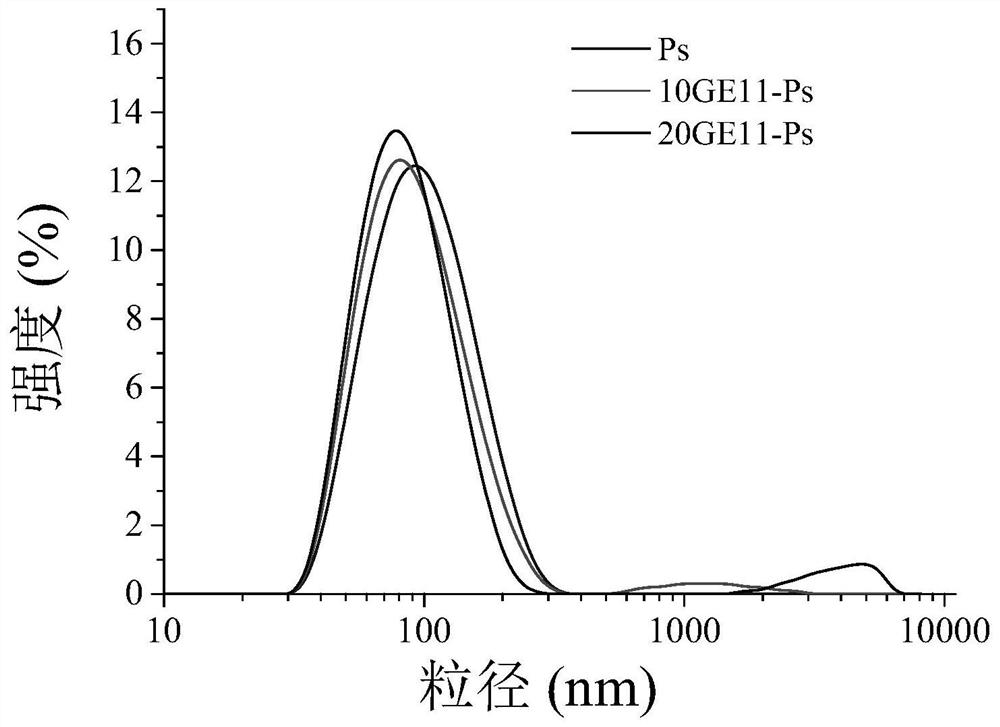
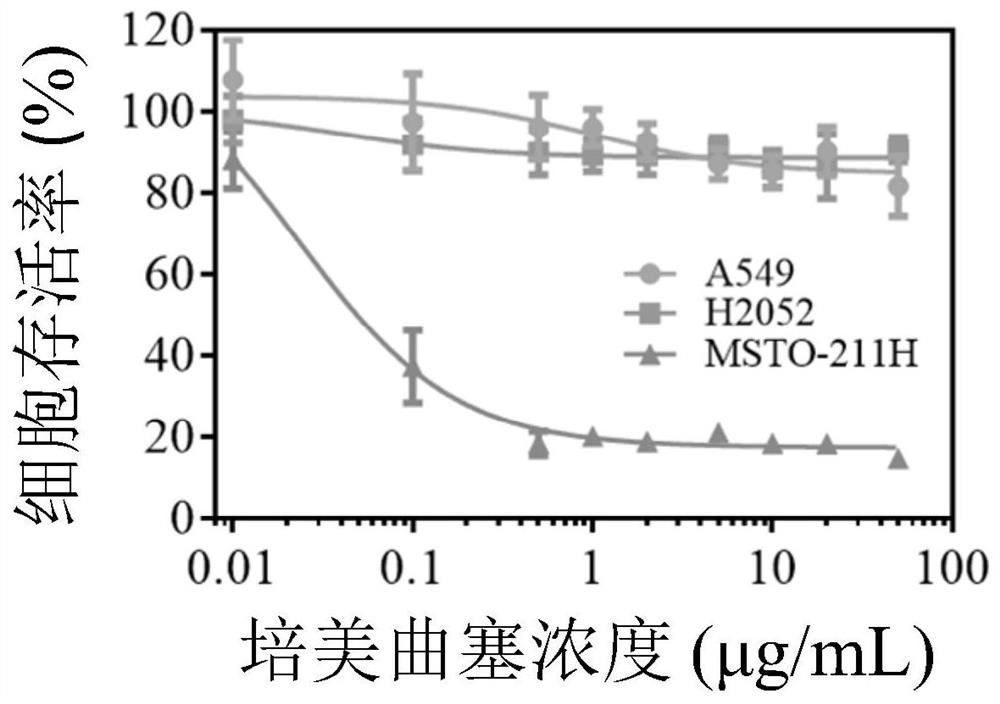
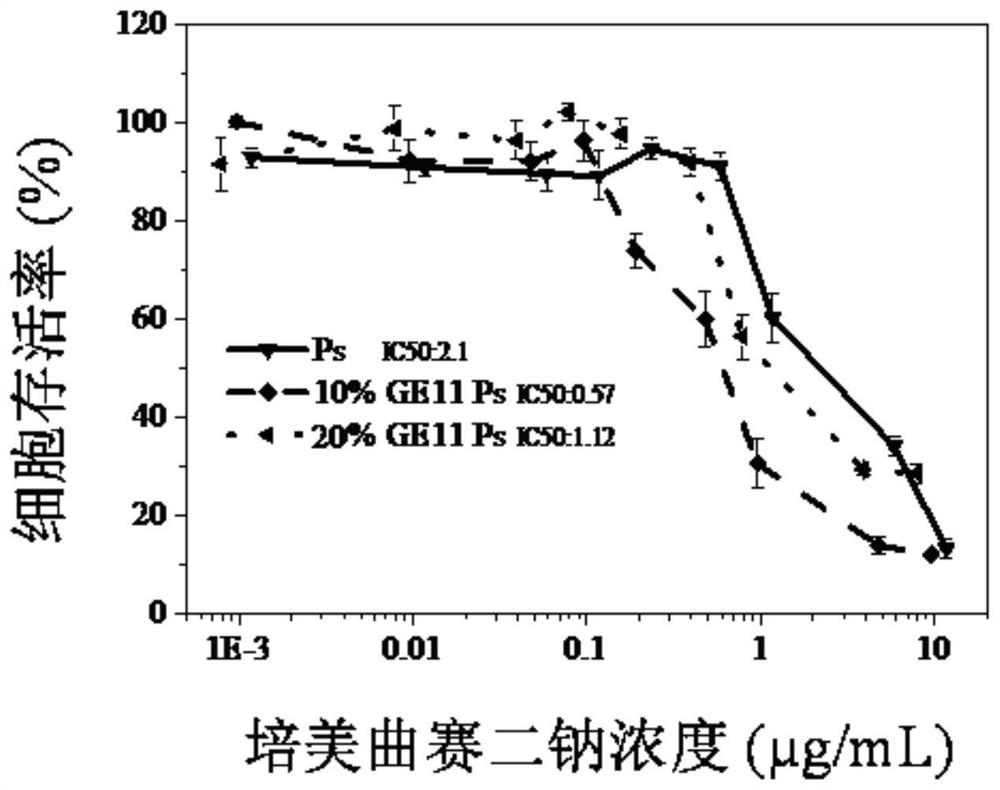
Carbonic ester polymer vesicle for micromolecule-carrying medicine, and preparation method and application of carbonic ester polymer vesicle
A technology of carbonate polymers and small molecules, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve low bioavailability, kidney toxicity and side effects, and short cycle time and other issues, to achieve enhanced anti-tumor effect, good biocompatibility, and long circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0048] The polymers were prepared according to the prior art.
[0049] In a nitrogen glove box, MeO-PEG-OH (Mn=5.0 kg / mol, 0.50 g, 100 μmol), TMC (1.52 g, 14.55 mmol) and DTC (0.23 g, 1.18 mmol) were successively weighed and dissolved in di In methyl chloride (DCM, 7.0 mL), the catalyst diphenyl phosphate (DPP, DPP / OH molar ratio is 10 / 1) was added with stirring. The airtight reactor was sealed and placed in an oil bath at 40°C under magnetic stirring for 2 days; triethylamine was terminated, precipitated twice in glacial ether, filtered by suction, and dried in vacuum to obtain PEG5k-P (DTC2k-TMC15k).
[0050] The targeted carbonate polymer is GE11-PEG-P (TMC-DTC). In the nitrogen environment of the glove box, the macromolecular initiator NHS-PEG-OH (75 mg, 10 μmol), dithiopentane trimethylene Carbonate (DTC, 20 mg, 0.1 mmol) and trimethylene carbonate (TMC, 230 mg, 2.25 mmol) were fully dissolved in 1.6 mL of dichloromethane (DCM), and then rapidly accelerated DPP ( 25 mg,...
Embodiment 1
