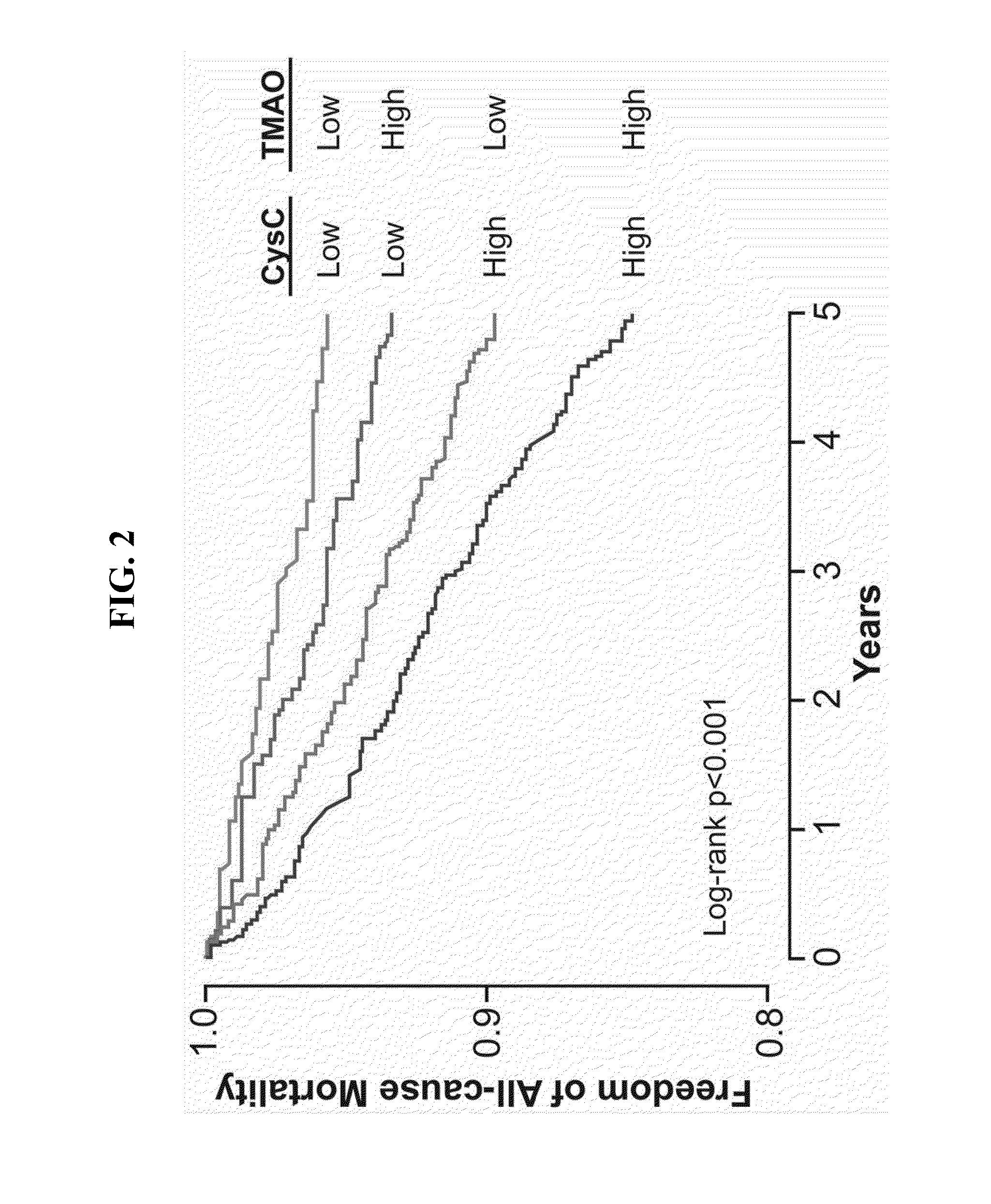
The Gut Microbiota-Dependent TMAO Pathway Contributes to Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease
[0057]This example describes an examination of the contribution of TMAO to the development of renal insufficiency and mortality risk in chronic kidney disease (CKD).
Methods
[0058]Human Studies.
[0059]The human population studied is a single-center, prospective cohort approved by the Cleveland Clinic Institutional Review Board. Adult subjects (ages 18 years and above) were included who underwent elective diagnostic coronary angiography for cardiac evaluation from 2001-2007 as previously described (9). Subjects with known acute coronary syndromes or revascularization procedures within 30 days of enrollment, or history of congenital heart disease, were excluded. After informed consent, fasting plasma blood samples were collected using ethylenediaminetetraacetic acid tubes prior to any drug administration via the arterial sheath, and immediately processed...
example 2
ACE (Acetylsalicylic Acid) Reduction of TMAO Production In Vivo
[0079]This Example investigated the question of whether aspirin can aspirin (ASA) mitigate the prothrombotic phenotype associated with high choline or TMAO related diet. And if so, is there evidence that aspirin therapy itself impacts TMAO production in vivo, and could this be a mechanism through which aspirin promotes some of its cardiovascular benefit.
