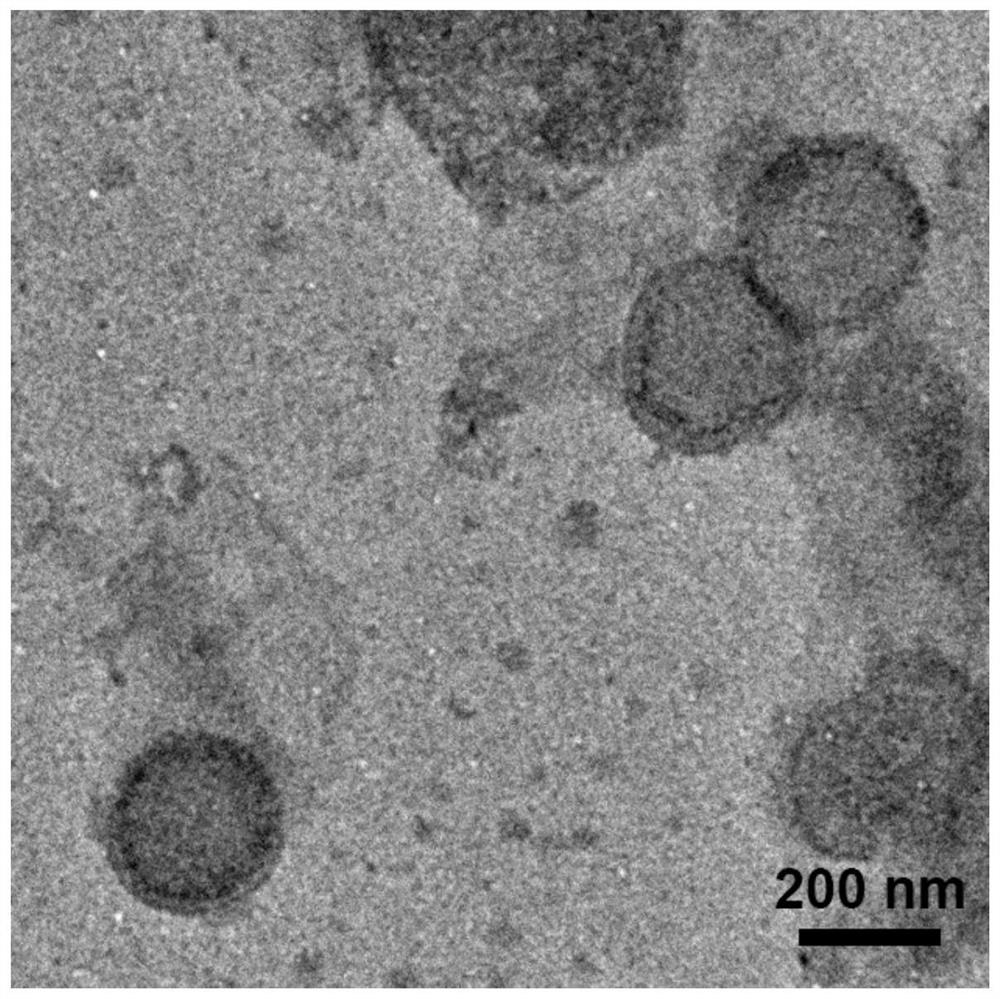
RNS-response-based 'nervous vascular unit' regulation and control targeted liposome drug delivery system and preparation method and application thereof
A technology targeting liposomes and drug delivery systems, applied in the field of anti-ischemic stroke, can solve problems such as disappointment, achieve less leakage, improve delivery, and improve therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Preparation of N-(2-aminophenyl)tetradecamide:
[0040] Myristic acid (10mmoL) was dissolved in tetrahydrofuran (150mL), placed in a round bottom flask, and then added 6-chlorobenzotriazole-1,1,3,3-tetramethyluronium hexafluorophosphate ( HCTU) (12mmoL) was stirred for 10 minutes; o-phenylenediamine (10mmoL) was dissolved in acetonitrile (10mL), and the above solution was added and stirred for 15min; triisopropanolamine (30mmoL) was added, and the mixture was stirred overnight at room temperature to Make sure to react completely. The organic solvent was removed by rotary evaporation, and the crude product was purified by chromatography using ethyl acetate:petroleum ether (2:1) as the eluent after extraction.
