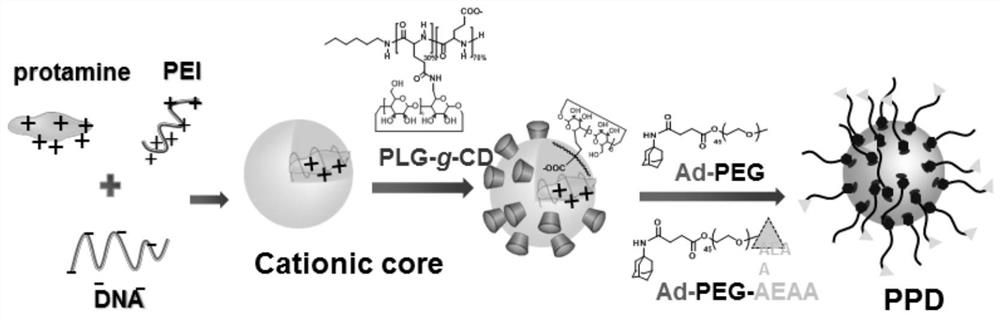
Virus-like structure gene vector, drug delivery system, and preparation method and application thereof
A structural gene and gene drug technology, applied in the field of biomedicine, can solve the problems of lack of non-viral gene delivery vectors, poor targeting of gene transfection, inability to achieve targeted delivery, etc., and achieve strong anti-tumor immune response and tumor growth inhibition. , good effect of gene transfection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
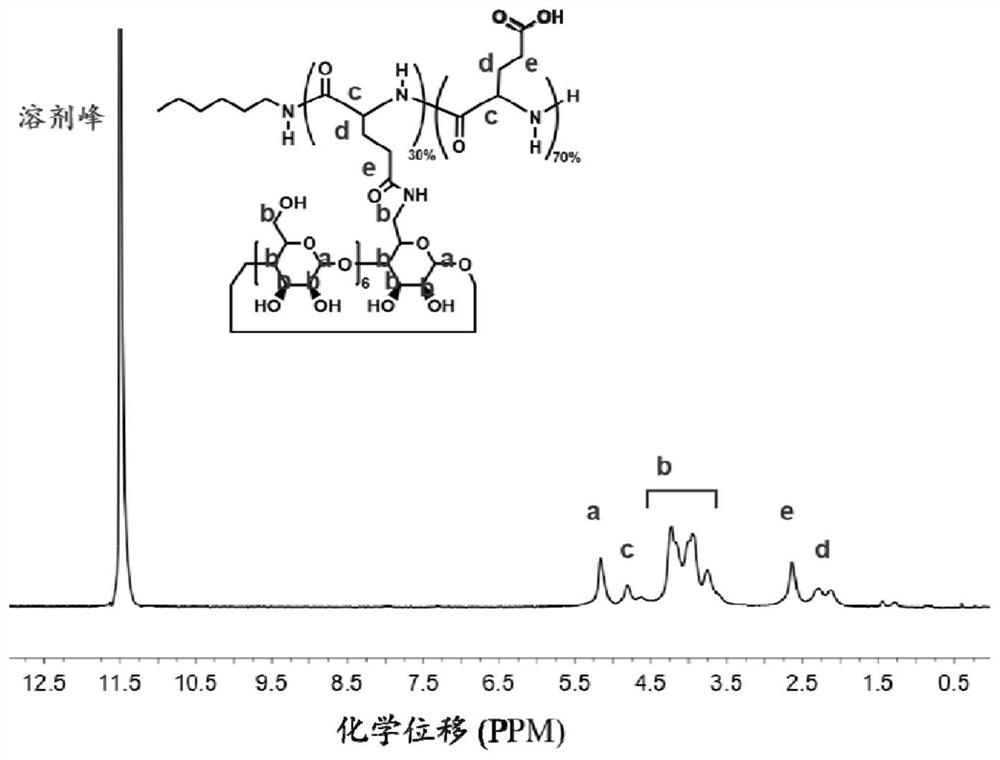
[0041] The preparation of embodiment 1 poly (L-glutamic acid)-graft cyclodextrin (PLG-g-CD)
[0042] 300 mg of PLG (with approximately 120 L-glutamic acid repeating units), 64 mg of N,N-diisopropylethylamine (DIPEA) and 184 mg of 2-(7-azabenzotriazole)- N,N,N',N'-Tetramethyluronium hexafluorophosphate (HATU) was dissolved in 10mL of N,N-dimethylformamide (DMF) solution, stirred at room temperature until all solid substances were dissolved, and 900mg of aminocyclodextrin (CD-NH 2 ) was dissolved in 3 mL of DMF, added to the above DMF mixture, and reacted at room temperature for 48 h. The product was purified by dialysis with sterile water for injection, and freeze-dried to obtain the final product PLG-g-CD.
