Macrophage membrane coated arginine deiminase/catalase/IR780 nanoparticle, preparation method and application
An arginine deiminase and catalase technology, applied in biochemical equipment and methods, oxidoreductases, enzymes, etc., can solve the problems of poor stability, low bioavailability, poor targeting, etc. Effects of poor stability, high immunogenicity, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
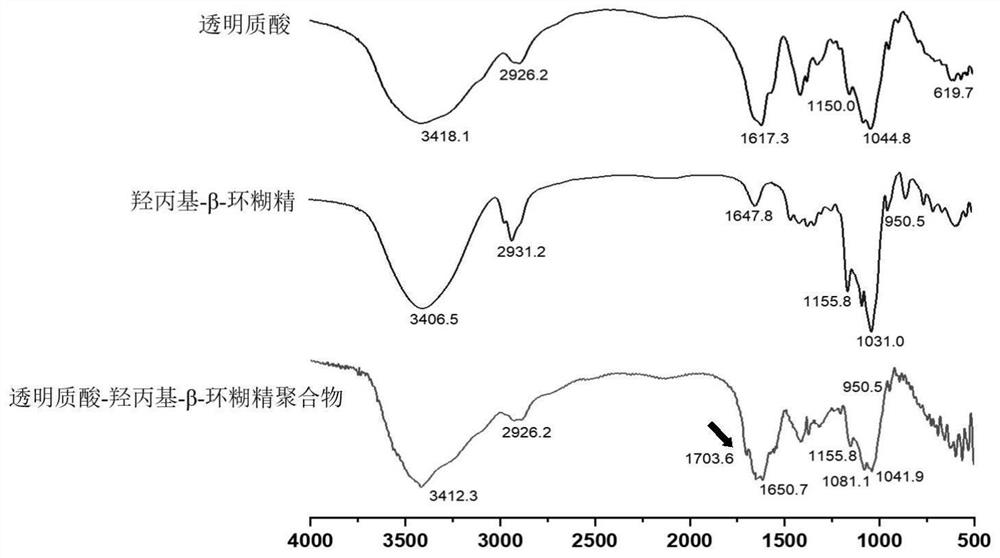
[0032] Macrophage membrane-coated arginine deiminase / catalase / IR780 nanoparticles include arginine deiminase / catalase / IR780 nanoparticles and macrophage membrane coating. Macrophage membrane-coated arginine deiminase / catalase / IR780 nanoparticles formula has the weight-to-number ratio of each component: 4.5 parts of hyaluronic acid, 2.5 parts of hydroxypropyl-β-cyclodextrin, 3 parts of lecithin, 1 part of cholesterol, 1 part of distearoylphosphatidylethanolamine-polyethylene glycol 2000, 200 parts of bis(2-hydroxyethylamino)tris(hydroxymethyl)methane-hydrochloride buffer, The buffer concentration is 50 mM, the buffer pH is 6.5, the content of arginine deiminase is 0.1 mg / mL, the content of catalase is 0.05 mg / mL, and the content of IR780 is 0.01 mg / mL.
[0033]Preparation method: (1) The preparation method of HA-HCD: dissolve the hyaluronic acid of prescription amount in anhydrous formamide, add 1-ethyl-3-[3-(dimethylamino)propyl]carbodiethylene After stirring the amine hydroc...
Embodiment 2
[0035] Macrophage membrane-coated arginine deiminase / catalase / IR780 nanoparticles include arginine deiminase / catalase / IR780 nanoparticles and macrophage membrane coating. Macrophage membrane-coated arginine deiminase / catalase / IR780 nanoparticles formula has the weight-to-number ratio of each component: 5.1 parts of hyaluronic acid, 3.1 parts of hydroxypropyl-β-cyclodextrin, 5.0 parts of lecithin, 2.8 parts of cholesterol, 2.0 parts of distearoylphosphatidylethanolamine-polyethylene glycol 5000, 636 parts of dipotassium hydrogen phosphate-phosphate buffer, the buffer concentration is 120mM, the buffer pH is 6.0, arginine The content of acid deiminase is 0.1 mg / mL, the content of catalase is 0.15 mg / mL, and the content of IR780 is 0.02 mg / mL.
[0036] Preparation method: (1) The preparation method of HA-HCD: dissolve the hyaluronic acid of prescription amount in anhydrous formamide, add 1-ethyl-3-[3-(dimethylamino)propyl]carbodiethylene After stirring the amine hydrochloride in...
Embodiment 3
[0038] Macrophage membrane-coated arginine deiminase / catalase / IR780 nanoparticles include arginine deiminase / catalase / IR780 nanoparticles and macrophage membrane coating. Macrophage membrane-coated arginine deiminase / catalase / IR780 nanoparticles formula has the following weight-to-number ratios of components: 5.6 parts of hyaluronic acid, 3.5 parts of hydroxypropyl-β-cyclodextrin, 5.6 parts of lecithin, 3.6 parts of cholesterol, 1 part of distearoylphosphatidylethanolamine-polyethylene glycol 2000, 1.1 parts of distearoylphosphatidylethanolamine-polyethylene glycol 5000, tris-hydrochloric acid 690 copies of salt buffer solution, the buffer concentration is 130mM, the buffer solution pH is 6.3, the content of arginine deiminase is 0.1mg / mL, the content of catalase is 0.12mg / mL, and the content of IR780 is 0.03mg / mL mL.
[0039] Preparation method: (1) The preparation method of HA-HCD: dissolve the hyaluronic acid of prescription amount in anhydrous formamide, add 1-ethyl-3-[3-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com