Preparation method of briracetam and intermediate compound
A technology for compounds and intermediates, applied in the field of compound synthesis, can solve the problems of harsh reaction conditions, explosion risks, and high industrial scale-up costs, and achieve the effect of mild reaction conditions and a simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
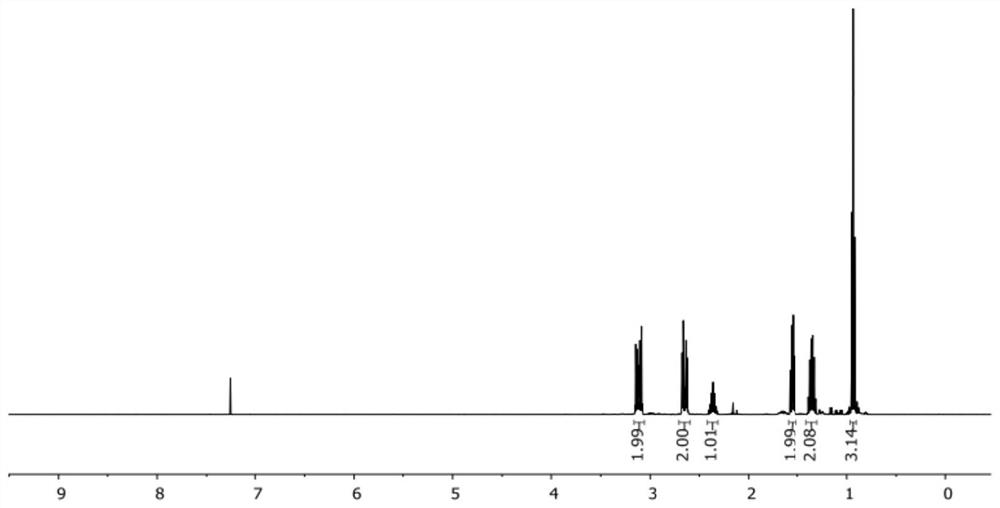
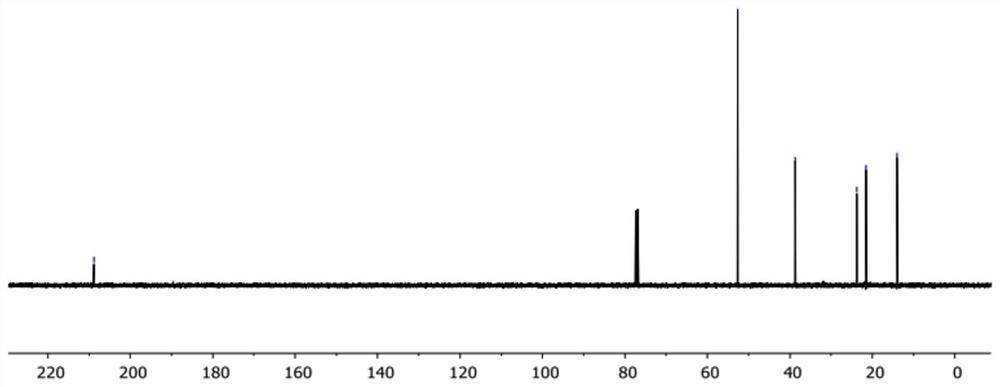
[0110] Preparation of compound 1
[0111]
[0112] Trichloroacetyl chloride (2.24 mL, 20 mmol) and phosphorus oxychloride (1.02 mL, 11.0 mmol) were dissolved in ether (10 mL), and the solution was slowly added dropwise to a solution containing 1-pentene (1.09 mL, 10 mmol) , diethyl ether (20 mL) and zinc-copper reagent (Zinc-Copper couple, CAS#: 53801-63-1, 1.96 g, 30.0 mmol) in a flask. The reaction was heated to 40°C and stirred for 2 hours, and then naturally cooled to room temperature and stirred for 8 hours. Then, the solution was filtered through celite, and 80 mL of n-hexane was added to the filtrate to precipitate zinc chloride salts. The clear solution was obtained by filtration, washed with water, saturated sodium bicarbonate solution and saturated brine successively, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and evaporated to remove the solvent to obtain a pale yellow oil (compound 5) (1.69 g), yield 94%, proceed directly to the ...
