Oxide solid electrolytes based on lithium halide-doping and low-temperature sintering method thereof
A solid electrolyte, low-temperature sintering technology, applied in electrolytes, circuits, electrical components, etc., can solve the problems of increasing the solid-solid interface resistance, deteriorating battery performance, and deviating from the balance ratio of compound components, reducing grain boundary resistance and reducing raw materials. Easy to obtain, avoid the effect of lithium volatilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
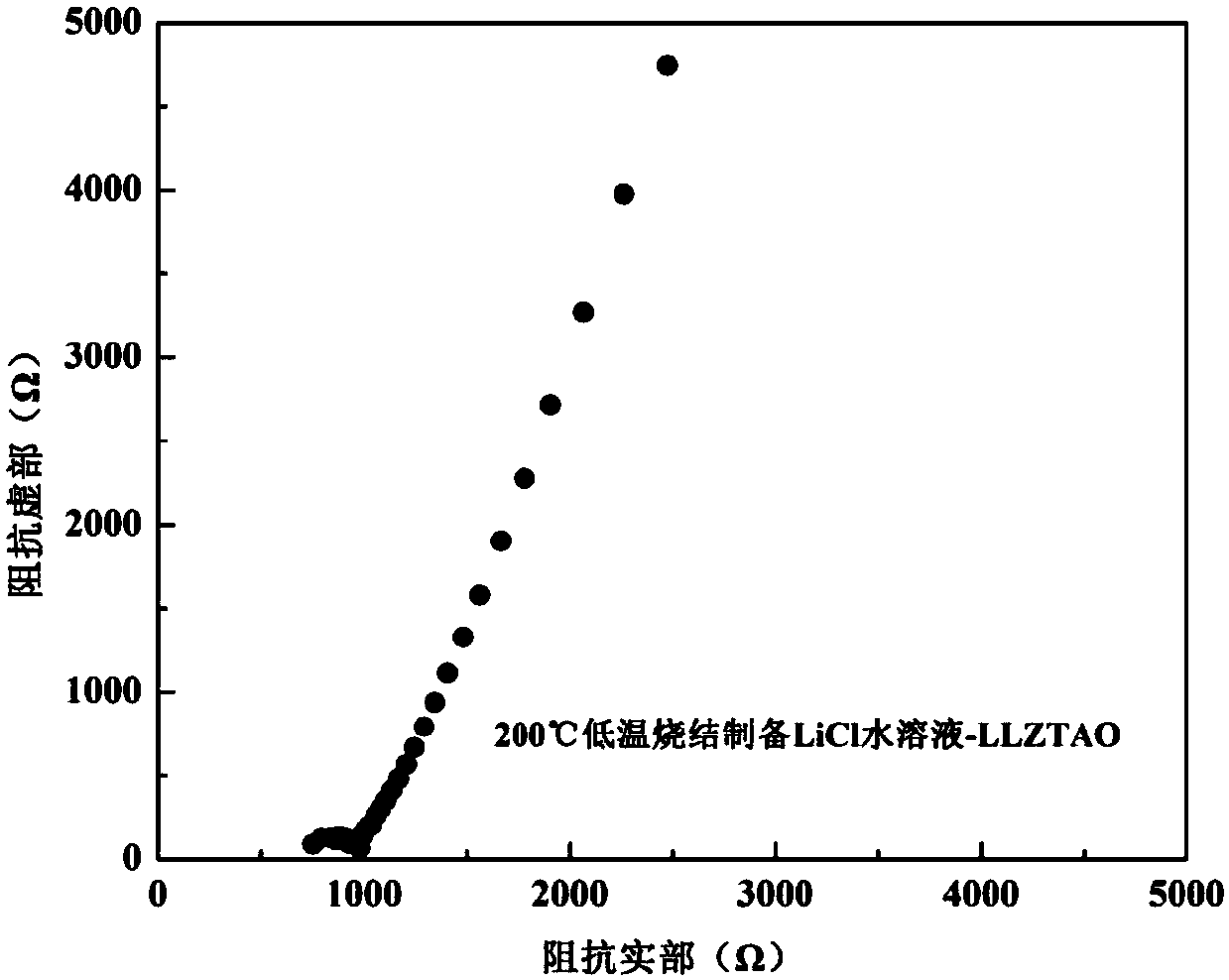
[0023] 1.2223g Li 2 CO 3 (>99%), 2.3412g La 2 o 3 (>99%), 1.0306g ZrO 2 (>99%), 0.4288g TaCl 5 (>99%) and 0.0603g Al 2 o 3 (>99%) mixed in the glove box, under the protection of high-purity argon (99.999%), with a ball mill at 200 rpm, ball milled for 8h, and then the powder after ball milling was put into an alumina crucible and placed in a muffle In the furnace, heat up to 900°C at a heating rate of 2.5°C / min, keep the temperature for 5 hours, and finally cool to room temperature in the furnace. The cooled block was taken out of the crucible, put into a glove box and manually ground into a powder with a particle size of 20 μm in a mortar to obtain a cubic phase Li 