A kind of multi-linked dry powder inactivated vaccine of clostridium sheepis-abamectin compound preparation
A technology of abamectin and compound preparations, which is applied in the direction of vaccines, veterinary vaccines, antibacterial drugs, etc., and can solve problems such as wasting material and financial resources, disturbing animals, and animal stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
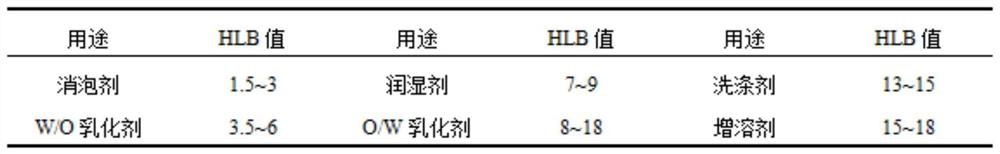
[0047] 1. Accurately weigh 0.100g of avermectin powder into a sterilized ampoule, and add 0.75g of toluene and 0.25g of co-solvent DMF into it. After the drug is completely dissolved, add compound emulsifier Span-20 0.1g and Tween-20 0.3g. Mix well to form the oil phase.
[0048] 2. Take 8.0 g of 20% aluminum hydroxide physiological saline, add 0.05 g of thickener (sodium carboxymethyl cellulose) and 0.4 g of antifreeze agent (propylene glycol) into it, and mix well to form the water phase.
[0049] 3. Drop the oil phase into the water phase, and stir with a high-shear emulsifier at 12,000 rpm for 10 minutes in a sterile state to obtain a well-dispersed abamectin-in-water emulsion (EW);
[0050] 4. Take 1mL of abamectin aqueous emulsion and 1 dose of Clostridium hiatus dry powder vaccine, shake and mix well to obtain a compound preparation.
Embodiment 2
[0051] The development and quality analysis of embodiment 2 clostridioides disease multi-linked dry powder inactivated vaccine-abamectin compound preparation
[0052] 1 Materials and methods
[0053] 1.1 Materials
[0054] 1.1.1 Main equipment and instruments
[0055] Y10 high-shear emulsifier, Shanghai Yicheng Electromechanical Co., Ltd.; AY-120 analytical balance, Japan Shimadzu Corporation; Rise-2006 wet laser particle size analyzer, Jinan Runzhi Technology Co., Ltd.; LC-20AT high-efficiency Liquid chromatograph, Japan Shimadzu Corporation; KQ-700DV CNC ultrasonic cleaner, Kunshan Ultrasonic Instrument Co., Ltd.; SHB-Ⅲ circulating water multi-purpose vacuum pump, Zhengzhou Century Shuangke Experimental Instrument Co., Ltd.; single-channel pipette , Thermo Fisher Scientific (China) Co., Ltd.; PHS-25C acidity meter, Shanghai Yulong Instrument Co., Ltd.; rotary viscometer, Shanghai Hongji Instrument Equipment Co., Ltd.; DHG-9240 blast drying oven, Shanghai No. 1 Heng Scienc...
Embodiment 3
[0285] The immunological research of embodiment 3 clostridium hirsutum multi-linked dry powder inactivated vaccine-abamectin compound preparation
[0286] 1 Immunological potency detection of compound preparation
[0287] 1.1 Rabbit serum antibody detection
[0288] Six big-eared rabbits were taken, numbered 1-6, and 5 mL of blood was collected from each animal before injection, the serum was separated, and stored at -20°C for future use. Rabbits 1 to 4 were the test group, and each rabbit was subcutaneously injected with 1 mL of the compound preparation. Rabbit No. 5 was injected with 1 mL of vaccine subcutaneously as a positive control, and rabbit No. 6 was injected with 1 mL of normal saline as a blank control. After 18 days, blood was collected, serum was separated, and stored at -20°C for antibody detection.
[0289] Mix equal amounts of No. 1 to No. 4 sera, and take 0.4 mL of the mixed serum and 0.8 mL of Clostridium putrefaciens toxin (containing 4 mouse MLDs) and Clo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com