Embodiment 2
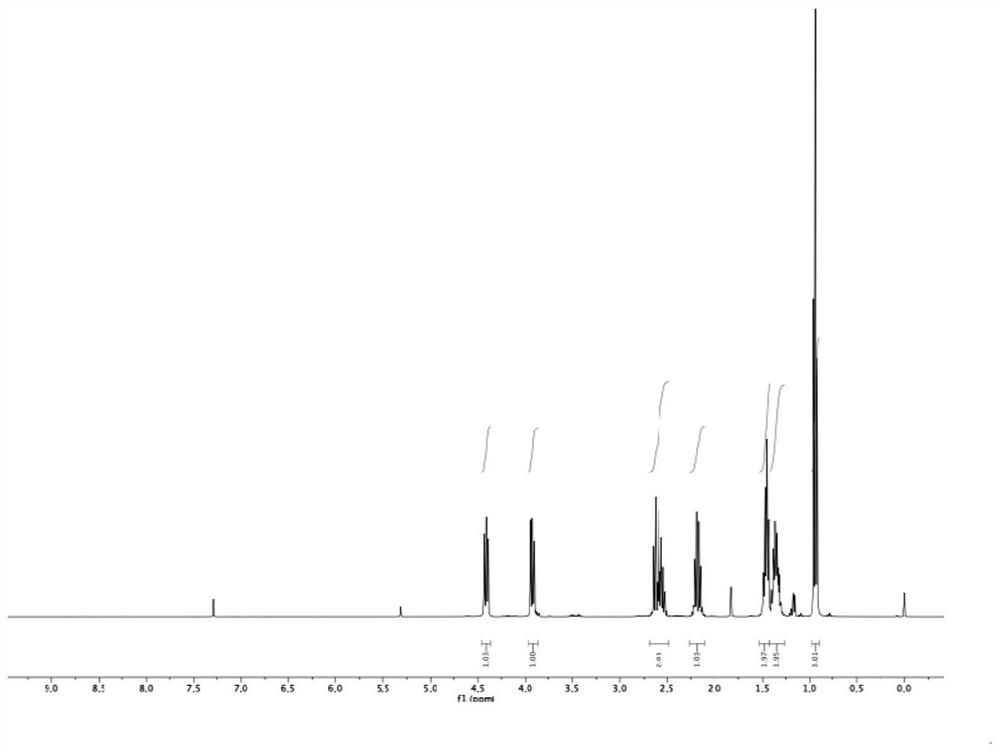
[0118] Preparation of compound 2
[0119]
[0120] Dissolve 0.39 g of ligand compound L2 and 0.24 g of scandium trifluoromethanesulfonate in 100 mL of ethyl acetate, and stir at 35 ° C for 1 hour to obtain a catalyst 1Sc (OTf) with a concentration of 0.005 M 3 -RaPr 3 ethyl acetate solution.
[0121] To a dry reaction vial under nitrogen protection, 112 mg (1.0 mmol) of compound 1, and 10 mL (0.05 mmol) of a solution of catalyst 1 prepared as described above were added. After adding 10 mL of ethyl acetate, the temperature was lowered to -20°C, 207 mg (1.2 mmol) of m-chloroperoxybenzoic acid was added, and the reaction was continued to stir at -20°C for 20 hours. Then, saturated potassium carbonate solution was added to quench the reaction, and the reaction solution was extracted three times with dichloromethane. The pure compound 2 was isolated by normal phase column chromatography on silica gel with a yield of 83% and a chiral purity of 99.8%.
[0122] Structural data ...
Embodiment 3
[0126] Preparation of compound 2
[0127]
[0128] Installed (PhCN) 2 PdCl 2 (10 mg, 0.025 mmol, 5.0 mol %) and Ligand Compound L7 (13 mg, 0.0275 mmol, 5.5 mol %) were added to a bottle of 2 mL of dry tetrahydrofuran, and stirred at room temperature for 1 hour. Then AgSbF6 (17 mg, 0.05 mmol, 10 mol%) was added, stirring was continued for 1 hour, and filtered to obtain a solution of catalyst 2. Compound 1 (56 mg, 0.5 mmol) was added to the filtrate, the reaction solution was cooled to -40°C with stirring, carbamide peroxide (61 mg, 0.65 mmol) was added, and the mixture was stirred at -40°C for 8 hours. Concentrated under pressure, purified compound 2 was obtained by column separation, the yield was 72%, and the chiral purity was 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com