Preparation method of polyamidoamine dendritic macromolecular structure modifier and application thereof
A polyamidoamine-type, macromolecular structure technology, applied in the direction of antibacterial drugs, can solve the problems of cytotoxicity, in-depth research and application, and achieve the effect of low synthesis cost, good water solubility, and inhibition of expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Synthesis of LED209 carboxyl derivatives
[0033] The following are the synthesis routes of two carboxyl derivatives of LED209, which are para-position (para-) and meta-position (meta-) carboxyl groups. The synthesis routes are as follows:
[0034] .
[0035] (1) Synthesis of Compound 1: Take a 100 mL round bottom flask, add dichloromethane (DCM, 50 mL), and while stirring, add aniline (SM-2, 5.1 g, 54.8 mmol), triethylamine (11 g , 109 mmol) and p-acetamidobenzenesulfonyl chloride (SM-1, 11.68 g, 50 mmol), stirred at room temperature for 10 h. The next day, dilute the reaction solution with 100 mL DCM, add 30 mL of 1 N HCl solution to wash the organic phase 3 times, wash with Na 2 SO 4 After thorough drying and spin-drying, 12 g of the target compound was obtained with a yield of 82.7%. The theoretical molecular weight of compound 1 is 290.5, and the detection value M-1 peak is 289.45 (see Figure 8 ).
[0036] (2) Synthesis of compound 2: 1 N...
Embodiment 2
[0045] Embodiment 2: the synthesis of para / meta-PAMAM G3.0-LED209
[0046] Accurately weigh para-LED209-COOH (30 mg, 0.070 mmol) prepared in Example 1, G3.0 PAMAM-NH 2 (242 mg, 0.035 mmol) was dissolved in N,N-dimethylformamide (DMF, 10 mL), triethylamine (21 mg, 0.21 mmol) and 1-hydroxybenzotriazole (HOBT, 9.5 mg , 0.070 mmol), and the reaction solution was stirred overnight at room temperature. The reaction solution was purified by preparative chromatography (pre-HPLC) to obtain 55 mg of light yellow oil.
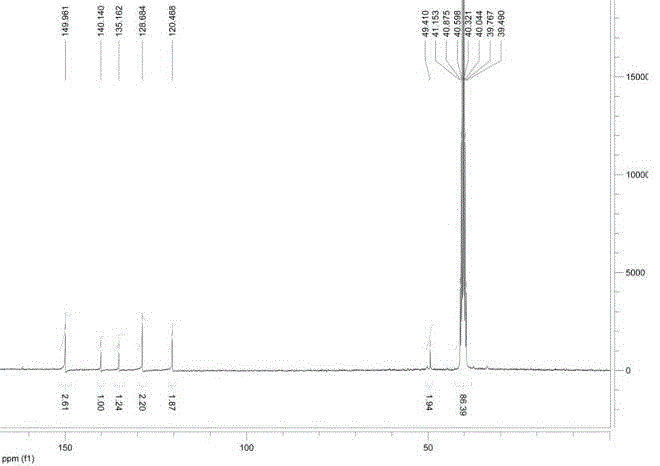
[0047] Accurately weigh the meta-LED209-COOH (30 mg, 0.070 mmol) prepared in Example 1, G3.0 PAMAM-NH 2 (242 mg, 0.035 mmol) was dissolved in N,N-dimethylformamide (DMF, 10 mL), triethylamine (21 mg, 0.21 mmol) and 1-hydroxybenzotriazole (HOBT, 9.5 mg , 0.070 mmol), and the reaction solution was stirred overnight at room temperature. The reaction solution was purified by preparative chromatography (pre-HPLC) to obtain 56 mg of a pale yellow oil. NMR was determined ...
Embodiment 3
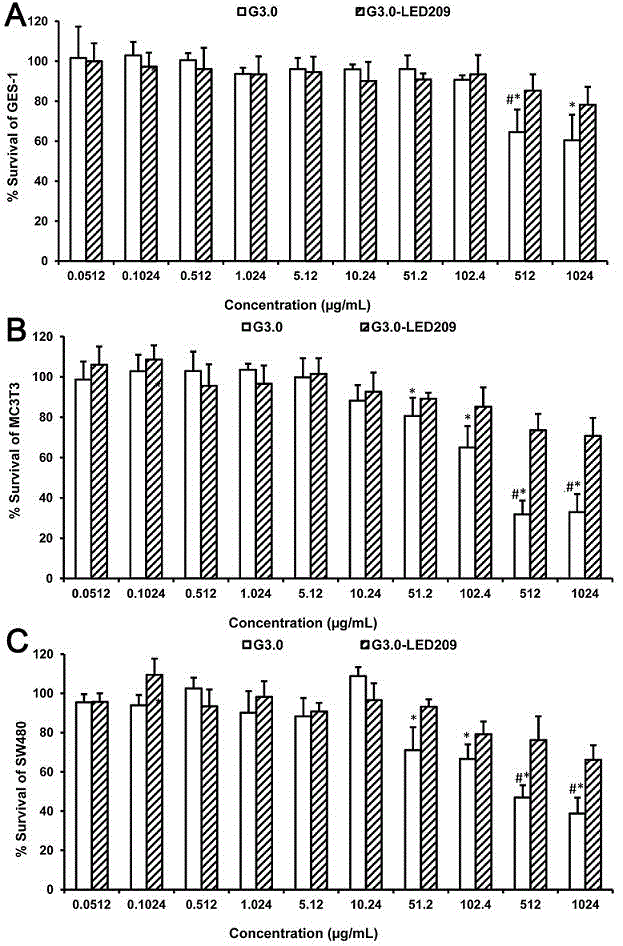
[0053] Example 3: In vitro antibacterial activity and cytotoxicity investigation of G3.0 PAMAM-LED209
[0054] 1. Materials and instruments
[0055] 1.1 Experimental strains
[0056] The 12 strains of Gram-negative bacteria, including 9 standard strains and 3 drug-resistant strains (see Table 2), were used in this part of the experiment.
[0057] 9 strains of standard strains and 3 strains of drug-resistant strains used in the test of table 2
[0058] Chinese name English name English abbreviations Standard strain Type strain TS Escherichia coli Escherichia coli ATCC 25922 E. coli Escherichia coli Escherichia coli K12MG1655 E. coli MG 1655 Pseudomonas aeruginosa 261H Pseudomonas aeruginosa ATCC 27853 P. aeruginosa Klebsiella pneumoniae Klebsiella pneumonia ATCC13883 K. pneumonia Salmonella Enteritidis 262H Salmonella enterica ATCC 9150 S. enteric Acinetobacter baumannii Ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com