[0041] Wherein, the structural formula of N-(2-aminophenyl)tetradecamide recorded by nuclear magnetic resonance spectrum is:
[0042] 1 H NMR (400MHz, DMSO-d 6 ,δ):δ:0.795-0.835(t,3H,CO(CH 2 ) 12 CH 3 ),1.162-1.270(m,20H,Ar-COCH 2 CH 2 (CH 2 ) 10 CH ...
Embodiment 2
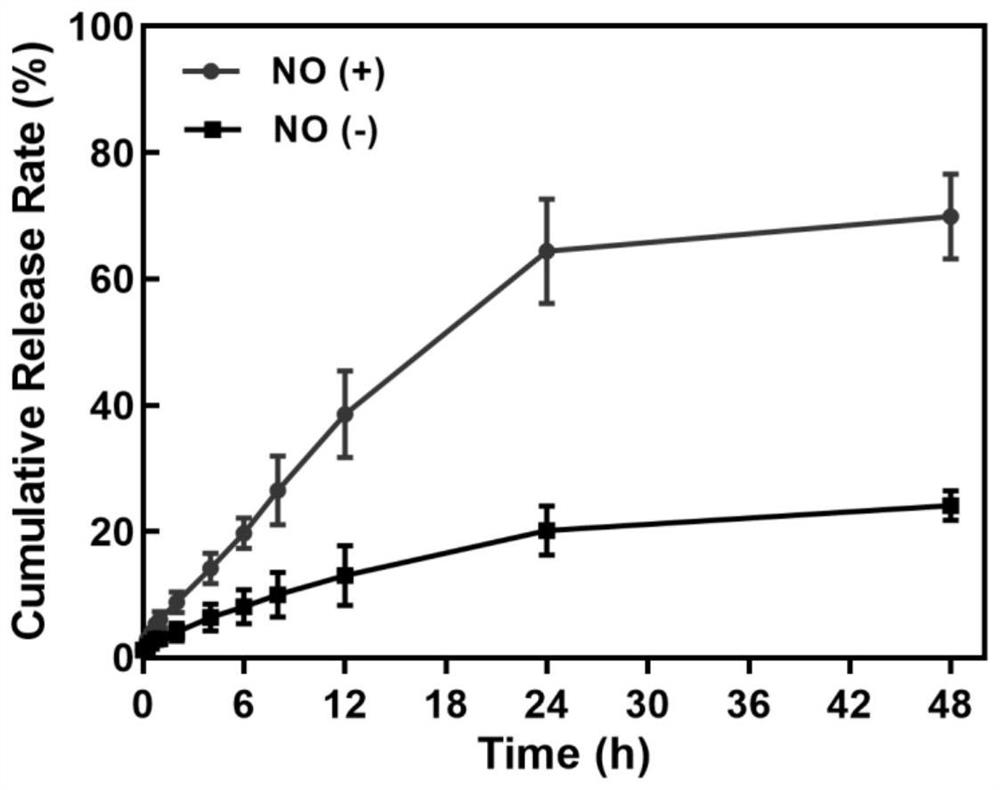
[0048]Example 2 The "neurovascular unit" based on RNS response regulates the in vitro release of targeted liposome drug delivery system
[0049] The dialysis method was used to investigate the in vitro release behavior of coumarin-6 in the liposome drug delivery system, wherein the liposome preparation method encapsulating coumarin-6 was carried out according to Example 1. In this release experiment, the concentration in the medium after the complete release of coumarin-6 was designed to be 20 μg / mL to ensure that the drug release was carried out in a sink state. The release media were saturated NO solution prepared in phosphate buffer (pH=7.4) containing 0.5% Tween-80 and phosphate buffer (pH=7.4) containing 0.5% Tween-80, respectively, and the R-Lipo - Put 2mL of C6 solution in a pre-expanded dialysis bag (MWCO=2.50kDa), tie the bag tightly, put it into a 50mL EP tube containing 25mL release medium, and release it in a constant temperature shaking box (37°C, 150rpm) In the ...
