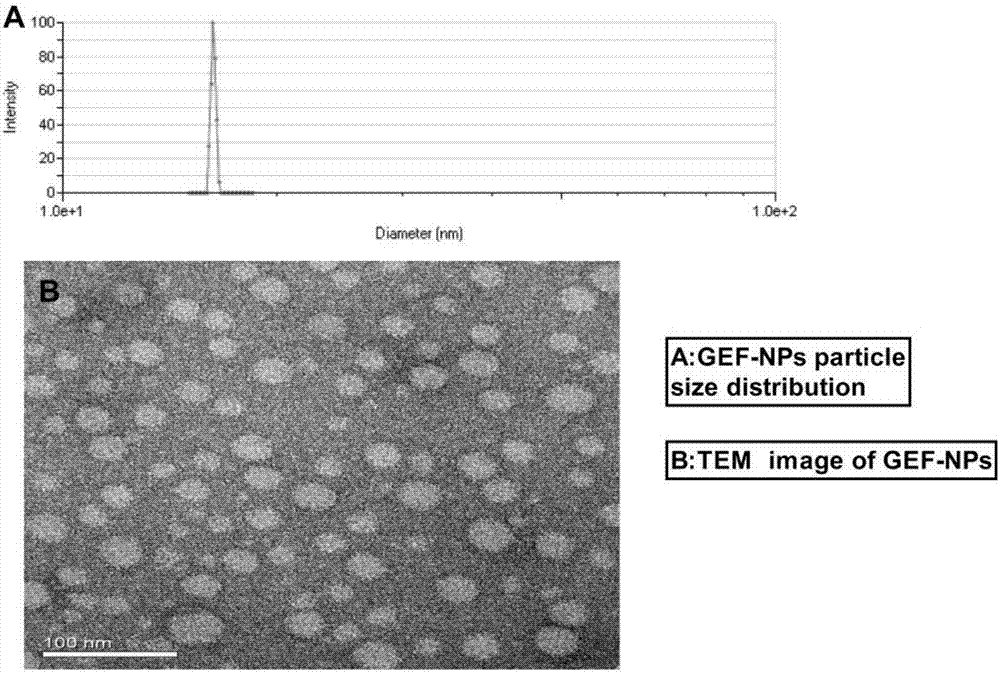
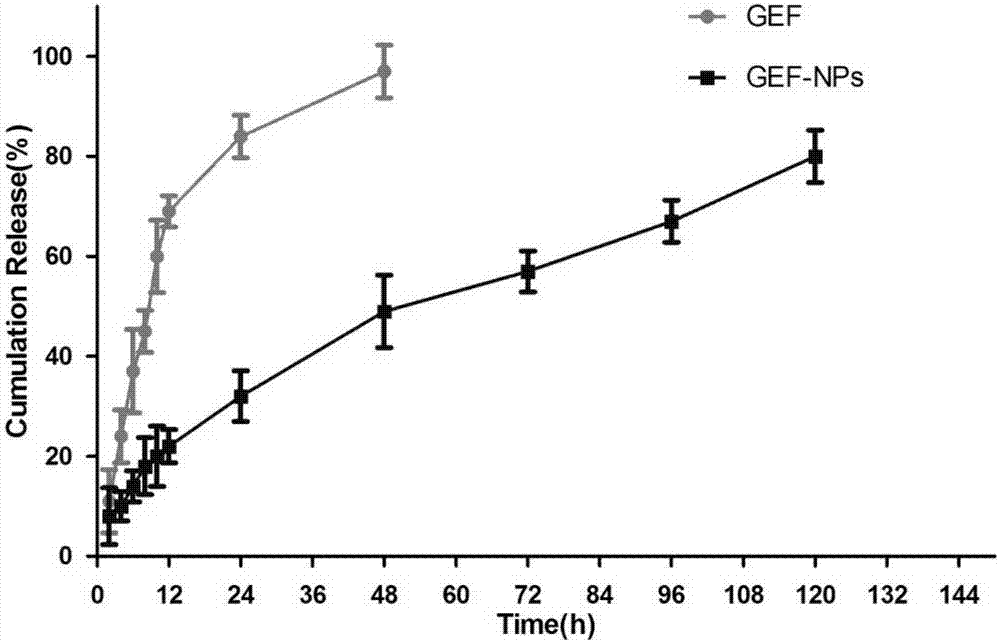
Nano-drug sustained release preparation for treating malignant tumor and preparation method thereof
A technology of sustained-release preparations and nano-drugs, applied in the field of medicine, can solve problems such as greater influence on solubility, many adverse reactions, slow oral absorption, etc., and achieve the effects of prolonging survival time, simple preparation method, and preventing agglomeration and precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation and application of PLA (polylactic acid) nano-sustained-release preparations loaded with gefitinib:
[0029] Prepared by emulsification volatilization method: Weigh 10 mg of gefitinib and 90 mg of PLA (polylactic acid) polymers and dissolve them in 5 ml of dichloromethane, then add 50 ml of 1% poloxamer F68 aqueous solution, and mix at high speed The mass machine was cut at a speed of 6000-15000 rpm for 5-20 minutes, and then the organic solvent was removed by rotary evaporation under reduced pressure at room temperature for 1 hour to obtain a nanoparticle suspension. The above-mentioned prepared nanoparticles were filtered and centrifuged. After several times, add a freeze-drying protection agent for freeze-drying storage, and the particle size of the nanoparticles prepared above is controlled within the range of 10-1000 nanometers.
[0030] Redissolve the above-mentioned gefitinib nano drug powder after freeze-drying in normal saline or glucose injection t...
Embodiment 2
[0032] Preparation and application of PEG-PCL (polymer of polyethylene glycol and polycaprolactone) nano-sustained-release preparations loaded with gefitinib:
[0033] Prepared by film emulsification method: Weigh 10 mg of gefitinib and 90 mg of PEG-PCL polymer and dissolve them in 5 ml of absolute ethanol, then remove the organic solvent by rotary evaporation under reduced pressure at room temperature for 1 hour, and the addition temperature is 10-65 Oscillating and redissolving in deionized water at ℃ to obtain a nanoparticle suspension. After filtering the above-mentioned nanoparticles, add a freeze-drying protective agent and freeze-dry them for storage. The particle size of the above-mentioned nanoparticles is controlled within the range of 10-1000 nanometers .
[0034] The gefitinib nano-drug freeze-dried powder prepared above was redissolved in normal saline or glucose injection to obtain the sustained-release injection of gefitinib nano-drug, and a mouse liver cancer m...
Embodiment 3
[0036]Preparation and application of Pluronic-PLGA (a polymer of poloxamer and polylactic-glycolic acid) nano-sustained-release formulation loaded with icotinib:
[0037] Prepared by self-assembly method: Weigh 20 mg of Icotinib and 80 mg of Pluronic-PLGA polymer dissolved in 5 ml of acetone, then slowly add dropwise to 50 ml of 1% poloxamer F68 aqueous solution, and then reduce at room temperature Press rotary evaporation for 1 hour to remove the organic solvent to obtain a nanoparticle suspension. After the above-mentioned nanoparticles are filtered and centrifuged for several times, they are added with a freeze-drying protective agent and freeze-dried. The particle size of the above-mentioned nanoparticles is controlled at In the range of 10-1000 nanometers.
[0038] The above-mentioned icotinib nano-medicine powder after freeze-drying was redissolved in normal saline or glucose injection to prepare gefitinib nano-medicine sustained-release injection, and a rat gastric canc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com