6.15 al 0.2 La 3 Zr 1.75 Ta 0.25 o 12 Solid electrolyte powder. Then 0.79g LiCl (>99%) was dissolved in 1ml deionized water to prepare LiCl solution, which was mixed with the prepared cubic phase Li 6.15 al0.2 La 3 Zr 1.75 Ta 0.25 o 12 Sufficient and uniform wetting, cold pressing into sheets and ...
Embodiment 2
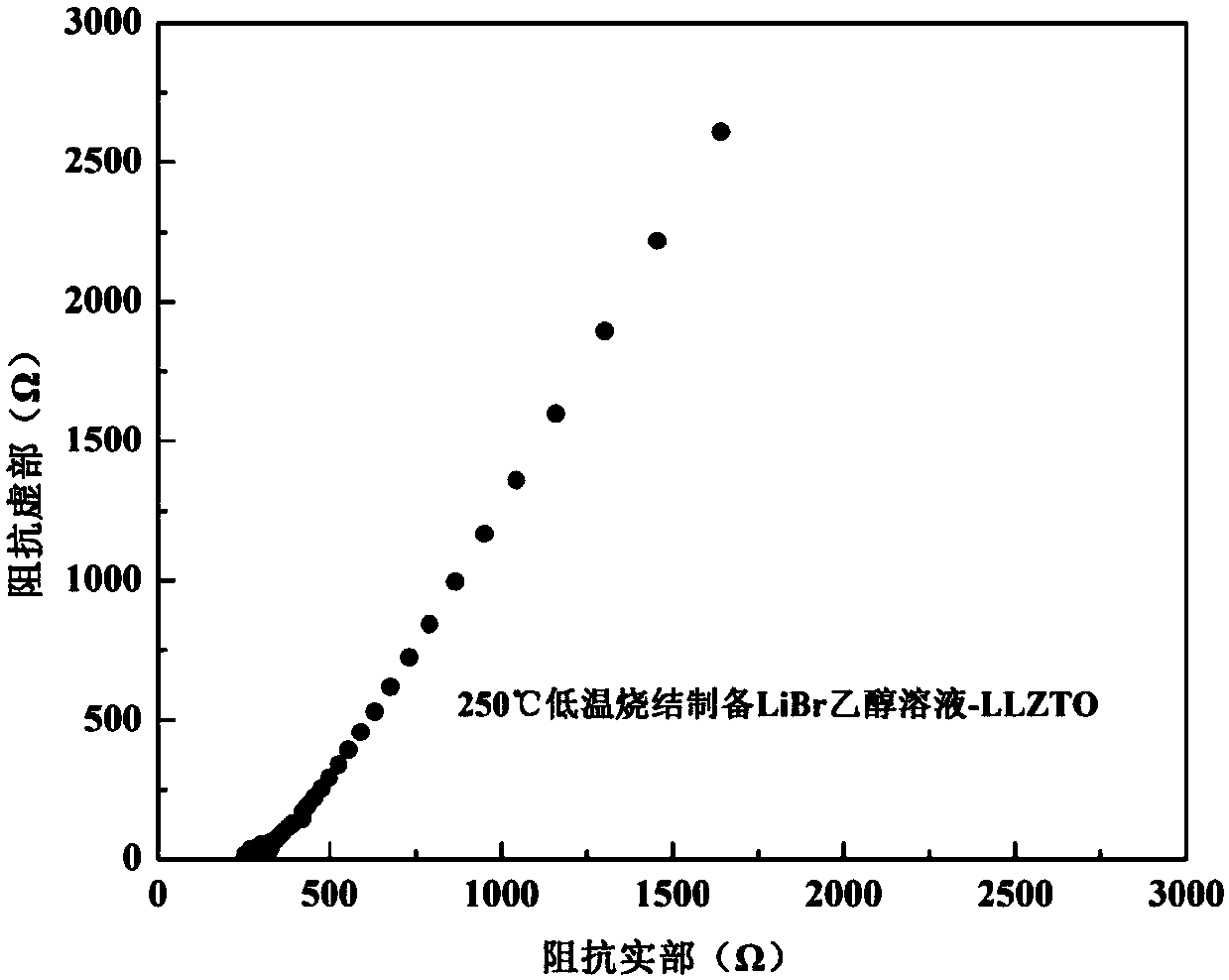
[0027] The purchased commercial cubic phase Li 6.75 La 3 Zr 1.75 Ta 0.25 o 12 Put the solid powder electrolyte into a glove box, and carry out ball milling under the protection of high-purity argon (99.999%). The ball milling adopts a low speed of 100 rpm, and the ball milling time is 1 hour, and is ground into a powder with a particle size of 18 μm. Then 0.26g LiBr was dissolved in 1ml ethanol to prepare LiBr solution, which was mixed with 5g cubic phase Li after ball milling 6.75 La 3 Zr 1.75 Ta 0.25 o 12 The solid powder electrolyte is fully and uniformly wetted, cold-pressed into a sheet, and hot-pressed and sintered at 250 ° C and 20 MPa for 0.5 hours to prepare a lithium bromide-doped oxide solid electrolyte.
[0028] Adopt the impedance test method test described in embodiment 1, such as image 3 As shown, the room temperature conductivity of the ionic conductor can be calculated from the intercept of the oblique line in the curve on the horizontal axis to be a...
Embodiment 3
[0030] 1.3762gLi 2 CO 3 (>99%), 2.3641g La 2 o 3 (>99%), 1.1983g ZrO 2 (>99%) and 0.0591g Al 2 o 3 (>99%) mixed in the glove box, under the protection of high-purity argon (99.999%), use a ball mill at 100 rpm, ball mill for 12h, then put the ball-milled powder into a corundum crucible and place it in a muffle furnace , heated to 850°C at a heating rate of 2°C / min, held for 6 hours, and finally cooled to room temperature with water. The cooled block was taken out from the crucible, put into a glove box and manually ground into powder with a mortar to obtain cubic phase Li 7.7 La 3 Zr 2 al 0.24 o 12 Solid electrolyte powder. 0.52g LiI is dissolved in the tetrahydrofuran solution of the 2M lithium borohydride of 1ml and is formulated into LiI solution, and it is mixed with the cubic phase Li that makes 7.7 La 3 Zr 2 al 0.24 o 12 Sufficient and uniform wetting, cold pressing into sheets and hot pressing and sintering at 100 ° C and 60 MPa for 5 hours to prepare a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity at room temperature | aaaaa | aaaaa |
| Conductivity at room temperature | aaaaa | aaaaa |
| Conductivity at room temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com