Embodiment 3
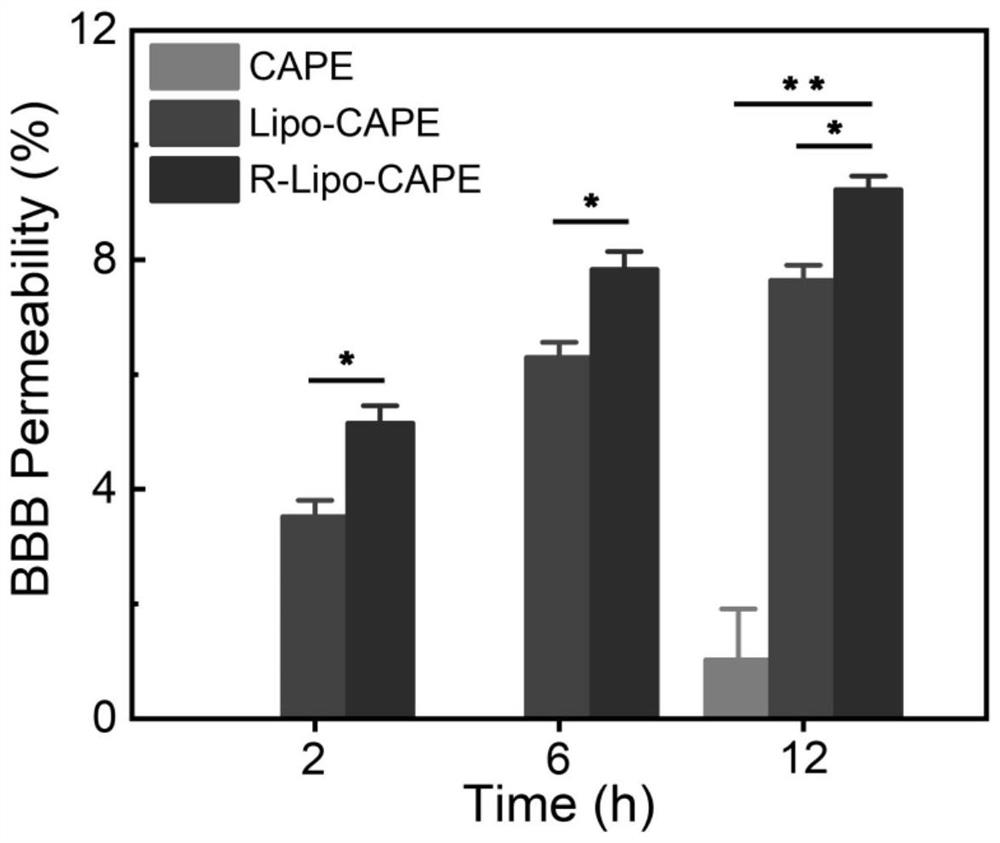
[0055] In vitro BBB permeability investigation of targeted liposome drug delivery system based on RNS response regulation of "neurovascular unit"
[0056] First establish the Transwell monolayer cell model: bEnd.3 cells in 5×10 4 Cells / well density were seeded in Transwell cell culture chambers (pore size 1.0 μm, surface area 0.33 cm 2 ), placed in a 24-well plate for routine culture for 3 days, when the cell confluence reached 90%, the culture solution was discarded, and the upper and lower chambers were replaced with 10 3 Nmol / L hydrocortisone in DMEM culture solution, cultivated for 3 days, to make the cell junction proteins compact and form a dense monolayer. Aspirate and discard the culture medium in the Transwell chamber, and establish the oxygen-glucose deprivation culture model (Oxygen-glucose deprivation, OGD) of bEnd.3 cells, that is, after washing with PBS, replace with sugar-free medium and culture in a tri-gas incubator for 12 hours, and then replace with The co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com