[0052] Push 100 μL of PEG5k-P at a concentration of 10 mg / mL into 900 μL of HEPES weakly alkaline buffer (5 mM, pH: 8.0) containing 20 mM calcium acetate at room temperature using a syringe pump at a pumping rate of 25 mL / min (TMC15k-DTC2k) in DMSO solution, and then the two items were mixed using magnetic stirring (280 rpm). After forming empty vesicles, add acid buffer (0.4M HAc / NaAc with pH 4.0) to adjust to acidic conditions (pH 4.5 ), then add 20 μL of PEM with a concentration of 5 mg / mL (dissolved in purified water) of negatively charged small molecule drugs, mix well and shake in a shaker at 37°C for 12 hours to fully cross-link. Then, dialysis (MWCO: 3500) was performed against phosphate PB buffer (10 mM, pH 7.4) for 8 h to remove organic solvents and free small molecule drugs, during which the medium was changed 5 times, thereby obtaining drug-loaded reversibly core-crosslinked vesicles.
[0053] Change the calcium acetate, and keep the rest unchanged, for a comparati...
Embodiment 2
[0076] Example 2 Cross-linked targeting vesicle PEM-GE11-Ps loaded with small molecule pemetrexed disodium and its release in vitro
[0077] First mix and dissolve PEG5k-P(TMC15k-DTC2k) and GE11-PEG7.5k-P(TMC15k-DTC2k) in DMSO (10mg / mL) at a weight ratio of 9:1 and 4:1 to obtain two groups of targeted / non-targeted To the mixed polymer solution, PEG5k-P(TMC15k-DTC2k) was dissolved in DMSO (10mg / mL) to obtain a set of non-targeted mixed polymer solutions.
[0078] With reference to the preparation method of Example 1, add 20 mM Ca(Ac) to three groups of 900 μL at room temperature. 2 In HEPES (5 mM, pH: 8.0) weakly alkaline buffer solution, use a syringe pump to push 100 μL of two groups of targeting / non-targeting mixed polymer solutions with a concentration of 10 mg / mL at a pumping rate of 25 mL / min, One set of non-targeted mixed polymer solutions; then two mixed with magnetic stirring (280 rpm), after the formation of empty vesicles, then add acidic buffer (0.4M HAc / NaAc at pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com