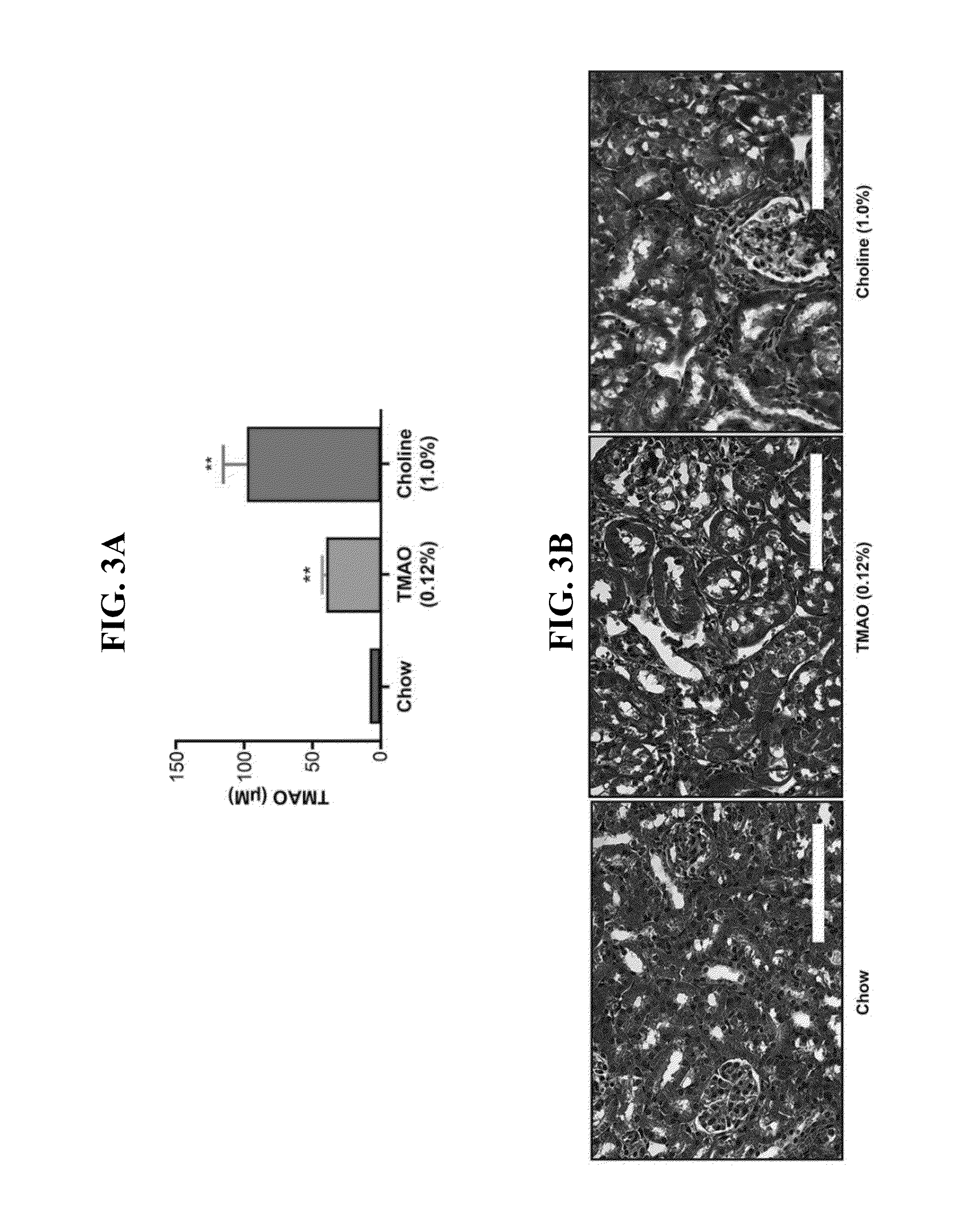
[0080]In the first part of this Example, the impact of aspirin (ASA) therapy on the prothrombotic phenotype provoked by chronic choline supplementation in the diet was examined. This diet elevates TMAO, and provokes enhanced platelet responsiveness and thrombosis. C57BL / 6J females mice were placed on a chemically defined diet comparable to the normal mouse feed (chow) vs. an identical diet supplemented with 0.5 gm % choline (choline). Each of these two groups of mice were then split, and half placed on aspirin therapy at a dose comparable to that used in humans (ASA 4 mg / ...
example 3
Identification of yeaW and yeaX as TMA Lyases
[0088]As part of this example, microbial enzymes of unknown function were searched for that were clustered with those known to synthesize or use either malate or succinate (potential products formed following carntine utilization) and a presumed betaine-carnitine-choline transporter. One potential candidate was gene pair previously called yeaW / X in E. Coli DH10B (yeaW (dioxygenase), GeneID: 6060925 and yeaX (oxidoreductase), GeneID: 6060982). Using a modified pET20 plasmid, E. coli BL21pLysS was transformed with each allele and subsequently individually purified recombinant yeaW and yeaX from bacterial lysates using the 8×His metal affinity chromatography. When the purified proteins are combined, the yeaW / X complex demonstrated carnitine TMA lyase activity (monitored by d9-TMA production from d9-carnitine). Interestingly, further characterization of the recombinant microbial yeaW / X complex revealed substrate promiscuity, catalyzing produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Spectrum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com