[0043] The PLG-g-CD is analyzed, the results can be found in figure 2 , figure 2 For the PLG-g-CD obtained in Example 1 1 H NMR spectrum.
Embodiment 2
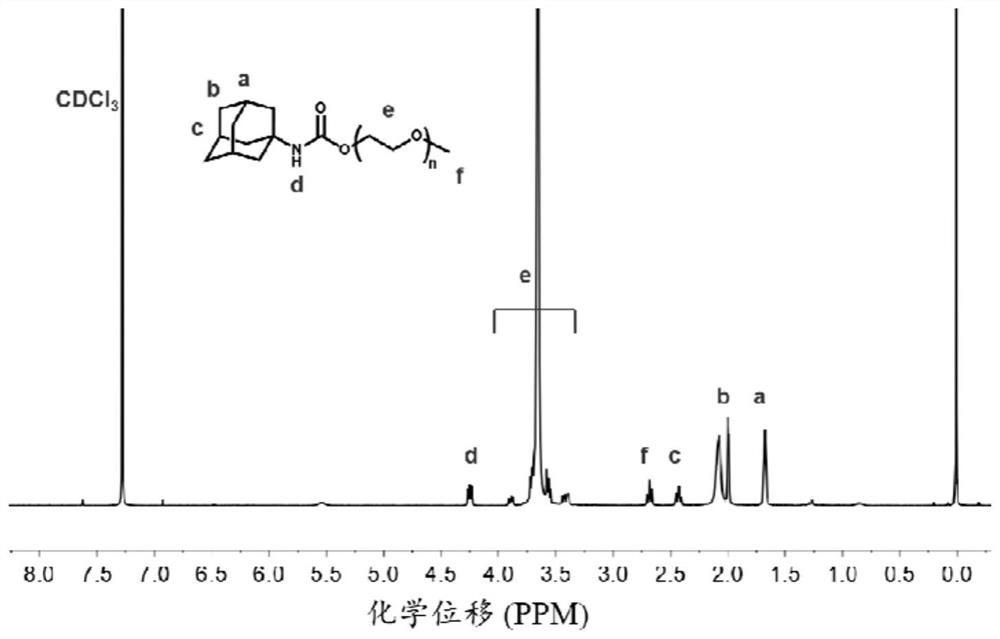
[0044] The preparation of embodiment 2 amantadine-polyethylene glycol (Ad-PEG)
[0045] 120 mg of polyethylene glycol (PEG, molecular weight 2000Da), 12 mg of DIPEA and 32 mg of HATU were dissolved in 3 mL of DMF solution, and 16 mg of aminoamantadine (Ad-NH 2 ) was dissolved in 4 mL of DMF, and added to the above DMF reaction system, and reacted for 48 h under stirring at room temperature. The product was purified by dialysis with sterile water for injection, and freeze-dried to obtain the final product Ad-PEG.
[0046] The Ad-PEG is analyzed, the results can be found in image 3 , image 3 for the obtained Ad-PEG 1 HNMR spectrum.
Embodiment 3
[0047] Embodiment 3 Preparation of amantadine-polyethylene glycol-aminoethylanisamide (Ad-PEG-AEAA)
[0048] First prepare aminoethylanisamide (AEAA): 410mg of 2-bromoethylamine and 800mg of DIPEA were dissolved in 4mL of acetonitrile, 300mg of p-methoxyphenylacetyl chloride was dissolved in 3mL of acetonitrile, and the above two solutions Mix and react for 6h under stirring at room temperature.
[0049] Next, bond AEAA on one side of the bifunctional PEG: add 200 mg of hydroxypolyethylene glycol amino (HO-PEG-NH 2 ), stirred and reacted at 80°C for 12h.
[0050] Finally, amantadine (Ad) was bonded to the other end of the bifunctional PEG: 60 mg of N,N'-carbonyldiimidazole (CDI) was dissolved in 2 mL of acetonitrile and slowly added to the above mixed solution, and reacted at 50 °C 5h to activate CDI, 160mg of Ad-NH 2 Dissolved in 4mL DMF and injected into the above reaction system, stirred at 50°C for another 48h. The product was purified by dialysis with sterile water fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com